Abstract
Candida albicans, the most common human fungal pathogen, is particularly problematic for immunocompromised individuals. The reversible transition of this fungal pathogen to a filamentous form that invades host tissue is important for its virulence. Although different signaling pathways such as a mitogen-activated protein kinase and a protein kinase A cascade are critical for this morphological transition, the function of polarity establishment proteins in this process has not been determined. We examined the role of four different polarity establishment proteins in C. albicans invasive growth and virulence by using strains in which one copy of each gene was deleted and the other copy expressed behind the regulatable promoter MET3. Strikingly, mutants with ectopic expression of either the Rho G-protein Cdc42 or its exchange factor Cdc24 are unable to form invasive hyphal filaments and germ tubes in response to serum or elevated temperature and yet grow normally as a budding yeast. Furthermore, these mutants are avirulent in a mouse model for systemic infection. This function of the Cdc42 GTPase module is not simply a general feature of polarity establishment proteins. Mutants with ectopic expression of the SH3 domain containing protein Bem1 or the Ras-like G-protein Bud1 can grow in an invasive fashion and are virulent in mice, albeit with reduced efficiency. These results indicate that a specific regulation of Cdc24/Cdc42 activity is required for invasive hyphal growth and suggest that these proteins are required for pathogenicity of C. albicans.
In response to intra- and extracellular signals, the Rho family GTPase Cdc42 and its GDP-GTP exchange factors are crucial for cytoskeletal reorganization and transcriptional activation in eukaryotes. In Saccharomyces cerevisiae, Cdc42 and its exchange factor Cdc24 are necessary for polarized growth during budding and mating (14). In addition, Cdc42 has been shown to be required for pseudohyphal growth in S. cerevisiae (27, 28), a developmental state similar in many respects to hyphal growth of fungal pathogens (20). At least two signaling pathways are important for pseudohyphal growth: a cyclic AMP (cAMP)-dependent protein kinase pathway and a mitogen-activated protein (MAP) kinase-dependent pathway (28, 32, 33, 36). Ras signaling, via Ras2, is important in both of these pathways (16, 23, 28). Three of the four kinases in the MAP kinase cascade are also required for signaling in response to pheromone (8, 24, 35).
Dominant cdc42 mutants have been used to demonstrate that this G protein is involved in the MAP kinase signaling required for pseudohyphal growth (28). More recently, cdc42 mutants specifically defective in pseudohyphal growth have been isolated (27). Characterization of these mutants suggests that interactions between this G protein and PAK family kinases (Ste20 and Skm1) and Cdc42 and the CRIB domain containing proteins Gic1 and Gic2 are important for pseudohyphal growth. An interaction between GTP bound Cdc42 and Ste20, via its CRIB domain, is necessary for pseudohyphal growth (17). This interaction antagonizes the auto-inhibitory effect of the CRIB domain on Ste20 protein kinase activity, resulting in the activation of the kinase.
Although Cdc24 is necessary for polarized growth in S. cerevisiae, its role in pseudohyphal growth is less clear. In contrast to S. cerevisiae, CDC24 is not essential for viability in Schizosaccharomyces pombe (7). However, CDC24 appears to be essential for viability in the filamentous fungi Ashbya gossypii (42). Homokaryotic A. gossypii cdc24 mutant spores grow in an isotropic fashion, are unable to polarize, and do not induce hyphal growth, suggesting that this exchange factor may be necessary for hyphal filament formation.
In contrast to S. cerevisiae, which can only form pseudohyphae characterized by chains of elongated buds with constrictions at cell-cell junctions, the most common human fungal pathogen (34) Candida albicans can form true hyphae that have parallel cell walls lacking constrictions at the point of germ tube emergence. Depending on its environment, this pathogen can exist either as an ellipsoidal yeast-form or different filamentous (hyphal and pseudohyphal) forms (5, 26). A range of host signals, including temperature, pH, and serum, trigger these morphological changes (31). Similar to S. cerevisiae, both a MAP kinase cascade and a cAMP signaling pathway are necessary for the C. albicans yeast-to-hyphal transition (2, 5, 9, 19, 21, 26, 38, 39). Ras1 has been implicated in both C. albicans hyphal growth pathways (10, 18). Disruption of genes encoding proteins of either pathway results in strains with reduced virulence (19, 21), suggesting that the yeast-to-hyphal transition is important for pathogenicity.
