Abstract
We have previously demonstrated that glycosylphosphatidylinositol (GPI) anchors strongly influence protein trafficking in the procyclic insect stage of Trypanosoma brucei (M. A. McDowell, D. A. Ransom, and J. D. Bangs, Biochem. J. 335:681-689, 1998), where GPI-minus variant surface glycoprotein (VSG) reporters have greatly reduced rates of endoplasmic reticulum (ER) exit but are ultimately secreted. We now demonstrate that GPI-dependent trafficking also occurs in pathogenic bloodstream trypanosomes. However, unlike in procyclic trypanosomes, truncated VSGs lacking C-terminal GPI-addition signals are not secreted but are mistargeted to the lysosome and degraded. Failure to export these reporters is not due to a deficiency in secretion of these cells since the N-terminal ATPase domain of the endogenous ER protein BiP is efficiently secreted from transgenic cell lines. Velocity sedimentation experiments indicate that GPI-minus VSG dimerizes similarly to wild-type VSG, suggesting that degradation is not due to ER quality control mechanisms. However, GPI-minus VSGs are fully protected from degradation by the cysteine protease inhibitor FMK024, a potent inhibitor of the major lysosomal protease trypanopain. Immunofluorescence of cells incubated with FMK024 demonstrates that GPI-minus VSG colocalizes with p67, a lysosomal marker. These data suggest that in the absence of a GPI anchor, VSG is mistargeted to the lysosome and subsequently degraded. Our findings indicate that GPI-dependent transport is a general feature of secretory trafficking in both stages of the life cycle. A working model is proposed in which GPI valence regulates progression in the secretory pathway of bloodstream stage trypanosomes.
All eukaryotes utilize glycosylphosphatidylinositol (GPI) as an alternative method for attaching proteins to the lipid bilayer (32). GPI anchor attachment to a protein, directed by a C-terminal hydrophobic GPI attachment peptide (GPI peptide), occurs via a transamidation reaction in the endoplasmic reticulum (ER). This results in cleavage of the GPI peptide from the protein and attachment of a preformed GPI anchor to the mature C terminus. There is a growing body of evidence suggesting that GPI anchors are also involved in protein trafficking (32). In polarized epithelial cells, GPI anchors may play a role in post-Golgi sorting by targeting proteins to the apical surface through its association with cholesterol and sphingolipid-rich microdomains (8, 26). GPI anchors have also been implicated to function in ER exit in both mammalian cells and yeast. When GPI attachment is compromised either specifically by mutating the GPI peptide (13, 15) or globally by blocking GPI synthesis (17), the resulting unprocessed proteins cannot leave the ER. However, since the GPI peptide is present in these cases, it is difficult to assess whether ER retention is due to the presence of the GPI peptide or the absence of a GPI anchor.
Our laboratory has investigated the role of GPI structures in the intracellular trafficking of membrane proteins in African trypanosomes (Trypanosoma brucei spp.), the parasitic protozoan responsible for African sleeping sickness. The trypanosomal life cycle alternates between the midgut of the tsetse fly and the bloodstream of mammals. Trypanosomes have a different GPI-anchored protein surface coat corresponding to each stage of the life cycle. Monomeric procyclin is the coat component expressed in insect-stage trypanosomes, whereas variant surface glycoprotein (VSG) homodimers comprise the densely packed uniform coat on the cell surface of bloodstream trypanosomes. Our previous studies explored the role of GPI anchors in secretory trafficking by heterologous expression of VSG, with or without its GPI attachment peptide, in transformed procyclic trypanosomes (29). Deletion of the GPI peptide reduces the rate of VSG transport fivefold relative to GPI-anchored controls (t1/2 ∼5 h versus t1/2 ∼1 h), resulting in the accumulation in the ER. This reduced rate does not appear to be due to misfolding because GPI-minus VSG dimerizes efficiently. Furthermore, when a second soluble reporter, BiPN, is coexpressed, it is exported rapidly (t1/2 ∼1 h), indicating that there is no defect in general secretion due to overexpression of mutated VSG reporters. These results suggest that GPI anchors act as positive forward transport signals in the early secretory pathway of procyclic trypanosomes.
We now investigate whether GPI anchors influence the secretion of GPI-anchored proteins in bloodstream trypanosomes in order to determine if GPI-dependent trafficking is stage specific. Like procyclic trypanosomes, steady-state GPI-minus VSG resides in the ER, suggesting that the absence of a GPI anchor leads to a delay in ER exit. Unlike procyclics, however, VSG lacking its GPI anchor is not efficiently secreted. Instead, the majority of it is intracellularly degraded. GPI-minus VSG is protected from degradation by FMK024, an inhibitor of the major lysosomal protease trypanopain (11), suggesting that it is mistargeted to the lysosome and subsequently degraded. Our findings indicate that GPI-dependent transport in bloodstream stage trypanosomes not only manifests itself in the early secretory pathway, as it does in procyclics, but also in the late secretory pathway. Therefore, GPI-dependent protein trafficking is a general feature of secretory trafficking in both stages of African trypanosomes and also has stage-specific aspects.
MATERIALS AND METHODS
Maintenance and manipulation of trypanosomes.
All stable transformants were generated in cultured bloodstream forms of the Lister 427 strain of Trypanosoma brucei brucei. VSG117 reporters were introduced into MITat 1.2 cells (expressing endogenous VSG221), and VSG221 reporters were introduced into MITat 1.4 cells (expressing endogenous VSG117). The maintenance, transformation, and radiolabeling of cultured bloodstream cells have been previously described (1). The proteasomal inhibitor lactacystin (Calbiochem, San Diego, Calif.) and the thiol protease inhibitor FMK024 (morpholinourea-phenylalanine-homophenylalanine-fluoromethyl ketone; Enzyme Systems Products, Livermore, Calif.) were dissolved in dimethyl sulfoxide and added to cultures at dilutions of 1/100 or greater.