Very recently, Cdc42 function in the cellular proliferation and polarized growth of C. albicans has been presented (41). In this pathogen, the G protein Cdc42 is necessary for polarized growth, as is the case in S. cerevisiae. However, the function of its exchange factor, Cdc24, in the C. albicans transition to the hyphal state is unknown. To study the function of Cdc24 in C. albicans, we made strains in which the only copy of CDC24 is under the control of a regulatable promoter. We compared the vegetative growth, the yeast-to-hyphal transition, and virulence of this strain and similar strains in which CDC42, BUD1, and BEM1 are under the control of the same regulatable promoter. Our results show that Cdc24, like Cdc42, is necessary for C. albicans viability. Furthermore, strains ectopically expressing CDC24 or CDC42, but not BUD1 or BEM1, are unable to form invasive hyphal filaments and are avirulent. Together, our results indicate that specific regulation of Cdc24/Cdc42 activity is required for invasive hyphal growth and suggest a role for this GTPase module in pathogenicity.
MATERIALS AND METHODS
Culture media.
Yeast extract-peptone-dextrose (YEPD) medium or synthetic complete (SC) medium supplemented with 80 mg of uridine/liter was used (37), and strains were grown at 30°C unless otherwise indicated. Where indicated, 2.5 mM of methionine and cysteine was included in SC media. For methionine- and cysteine-free fetal calf serum (FCS; BRL Life Technologies), serum was dialyzed twice for 6 h by using MWCO 12-kDa tubing against 2 liters of phosphate-buffered saline (PBS). YEPD FCS and SC-M/C DFCS (dialyzed FCS) contained 0.5× medium and 50% serum. Amino acid analysis of YEPD, FCS, and DFCS revealed 1.0 mM, 0.2 mM, and undetectable levels of methionine, respectively.
Isolation of C. albicans CDC24, CDC42, BUD1, and BEM1.
Initially, we attempted to isolate CaCDC24 by complementation of the temperature-sensitive growth of an S. cerevisiae cdc24-4 strain by using a C. albicans genomic pRS202 library (gift from G. Fink). Since this approach was unsuccessful, degenerate oligonucleotides based on conserved amino acid sequences in S. pombe, Kluyveromyces lactis (A. Nern and R. A. Arkowitz, unpublished data), and S. cerevisiae Cdc24s were used to PCR amplify two different regions of CaCDC24 from a genomic library in pRS202. Exact-match oligonucleotides were then used to screen this library for a clone containing a 1-kb fragment between these two regions. This clone contained a part of the CaCDC24 sequence, encoding the first 444 amino acids. The remaining portion of CaCDC24 was isolated by PCR amplification of the library, and these two CaCDC24 fragments were ligated and cloned into pBluescript, resulting in pBSCaCdc24 that has a 5.1-kb C. albicans genomic DNA fragment containing the 2.5-kb CDC24 open reading frame (ORF), together with a 1.9-kb 5′ sequence and a 0.7-kb 3′ sequence. Genomic DNA fragments containing CaCDC42, CaBUD1, and CaBEM1 were isolated by suppression of the temperature-sensitive growth of a S. cerevisiae cdc24-4 strain by using the C. albicans genomic pRS202 library. All isolated DNA fragments were sequenced, and 1.7-kb (576-bp ORF), 2-kb (820-bp ORF), and 3-kb (1911-bp ORF) fragments were subcloned into pBluescript, yielding pBSCaCDC42, pBSCaBUD1, and pBSCaBEM1, respectively.
Mutant strains construction.