Immunoprotocols, antibodies, and electrophoresis.
Immunoprecipitation, electrophoresis, fluorography, and phosphorimaging have all been reported previously (1, 4). All cell lysates and media fractions were supplemented with protease inhibitor cocktail (2 μg each of leupeptin, antipain, chymostatin, and pepstatin/ml) plus 0.1 mM tosyllysine chloromethyl ketone. Rabbit anti-BiP, anti-VSG117, anti-VSG221, and mouse anti-p67 have already been described (1, 3, 29). Rabbit anti-VSG117 sera were first negatively selected over a VSG221 column in order to remove any antibodies recognizing the cross-reacting determinant epitope (42). The resultant flowthrough was next positively selected over a VSG117 column. Affinity-purified anti-VSG221 was prepared similarly by first negative selection with a VSG117 column and then positive selection with a VSG221 column. The resultant affinity-purified anti-VSG117 and anti-VSG221 were confirmed to be monospecific by immunoblotting (data not shown).
Construction of reporter genes.
The construction of the VSG117, VSG221, and BiPN reporter genes (Fig. 1) has been described previously (3, 4). All reporters were cloned into the bloodstream stage constitutive expression vector pXS5, which has been previously described (1). Transfection of cultured bloodstream stage trypanosomes with linearized vector and subsequent selection with G418 has been described previously (1).
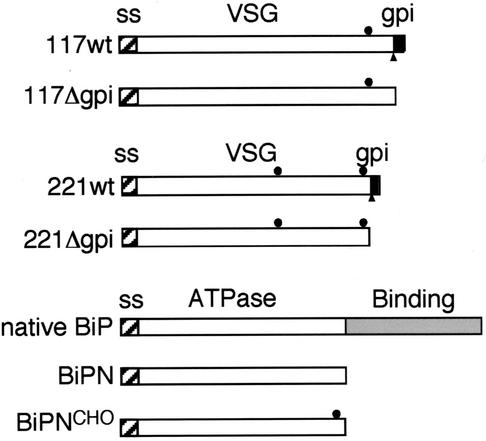
FIG. 1.
Diagram of secretory reporters. Diagrammatic representations of VSG 117, VSG 221, and BiP reporters are shown (scale approximate.) The hatched boxes denote N-terminal signal sequences. The filled circles indicate N-linked glycans. The black boxes signify the GPI attachment peptide, and the filled triangle shows the site of cleavage and GPI attachment to VSG. The native BiP structure is shown for comparison. The BiP ATPase and peptide binding domains are indicated.
Velocity sedimentation analysis.
Bloodstream cells (5 × 108) were pulse-labeled with 35S-labeled Met-Cys (Expre35S35S; Dupont NEN, Boston, Mass.) for 5 min and then chased for 5 min. Cells were then washed with HEPES buffered saline (50 mM HEPES-NaOH [pH 7.5], 50 mM NaCl, 5 mM KCl, 70 mM glucose) and lysed in TEN buffer (50 mM Tris-HCl, pH 7.5; 150 mM NaCl; 5 mM EDTA) with 1% NP-40. Extracts were clarified (30 min at 100,000 × g by using a Beckman 70.1 Ti rotor), then loaded onto an 11-ml linear 10 to 20% (wt/vol) sucrose gradients in 50 mM Tris-HCl (pH 7.5) with 0.1% NP-40, and centrifuged for 24 h (200,000 × g at 25°C by using a Beckman SW41 Ti rotor). After centrifugation, 650-μl fractions were collected from the top, and both radiolabeled endogenous VSGs and transgenic VSGΔgpi reporters were detected by specific immunoprecipitation.
Immunofluorescence.
Immunostaining of fixed and permeabilized bloodstream stage cells has been previously described (1). Specific staining was visualized with the appropriate Alexa 488- and Alexa 633-conjugated secondary reagents (Molecular Probes, Seattle, Wash.). Serial image Z-stacks (0.2-μm increments) were collected at ×100 magnification on a motorized Zeiss Axioplan IIi equipped with a rear-mounted excitation filter wheel, a triple-pass (DAPI [4′,6′-diamidino-2-phenylindole]-fluorescein isothiocyanate-Texas red) emission cube, differential interference contrast optics, and a Zeiss AxioCam B&W charge-coupled device camera. Fluorescence images were deconvolved by a constrained iterative algorithm, pseudocolored, and merged by using OpenLabs 3.0 software (Improvision, Inc., Lexington, Mass.).
RESULTS
Soluble secretion in bloodstream stage trypanosomes.
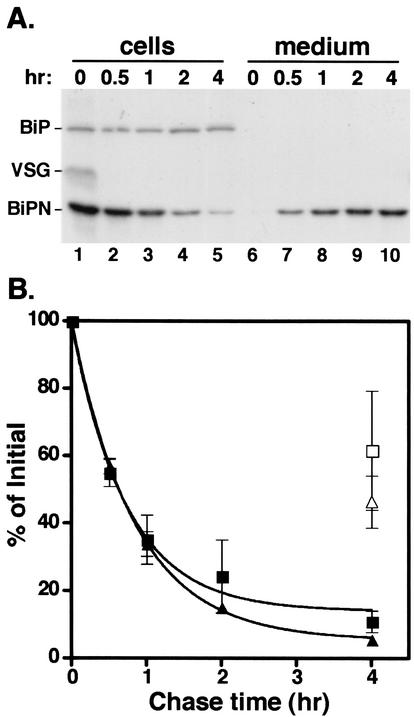
We wanted to study the role of GPI anchors in secretory trafficking, but since there are no characterized endogenous soluble secretory proteins in either stage of the trypanosome life cycle, we sought first to determine whether soluble secretion is possible in bloodstream stage parasites. Extracellular parasite-derived proteolytic activities have been reported, but it is not clear if these are actively secreted from cells (30, 36). However, our laboratory has previously engineered and characterized a soluble reporter (BiPN; Fig. 1) that is actively secreted from transgenic procyclic trypanosomes (3). Using a constitutive expression vector, BiPN was transfected into bloodstream cells expressing endogenous VSG221. In pulse-chase experiments (Fig. 2A), BiPN is initially cell associated but rapidly disappears from cell fractions (lanes 1 to 5) concomitant with its appearance in the media (lanes 6 to 10). Endogenous, full-length BiP is also detected in this assay but remains cell associated (lanes 1 to 5). As expected, a small amount of endogenous VSG transiently coprecipitates with BiP at the beginning of the chase (lane 1). We have previously shown that VSG physically associates with BiP as part of its normal protein-folding pathway (3). The kinetics of BiPN secretion were quantified by phosphorimager analysis (Fig. 2B).
FIG. 2.
Secretion of BiPN. (A) Bloodstream 221 cells expressing transgenic BiPN reporter were pulse-radiolabeled for 10 min with 35S-labeled Met-Cys and then chased for 4 h. BiP polypeptides were immunoprecipitated from cell and medium fractions at the indicated times. Samples were then analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and fluorography. Each lane has 5 × 106 cell equivalents. The positions of endogenous BiP, VSG, and the BiPN reporter have been indicated. (B) The experiment shown in panel A was performed in triplicate, and the amount of both the cell-associated and secreted forms of the two different BiPN reporters were quantified by phosphorimaging. Cell-associated (solid symbols) and medium-accumulated (open symbols; only the final datum point is shown) BiPN as a percentage of time zero (squares, means ± the standard error) are plotted as a function of chase time. The same analysis was also performed on a glycosylated version of BiPN (BiPNCHO, triangles).
In order to determine whether glycosylation affects secretory kinetics, the same analysis was also performed on a glycosylated version of BiPN (BiPNCHO; Fig. 1). BiPN and BiPNCHO are exported from bloodstream cells with t1/2 values of ∼36 and 38 min, respectively. Pulse-labeling of transgenic BiPNCHO cells in the presence of tunicamycin, an inhibitor of N glycosylation, confirms that all of the BiPNCHO detected is glycosylated (data not shown). Therefore, the addition of an N-glycan has no effect on the secretion kinetics of BiPN. These results demonstrate that the flagellar pocket is not an a priori barrier to the export of soluble proteins in bloodstream trypanosomes.
Fate of GPI-minus VSGs.
Two transgenic bloodstream cell lines expressing GPI-minus VSG reporters (Fig. 1) were generated. Trypanosomes expressing endogenous VSG221 were stably transfected with 117Δgpi, whereas trypanosomes expressing endogenous VSG117 were transfected with 221Δgpi. These reporters have stop codons engineered immediately downstream of the GPI attachment site, thereby preventing GPI addition and membrane attachment. GPI-anchored versions of these VSGs have also been shown not to heterodimerize in similar transfection experiments (33). Pulse-chase analyses were conducted with both cell lines in order to examine the GPI dependence of secretory transport in this stage of the life cycle (Fig. 3). In the case of 117Δgpi (Fig. 3A), a doublet of newly synthesized polypeptides is seen at time zero (lane 1). Experiments with tunicamycin indicate that the lower band represents newly synthesized 117Δgpi that has not yet received its single N-glycan (data not shown). Nonglycosylated 117Δgpi is rapidly converted to the mature form, which then disappears from the cell fraction as a function of chase time (lanes 2 to 5). Delayed core glycosylation has been observed previously with VSGs (2, 19). Surprisingly, very little of the mature soluble 117Δgpi reporter is exported to the media at any time point (lanes 6 to 10). In contrast, endogenous GPI-anchored VSG 221 remains fully cell associated throughout the chase period (lanes 11 and 12). Similarly, transgenic 221Δgpi disappears from trypanosomes over a 4-h period, yet little is recovered in the media (Fig. 3B), whereas endogenous VSG 117 remains cell associated throughout the duration of the chase. The upper band seen in Fig. 3B (lanes 4 and 5) is most likely endogenous VSG 117 coimmunoprecipitating with 221Δgpi as a nonspecific contaminant. Disappearance of the GPI-minus VSG reporters was quantified to determine the precise rate of degradation (Fig. 3C). 117Δgpi and 221Δgpi have turnover rates with t1/2 values of 90 and 47 min, respectively. Approximately 5% of initial 117Δgpi and ∼25% of 221Δgpi accumulate stably in the media, indicating that little VSG is secreted. These data suggest that most GPI-minus VSG is subject to rapid degradation in an internal location. We have also tested transgenic GPI-anchored versions of VSG117 and VSG221 as appropriate controls for reporter expression and recovery (data not shown). In each case the transgenic GPI-anchored VSGs were quantitatively recovered at the end of the chase period, as we have demonstrated for the endogenous VSGs (Fig. 3). These results differ dramatically from those seen in procyclic trypanosomes where GPI-minus VSGs are efficiently (>90%) secreted to the media, albeit with fivefold-slower transport kinetics compared to their GPI-anchored counterparts (29).