Gene disruption cassettes were created by replacing each ORF with a 2.0-kb CaHIS1 fragment from pGEMHIS1 (43). pBSCacdc24::HIS1 was created by replacing a 2.7-kb CaCDC24 HpaI fragment (which includes 0.3 kb 5′ of the CDC24 ATG) with HIS1. This cassette replaces all but the last 21 amino acids of Cdc24. In order to construct CaCDC42, CaBUD1, and CaBEM1 gene disruption cassettes, a unique BamHI restriction site was introduced 5′ of the ATG of each ORF. Two internal XbaI sites in CaCDC42 were removed by blunting and religation and subsequently a similar approach was used to introduce unique XbaI and NheI restriction sites 3′ of each CaCDC42 and CaBUD1 stop codon, respectively. Each ORF, BamHI/XbaI for CaCDC42, BamHI/NheI for CaBUD1, and BamHI/PacI (a restriction site present 200 bp 5′ of the stop codon) for CaBEM1 was then replaced with the HIS1 fragment. Deletion cassettes were released by digestion, transformed into BWP17 (a ura− his− arg− derivative of SC5314) (43), and selected for histidine prototrophy. Heterologous disruption strains (Wild-type/Δ) of CaCDC24, CaCDC42, CaBUD1, and CaBEM1 grew normally. For methionine regulated expression, a fragment of each gene containing the ATG start codon was cloned into either pCaDIS (6) or a modified version of this plasmid in which an MfeI site had been removed. We used 464-, 339-, 197-, and 418-bp fragments for CaCDC24, CaCDC42, CaBUD1, and CaBEM1, respectively. Each pCaDIS plasmid was linearized within the CaCDC24, CaCDC42, CaBUD1, and CaBEM1 gene fragments by using a ClaI, StuI, MfeI, and SacI sites, respectively, and transformed into the appropriate heterozygote strain. Transformants were selected for uridine prototrophy, resulting in Δ/pMet gene strains. Correct integrants were identified by PCR, and two independent isolates of each strain were analyzed for growth. The CaCDC24 ORF expressed from its native promoter was reintroduced at the RP10 locus (29) on linearized pCaEXPA plasmid. pCaEXPA plasmid was constructed from pCaEXP (6), which contains URA3 and a MET3 promoter. First, a 1.4-kb HindIII fragment of pCaEXP, containing URA3, was replaced with a 2.0-kb ARG4 fragment from pRSARGΔSpeI (43). Subsequently, the MET3 promoter was removed by digestion and a 4.7-kb CaCDC24 fragment (including the 2.5-kb ORF and 1.5 kb of 5′ sequence) from pBSCaCDC24 was inserted by ligation. pCaEXPA or pCaEXPACaCDC24 were targeted to the RP10 locus after StuI digestion, transformation, and selection for arginine prototrophy. Correct integration of pCaEXPACaCDC24 was identified by PCR.
For mouse virulence assays, a URA+ wild-type strain was made by transforming StuI-digested pCaEXPGFP (pRSC4b [6]) into BWP17. The resulting strain contains URA3, followed by the MET3 promoter and green fluorescent protein (GFP) integrated at the RP10 locus and was also used for immunoblot analyses. A completely prototrophic wild-type strain (URA+, HIS+, and ARG+) was made by sequential transformation of BWP17. First, this strain was transformed by NruI-digested pGEMHIS1 (43). HIS+ prototrophs were subsequently transformed with NotI-digested pRS-ARG-URA-BN (pRS314 containing CaURA3 and CaARG4 [a gift from A. Mitchell]), and histidine, uridine, and arginine prototrophs were selected. A prototrophic Δcdc24/CDC24 strain was constructed, as described above, by using pRS-ARG-URA-BN, and a prototrophic Δcdc24/pMetCDC24 strain was made by using StuI-linearized pCaEXPA.