FIG. 3.
Fate of GPI-minus VSG. (A and B) Transgenic cell lines expressing GPI-minus VSG reporters 117Δgpi (A; endogenous VSG 221) or 221Δgpi (B; endogenous VSG 117) were pulse-chase radiolabeled as in Fig. 2. Immunoprecipitated VSG polypeptides were analyzed by SDS-PAGE and fluorography. All VSGΔgpi samples have 5 × 106 cell equivalents per lane. Immunoprecipitations of endogenous VSGs (A; VSG 221, 5 × 105 cell equivalents per lane; B, VSG 117, 2.5 × 105 cell equivalents per lane) were analyzed as internal GPI-anchored controls. (C) Cell-associated (solid symbols) and medium-accumulated (open symbols) VSGΔgpi reporters (117Δgpi, squares; 221Δgpi, triangles) were quantified by phosphorimaging in three independent experiments. Cell-associated and secreted VSGΔgpis as a percentage of time zero (mean ± the standard error) are plotted as a function of the chase time.
VSGΔgpi folding and/or dimerization.
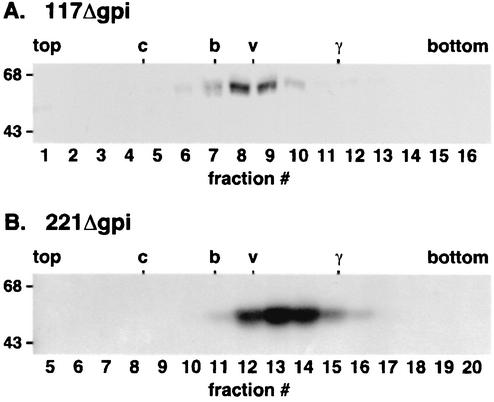
One possible explanation for the failure of VSGs to be secreted is misfolding and subsequent retention by ER quality control mechanisms. Since endogenous GPI-anchored VSG is a homodimer of 55- to 60-kDa subunits, we use dimerization as a hallmark for proper folding. Extracts of cells that had been pulse radiolabeled and chased briefly were fractionated by velocity sedimentation. The positions of the newly synthesized GPI-minus reporters were determined relative to internal molecular mass markers and to endogenous GPI-anchored VSG (Fig. 4). Both 117Δgpi (Fig. 4A) and 221Δgpi (Fig. 4B) sedimented at a position between internal BSA (67-kDa) and bovine gamma globulin (∼170-kDa) markers, at a region coincident with endogenous homodimeric VSG. These data indicate that the GPI-minus reporters are rapidly folded and assembled into homodimers in the absence of GPI-mediated membrane association.
FIG. 4.
Dimerization of GPI-minus VSG. Detergent extracts of radiolabeled transgenic bloodstream cells (5-min pulse, 5-min chase) were fractionated by velocity sedimentation. The sedimentation positions of transgenic 117Δgpi (A) and 221Δgpi (B) and endogenous, GPI-anchored VSG (data not shown) were determined by immunoprecipitation. Samples of each fraction were analyzed by SDS-PAGE, and the sedimentation positions of internal molecular mass standards (c = carbonic anhydrase, 31 kDa; b = bovine serum albumin, 68 kDa; γ = bovine gamma globulin, 170 kDa) were determined by Coomassie blue staining. The peak positions of internal markers and endogenous VSG are indicated. The scale refers to the molecular mass in kilodaltons.
VSGΔgpi degradation.
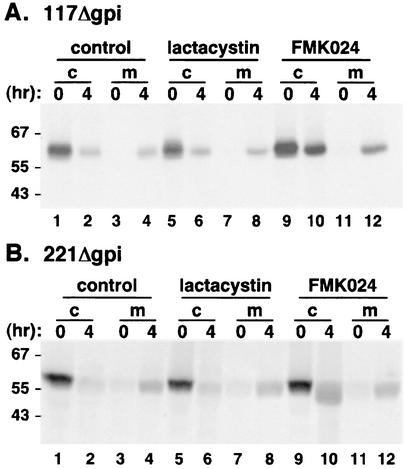
The two most well-characterized sites of protein turnover are the cytoplasm, where proteins are degraded by the proteasome and the lysosome. To determine the location of GPI-minus VSG degradation, pulse-chase experiments were performed in the presence of different inhibitors. Cells expressing 117Δgpi were pulsed and chased in untreated conditions, in the presence of 1 μM lactacystin, a proteasomal inhibitor (34), or in the presence of 2 μM FMK024, a potent cysteine protease inhibitor (Fig. 5A). Trypanopain, a cathepsin L-like thiol protease, is the major lysosomal protease in T. brucei and is irreversibly inhibited by FMK024-like compounds (27, 39). Little 117Δgpi is detected in untreated and lactacystin-treated cell fractions after 4 h (lanes 2 and 6), yet elevated levels of the labeled 117Δgpi are still present in the FMK024-treated cell fraction (lane 10). Similarly, 221Δgpi degradation (Fig. 5B) is unaffected by lactacystin and is blocked by FMK024, although at higher concentrations than is required for 117Δgpi. These results imply that proteolysis of the GPI-minus VSG reporters is not mediated by the proteasome but that turnover is most likely lysosomal.
FIG. 5.
Effect of FMK024 on VSGΔgpi degradation. Transgenic cell lines stably expressing 117Δgpi (A) or 221Δgpi (B) were pretreated for 1 h in the presence or absence of the proteasomal inhibitor lactacystin (1 μM) or the cysteine protease inhibitor FMK024 (117Δgpi, 2 μM; 221Δgpi, 20 μM). Cells were then radiolabeled (10-min pulse, 4-h chase) in the continued presence or absence of inhibitor. At the indicated chase times, aliquots (5 × 106 cell equivalents) were removed and 117Δgpi (A) or 221Δgpi (B) was immunoprecipitated from cell (c) and medium (m) fractions. Samples were analyzed by SDS-PAGE and fluorography.
Immunofluorescence assays were performed to determine the location of degradation more precisely (Fig. 6). In untreated bloodstream cells, both 117Δgpi and 221Δgpi colocalize with the ER molecular chaperone BiP (Fig. 6E and M, yellow) but not with the lysosomal membrane protein p67, which stains a discrete postnuclear vacuole (Fig. 6F and N, red). These staining patterns suggest that steady-state levels of the GPI-minus VSG reporters are located primarily in the ER. In FMK024-treated cells the GPI-minus VSG reporters continue to colocalize with BiP (Fig. 6G and O, yellow) but, in addition, are found in a prominent postnuclear compartment (green, arrowheads). This compartment was confirmed as the lysosome by robust colocalization of the GPI-minus VSG reporters with p67 in FMK024-treated cells (Fig. 6H and P, yellow, arrowheads). Inhibition of trypanopain is known to cause lysosomal swelling and engorgement in T. brucei (1) and, in these experiments, leads to a substantial accumulation of GPI-minus VSG in the lysosome. Taken together, these results suggest that GPI-minus VSGs are first retained in the ER and are then mistargeted to the lysosome where they are degraded.
FIG. 6.
Localization of VSGΔgpi in bloodstream cells. Bloodstream cells expressing either 117Δgpi (A to H) or 221Δgpi (I to P) were incubated for 2 h in the absence (A, B, E, F, I, J, M, and N) or presence (C, D, G, H, K, L, O, and P) of FMK024 (117Δgpi, 2 μM; 221Δgpi, 20 μM). Fixed and permeabilized cells were stained as follows: panels E, G, M, and O, anti-BiP (red) and anti-VSGΔgpi (green); panels F, H, N, and P, anti-p67 (red) and anti-VSGΔgpi (green). All samples were counterstained with DAPI to reveal the nucleus (n) and the kinetoplast (k). Three channel merged images are presented in which colocalization is represented as yellow. The corresponding differential interference contrast-DAPI merged images are presented above each panel. The insets in panels F to H and panels N to P are the corresponding single channel images (p67, red; VSGΔgpi, green) in the region of the lysosome.
DISCUSSION
Using the BiPN reporter, we have demonstrated for the first time that soluble secretion is possible in bloodstream stage African trypanosomes. This was previously established in procyclic trypanosomes (3) but, given the heightened endocytic activity of bloodstream parasites (37) and the obligate intersection of secretion and endocytosis in the flagellar pocket (28), this was an open question. Active export of endogenous proteins by the classical secretory pathway has never been demonstrated in any stage of the trypanosome life cycle. Our results reveal that the flagellar pocket allows secretion of soluble polypeptides. Furthermore, it now seems formally possible that the parasite might export endogenous factors that could influence pathogenicity in the mammalian host.
GPI anchors have been implicated in protein trafficking in other eukaryotic organisms (26, 32). We have previously demonstrated that GPI anchors are necessary for the proper transport of ectopically expressed VSG in procyclic trypanosomes (29). In this case, soluble GPI-minus VSG is exported from cells ∼5 times more slowly than transport of control GPI-anchored VSG to the cell surface. The reduced rate of secretion is manifested as an accumulation in the ER that is not easily accounted for by global misfolding. The implication is that GPI anchors provide essential forward trafficking information required for ER exit and that once exit is achieved, subsequent secretion proceeds in an unimpeded manner. Our current findings indicate that bloodstream stage trypanosomes also display GPI-dependent protein trafficking in the form of delayed ER exit, as suggested by the steady-state accumulation of GPI-minus VSG in the ER. In addition to influencing the transport of VSG in the early secretory pathway, GPI anchors also affect VSG trafficking in the late secretory pathway in bloodstream cells. Instead of being secreted quantitatively as it is in procyclics, only 5 to 25% of GPI-minus VSG is secreted, whereas the rest is targeted to the lysosome where it is degraded. The rate-limiting step in trafficking of endogenous GPI-anchored VSG, as judged by N-glycan processing, is ER-to-Golgi transport (t1/2 ∼15 min) (2, 18). For the GPI-minus reporters, lysosomal degradation provides a first approximation of the rate of intracellular transport (t1/2 ∼45-90 min), which is about three- to sixfold slower than the transport of native VSG. Unfortunately, efforts to directly measure the rate of ER exit using Golgi-specific N-glycan modifications of VSG221 as a hallmark for transport were unsuccessful. Nevertheless, given the obvious accumulation of GPI-minus VSG in the ER, our findings strongly suggest that ER exit is the rate-limiting step in transport. These results confirm and extend the recent findings of Bohme and Cross (6), who used a construct identical to the 117Δgpi reporter used here. In a broader study of the sequence specificity of GPI addition, these authors found that GPI-minus VSG was degraded by a nonproteasomal mechanism and suggested that turnover occurred in the lysosome.