For Western analyses, a plasmid derived from pCaDISCaCDC24 was used in which the MET3 promoter was replaced with the CDC24 promoter to determine the levels of 3xHACaCdc24 from its native promoter. This was accomplished by first introducing a unique BamHI site 5′ of the CDC24 ATG in pCaEXPACaCDC24 by site-directed mutagenesis and subsequently replacing a 1.8-kb AatII/BamHI fragment from pCaDISCaCDC24 with a 0.9-kb SwaI/BamHI CDC24 promoter fragment from the modified pCaEXPACaCDC24, yielding pCaCDC24. An amino-terminal triple-hemagglutinin (HA) epitope tag, followed by a Gly-Ala-Gly-Ala-Gly-Ala linker, was fused to CaCdc24 by PCR and cloned into pCaCDC24, resulting in pCa-3xHACDC24. The sequence of 3xHACDC24 was verified. This plasmid was linearized within CDC24 with ClaI, transformed into BWP17, and selected for uridine prototrophy, and the resulting strain, pCDC24-3xHACdc24, was used for examination of Cdc24 levels. In order to determine the levels of 3xHACdc24 from the MET3 promoter, a plasmid was constructed in which the 0.6-kb BamHI/PstI 3xHACDC24 fragment was cloned into pCaDis, resulting in pCaDis-3xHACDC24. This plasmid was then linearized within CDC24 ORF with ClaI and transformed into BWP17, and uridine prototrophs were selected, yielding the strain pMet3-3xHACdc24. Epitope-tagged strains were confirmed by PCR.
Southern and Western blot analyses.
For Southern analysis, genomic DNA was extracted from cells grown in YEPD. Five micrograms of DNA was digested with MfeI, and the resulting fragments were separated on a 1.2% agarose gel. DNA was transferred to Immobilon-nylon membrane (Millipore) and hybridized with a 1.1-kb MfeI fragment, which contained 0.4-kb 3′ of the CDC24 ATG, that was labeled with [α-32P]dCTP. Southern blots were visualized by using a Fuji phosphorimager. For immunoblot analyses, exponentially growing cells were broken with glass beads by agitation in a Ribolyser (Hybaid). Proteins were then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on an 8% gel and transferred onto a Protran nitrocellulose membrane (Schleicher & Schuell). The membranes were probed with either anti-HA (HA.11) monoclonal antibodies (1:1,000; Covence) or anti-GFP polyclonal antibodies (30), followed by enhanced chemiluminescence visualization (Amersham).
Microscopy.
For colony morphology, cells grown on agar containing media for 3 to 5 days at 30°C were imaged with a Leica MZ6 dissection scope at ×20 magnification. For cell morphology, cells were grown to exponential phase in the indicated media and then shifted at 37°C for 3 h in the presence of 1:1 (vol/vol) FCS or DFCS. Cells were then fixed for 1 h with formaldehyde (7.5%), resuspended in PBS, and imaged with a Leica DMR microscope by using a ×63 N/A 1.32 objective lens, by using differential interference contrast (DIC) optics. All images were obtained with a Princeton Instruments Micromax charge-coupled device camera.
Murine virulence assay.
Overnight cultures of C. albicans strains were diluted into YEPD and grown for an additional 4 h at 30°C. Cultures were collected by centrifugation and washed with PBS. Cells (106) were injected into the tail vein of 21- to 25-g male CD1 mice. Cell numbers were determined by using a hemacytometer, and viability was confirmed by plating cultures onto SC-M/C solid media. Ten mice were used per strain and were monitored for 6 weeks after injection.
RESULTS
Identification of C. albicans Cdc24 homolog.
Since attempts to isolate CaCDC24 by complementation of an S. cerevisiae cdc24 temperature-sensitive mutant were unsuccessful, degenerate oligonucleotides based on conserved amino acid sequences in S. pombe, K. lactis, and S. cerevisiae Cdc24s were used to PCR amplify two different regions of CDC24 from a C. albicans genomic library in pRS202. Exact-match oligonucleotides were then used to screen this library for a clone containing a partial CDC24 sequence. The remaining portion of CDC24 was isolated by PCR amplification of the library. The sequence of the C. albicans CDC24 ORF revealed three bases that were different from the CDC24 sequence Contig6-1854 (Stanford C. albicans genome sequencing project; http://www-sequence.stanford.edu/group/candida), the first change resulting in a methionine at amino acid 735 and the latter two changes being silent. C. albicans Cdc24 is 32% identical to its budding yeast counterpart, displaying 43% identity within the guanine nucleotide exchange factor domain (Fig. 1). A BLAST search of the human genome sequence with the C. albicans Cdc24 sequence revealed a number of human guanine nucleotide exchange factors with characteristic exchange factor domains that were ≤28% identical to the exchange factor domain. The overall sequence identity of C. albicans Cdc24 with different human exchange factors, such as RhoGEF KIAA1209 and Cdc42 GEF hPEM-2, is ca. 8%. In contrast, Cdc42 is highly conserved in many eukaryotes, with C. albicans Cdc42 having 88 and 77% identity to S. cerevisiae and human Cdc42, respectively.