One possible explanation for delayed ER exit is that the absence of a GPI anchor impairs the ability of VSG to properly fold, leading to retention by quality control machinery. Native VSG has been shown to physically interact with endogenous BiP during normal folding (3). However, misfolded proteins in the ER are typically retrotranslocated to the cytosol for degradation by the proteasome (7, 21), and our data establish that degradation of GPI-minus VSG is a lysosomal process. Furthermore, we find that newly synthesized GPI-minus VSG rapidly dimerizes, suggesting proper folding. VSG dimers are elongated structures with extensive faces of intermolecular contact throughout the N-terminal two-thirds of the monomers (5). Thus, assuming that proper quaternary structure requires proper secondary and tertiary structure, our results suggest that a defect or delay in folding cannot account for the observed ER accumulation of GPI-minus VSG. However, nothing is known about the structure of the VSG C-terminal domain, nor can the dimerization assay take into account small, localized conformational changes in VSG structure. Butikofer et al. found that enzymatic removal of the GPI diacylglycerol moiety significantly affects the reactivity of VSG with specific antibodies, presumably due to the induction of conformational changes (10). It is possible that similar changes in GPI-minus VSG could lead to ER retention by a quality-control mechanism. An alternative explanation for ER accumulation, as we have previously suggested for GPI-minus VSG in procyclic trypanosomes, is that the GPI anchor provides essential information for ER exit. Muniz et al. demonstrated in a yeast-derived in vitro system that GPI-anchored proteins exit the ER in a distinct vesicle population from endogenous transmembrane or soluble cargo (31), suggesting that GPI anchors can act as ER sorting determinants. The mechanism of sorting is not clear, but this work is consistent with GPI-mediated ER sorting in trypanosomes.
GPI anchors apparently also play a role in trafficking in the late secretory pathway, as deletion of the GPI peptide results predominantly in lysosomal targeting of truncated VSG in bloodstream stage trypanosomes. Interestingly, in bloodstream cells the major fate of BiPN, which is not N glycoslylated, is secretion (∼60%). Initially, it seemed possible that the N-glycans on the GPI-minus VSG reporters (117Δgpi, one glycan; 221Δgpi, two glycans [23]) might mediate lysosomal trafficking. However, there is no evidence for such targeting signals in trypanosomes, and the addition of an N-glycan to BiPN had no effect on its rate or extent of secretion. A more likely explanation is that GPI anchors are targeting proteins to the plasma membrane in a manner similar to that in polarized epithelial cells (22). In this system, GPI-anchored proteins associate with lipid rafts that are rich in cholesterol and sphingolipids and are thereby selectively targeted to the apical membrane (8, 26). VSG does segregate into detergent insoluble complexes, a hallmark of association with lipid rafts (16, 35). Furthermore, an increasing 50-fold density gradient of VSG exists in the trypanosome secretory pathway (ER < Golgi < endosomes/flagellar pocket < plasma membrane) (20). Bloodstream trypanosomes have an extraordinary rate of endocytosis (14) and perhaps GPI-minus VSG, lacking the proper membrane interaction, is delivered by default to the lysosome by “endocytic backflow.” We have reported a similar phenomenon with truncations of the lysosomal membrane protein p67 that are secreted from procyclic trypanosomes yet are still delivered to the lysosome in bloodstream cells (1). This begs the question of why BiPN is secreted more efficiently. We can only speculate about this paradox, but one explanation may be that BiPN is a small compact protein (45 kDa) (3), thus allowing more effective escape from backflow than the larger dimeric VSG constructs.
Finally, our results with GPI-minus VSG, in conjunction with the known behaviors of native VSG and transferrin receptor, present an intriguing situation. All of these proteins are members of the larger VSG superfamily (12). GPI-minus VSG is homodimeric; it has no GPI anchors and is rapidly delivered to the lysosome and degraded (t1/2 ∼0.75 to 1.5 h). Native VSG is homodimeric with two GPI anchors, and its steady-state location is the plasma membrane. It is an extremely stable protein and, despite being endocytosed and recycled (40, 41), is turned over (t1/2 ∼30 h) by GPI hydrolysis and release (9, 40). Strikingly, transferrin receptor is intermediate in all its behaviors. It is a heterodimer of ESAG6 and ESAG7 (25, 38). It has a single GPI anchor on ESAG6. Its steady-state location is the flagellar pocket, from which it is constantly endocytosed and recycled, and its turnover occurs in the lysosome with a t1/2 of ∼7 h (24). Thus a natural titration exists in bloodstream trypanosomes such that increasing GPI valence correlates directly with ultimate progression in the secretory pathway and inversely with rate of turnover. This speculation will have to be tested by experimental manipulation of GPI valence in heterologous dimeric reporters, but it does serve to underscore the role of GPI anchors in proper trafficking of VSG and assembly of the essential bloodstream surface coat.
Acknowledgments
We are indebted to Anant Menon for thoughtful discussion and comments.
This work was supported by National Institutes of Health grant AI35739 to J.D.B. J.D.B. is a Burroughs Wellcome Fund New Investigator in Molecular Parasitology. V.P.T. was supported by a GEM/NIH Ph.D. Science Fellowship, a National Science Foundation predoctoral fellowship, a UW-Madison Advanced Opportunity predoctoral fellowship, and the NIGMS UW-Madison Biotechnology Training Program.
REFERENCES
- 1.Alexander, D., K. Schwartz, A. Balber, and J. D. Bangs. 2002. Developmentally regulated trafficking of the lysosomal membrane protein p67 in Trypanosoma brucei. J. Cell Sci. 115:3253-3263. [DOI] [PubMed] [Google Scholar]
- 2.Bangs, J. D., N. Andrews, G. W. Hart, and P. T. Englund. 1986. Posttranslational modification and intracellular transport of a trypanosome variant surface glycoprotein. J. Cell Biol. 103:255-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bangs, J. D., E. M. Brouch, D. M. Ransom, and J. L. Roggy. 1996. A soluble secretory reporter system in Trypanosoma brucei: studies on endoplasmic reticulum targeting. J. Biol. Chem. 271:18387-18393. [DOI] [PubMed] [Google Scholar]
- 4.Bangs, J. D., D. M. Ransom, M. A. McDowell, and E. M. Brouch. 1997. Expression of bloodstream variant surface glycoproteins in procyclic stage Trypanosoma brucei: role of GPI anchors in secretion. EMBO J. 16:4285-4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blum, M. L., J. A. Down, A. M. Gurnett, M. Carrington, M. J. Turner, and D. C. Wiley. 1993. A structural motif in the variant surface glycoprotein of Trypanosoma brucei. Nature 362:603-609. [DOI] [PubMed] [Google Scholar]
- 6.Bohme, U., and G. A. M. Cross. 2002. Mutational analysis of the variant surface glycoprotein GPI-anchor signal sequence in Trypanosoma brucei. J. Cell Sci. 115:805-816. [DOI] [PubMed] [Google Scholar]
- 7.Bonifacino, J. S., and A. M. Weissman. 1998. Ubiquitin and the control of protein fate in the secretory and endocytic pathways. Annu. Rev. Cell Dev. Biol. 14:19-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown, D. A., and J. K. Rose. 1992. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 68:533-544. [DOI] [PubMed] [Google Scholar]
- 9.Bulow, R., C. Nonnengasser, and P. Overath. 1989. Release of the variant glycoprotein during differentiation of bloodstream to procyclic forms of Trypanosoma brucei. Mol. Biochem. Parasitol. 32:85-92. [DOI] [PubMed] [Google Scholar]
- 10.Butikofer, P., T. Malherbe, M. Boschung, and I. Roditi. 2001. GPI-anchored proteins: now you see 'em, now you don't. FASEB J. 15:545-548. [DOI] [PubMed] [Google Scholar]
- 11.Caffery, C. R., E. Hansell, K. D. Lucas, L. S. Brinen, A. A. Hernandez, J. Cheng, S. L. Gwalteny, W. R. Roush, Y.-D. Stierhof, M. Bogyo, D. Steverding, and J. H. McKerrow. 2001. Active site mapping, biochemical properties and subcellular localization of rhodesain, the major cysteine protease of Trypanosoma brucei rhodesiense. Mol. Biochem. Parasitol. 118:61-73. [DOI] [PubMed] [Google Scholar]
- 12.Carrington, M., and J. Boothroyd. 1996. Implications of conserved structural motifs in disparate trypanosome surface proteins. Mol. Biochem. Parasitol. 81:119-126. [DOI] [PubMed] [Google Scholar]
- 13.Conzelmann, A., A. Spiazzi, C. Bron, and R. Hyman. 1988. No glycolipid anchors are added to Thy-1 glycoprotein in Thy-1-negative mutant thymoma cells of four different complementation classes. Mol. Cell. Biol. 8:674-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coppens, I., F. R. Opperdoes, P. J. Courtoy, and P. Baudhin. 1987. Receptor-mediated endocytosis in the bloodstream form of Trypanosoma brucei. J. Protozool. 34:344-349. [DOI] [PubMed] [Google Scholar]
- 15.Delahunty, M. D., F. J. Stafford, L. C. Yuan, D. Shaz, and J. S. Bonifacino. 2027. 1993. Uncleaved signals for glycosylphosphatidylinositol anchoring cause retention of precursor proteins in the endoplasmic reticulum. J. Biol. Chem. 268:12017-12021. [PubMed] [Google Scholar]
- 16.Denny, P. W., M. C. Field, and D. F. Smith. 2001. GPI-anchored proteins and glycoconjugates segregate into lipid rafts in Kinetoplastida. FEBS Lett. 491:148-153. [DOI] [PubMed] [Google Scholar]
- 17.Doering, T. L., and R. Schekman. 1996. GPI anchor attachment is required for Gas1p transport from the endoplasmic reticulum in COP II vesicles. EMBO J. 15:182-191. [PMC free article] [PubMed] [Google Scholar]
- 18.Duszenko, M., I. Ivanov, M. A. J. Ferguson, H. Plesken, and G. A. M. Cross. 1988. Intracellular transport of a variant surface glycoprotein in Trypanosoma brucei. J. Cell Biol. 106:77-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferguson, M. A. J., M. Duszenko, G. S. Lamont, P. Overath, and G. A. M. Cross. 1986. Biosynthesis of Trypanosoma brucei variant surface glycoprotein: N glycosylation and addition of a phosphatidylinositol membrane anchor. J. Biol. Chem. 261:356-362. [PubMed] [Google Scholar]
- 20.Grunfelder, C. G., M. Engstler, F. Weise, H. Schwarz, Y. D. Stierhof, M. Boshart, and P. Overath. 2002. Accumulation of a GPI-anchored protein at the cell surface requires sorting at multiple intracellular levels. Traffic 3:547-559. [DOI] [PubMed] [Google Scholar]
- 21.Hampton, R. Y. 2002. ER-associated degradation in protein quality control and cellular regulation. Curr. Opin. Cell Biol. 14:476-482. [DOI] [PubMed] [Google Scholar]
- 22.Harder, T., and K. Simons. 1997. Caveolae, DIGs, and the dynamics of sphingolipid-cholesterol microdomains. Curr. Opin. Cell Biol. 9:534-542. [DOI] [PubMed] [Google Scholar]
- 23.Holder, A. 1985. Glycosylation of the variant surface antigens of Trypanosoma brucei. Curr. Top. Microbiol. Immunol. 117:57-74. [DOI] [PubMed] [Google Scholar]
- 24.Kabiri, M., and D. Steverding. 2000. Studies on the recycling of the transferrin receptor in Trypanosoma brucei using an inducible gene expression system. Eur. J. Biochem. 267:3309-3314. [DOI] [PubMed] [Google Scholar]
- 25.Ligtenberg, M. J. L., W. Bitter, R. Kieft, D. Sterverding, H. Janssen, J. Calafat, and P. Borst. 1994. Reconstitution of a surface transferrin binding complex in insect form Trypanosoma brucei. EMBO J. 13:2565-2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lisanti, M., and E. Rodriguez-Boulan. 1990. Glycolipid membrane anchoring provides clues to the mechanism of protein sorting in polarized epithelial cells. Trends Biochem. Sci. 15:113-118. [DOI] [PubMed] [Google Scholar]
- 27.Lonsdale-Eccles, J. D., and D. Grab. 1987. Lysosomal and non-lysosomal peptidyl-hydrolases of the bloodstream forms of Trypanosoma brucei. Eur. J. Biochem. 169:467-475. [DOI] [PubMed] [Google Scholar]
- 28.McConville, M. J., K. A. Mullins, S. C. Ilgoutz, and R. H. Teasdale. 2002. Secretory pathway of trypanosomatid parasites. Microbiol. Mol. Biol. Rev. 66:122-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDowell, M. A., D. A. Ransom, and J. D. Bangs. 1998. Glycosyl phosphatidylinositol-dependent secretory transport in Trypanosoma brucei. Biochem. J. 335:681-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morty, R. E., J. D. Lonsdale-Eccles, R. Mentele, E. A. Auerswald, and T. H. T. Coetzer. 2001. Trypanosome-derived oligopeptidase B is released into the plasma of infected rodents, where it persists and retains full catalytic activity. Infect. Immun. 69:2757-2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muniz, M., P. Morsomme, and H. Riezman. 2001. Protein sorting upon exit from the endoplasmic reticulum. Cell 104:313-320. [DOI] [PubMed] [Google Scholar]
- 32.Muniz, M., and H. Riezman. 2000. Intracellular transport of GPI-anchored proteins. EMBO J. 19:10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muñoz-Jordan, J., K. P. Davies, and G. A. M. Cross. 1996. Stable expression of mosaic coats of variant surface glycoproteins in Trypanosoma brucei. Science 272:1795-1797. [DOI] [PubMed] [Google Scholar]
- 34.Mutomba, M. C., T. Wah-Yuen, W. C. Hyun, and C. C. Wang. 1997. Inhibition of proteasome activity blocks cell cycle progression at specific phase boundaries in African trypanosomes. Mol. Biochem. Parasitol. 90:491-504. [DOI] [PubMed] [Google Scholar]
- 35.Nolan, D. P., D. G. Jackson, M. J. Biggs, E. D. Brabazon, A. Pays, F. Van Laethem, F. Paturiaux-Hanocq, J. F. Elliot, H. P. Voorheis, and E. Pays. 2000. Characterization of a novel alanine-rich protein located in surface microdomains in Trypanosoma brucei. J. Biol. Chem. 275:4072-4080. [DOI] [PubMed] [Google Scholar]
- 36.Okenu, D. M. N., K. N. Opara, R. I. Nwuba, and M. Nwagwu. 1999. Purification and characterisation of an extracellularly released protease of Trypanosoma brucei. Parasitol. Res. 85:424-428. [DOI] [PubMed] [Google Scholar]
- 37.Overath, P., Y.-D. Stierhof, and M. Wiese. 1997. Endocytosis and secretion in trypanosomatid parasites: tumultuous traffic in a pocket. Trends Cell Biol. 7:27-33. [DOI] [PubMed] [Google Scholar]
- 38.Salmon, D., M. Geuskens, F. Hanocq, J. Hanocq-Quertier, D. Nolan, L. Ruben, and E. Pays. 1994. A novel heterodimeric transferrin receptor encoded by a pair of VSG expression site-associated genes in T. brucei. Cell 78:75-86. [DOI] [PubMed] [Google Scholar]
- 39.Scory, S., C. R. Caffrey, Y. D. Stierhof, A. Ruppel, and D. Steverding. 1999. Trypanosoma brucei: killing of bloodstream forms in vitro and in vivo by the cysteine proteinase inhibitor Z-Phe-Ala-CHN2. Exp. Parasitol. 91:327-333. [DOI] [PubMed] [Google Scholar]
- 40.Seyfang, A., D. Mecke, and M. Duszenko. 1990. Degradation, recycling and shedding of Trypanosoma brucei variant surface glycoprotein. J. Protozool. 37:546-552. [DOI] [PubMed] [Google Scholar]
- 41.Webster, P., and D. J. Grab. 1988. Intracellular colocalization of variant surface glycoprotein and transferrin-gold in Trypanosoma brucei. J. Cell Biol. 106:279-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zamze, S. E., M. A. J. Ferguson, R. Collins, R. A. Dwek, and T. W. Rademacher. 1988. Characterization of the cross-reacting determinant (CRD) of the glycosyl-phosphatidylinositol membrane anchor of Trypanosoma brucei variant surface glycoprotein. Eur. J. Biochem. 176:527-534. [DOI] [PubMed] [Google Scholar]