FIG. 1.
Comparison of fungal Cdc24 guanine nucleotide exchange factors. (A) Protein sequence comparison of fungal Cdc24s. Percentage identity and similarity of Saccharomyces cerevisiae (S.c.), Kluyveromyces lactis (K.l.), Candida albicans (C.a.), and Schizosaccharomyces pombe (S.p.) Cdc24 proteins. The GenBank accession number for C. albicans CDC24 is AY208122. The guanine nucleotide exchange factor (GEF) and plekstrin homology domains are shown in gray and black, respectively. Numbers below the schematic representation of C. albicans Cdc24 indicate the percent similarity of each region with Saccharomyces cerevisiae Cdc24. Alignments were carried out by using the BLAST algorithm (1). (B) Protein sequence alignment of fungal guanine nucleotide exchange factor domain. Residues boxed in black and in gray are identical and similar to S.c. Cdc24, respectively. The black line above the sequences represents the S. cerevisiae Cdc24 GEF domain. S.c., S. cerevisiae; K.l., K. lactis; C.a., C. albicans; S.p., S. pombe.
Construction and analysis of cdc24 mutant strains.
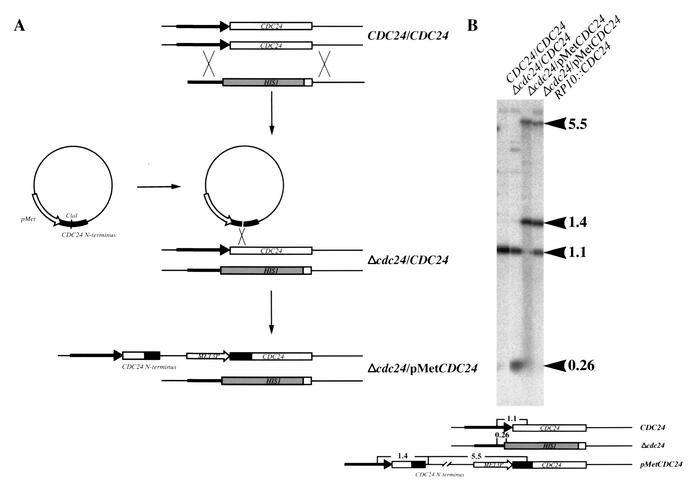
In order to examine the function of CDC24 in C. albicans vegetative and hyphal growth, strains were constructed in which one copy of the CDC24 ORF was replaced by the HIS1 marker and the MET3 promoter was integrated immediately 5′ of the ATG of the second copy of CDC24 (Fig. 2A). To confirm that any defects observed with the Δcdc24/pMetCDC24 strain were due to the loss of CDC24 function, an additional copy of CDC24 was reintroduced into this strain at the RP10 locus. Correct Δcdc24/CDC24, Δcdc24/pMetCDC24, and Δcdc24/pMetCDC24/RP10::CDC24 integrants were identified and confirmed by PCR (not shown) and Southern analysis (Fig. 2B).
FIG. 2.
Construction and Southern analysis of C. albicans cdc24 mutant strains. (A) Schematic representation of CDC24 strain construction. (B) Southern blot analysis of CDC24 mutant strains. Five micrograms each of total genomic DNA from the indicated strains was digested with MfeI and analyzed by Southern blotting. Blots were probed with a radiolabeled 1.1-kb CaCDC24 MfeI fragment. The size of the fragments is indicated in kilobases.
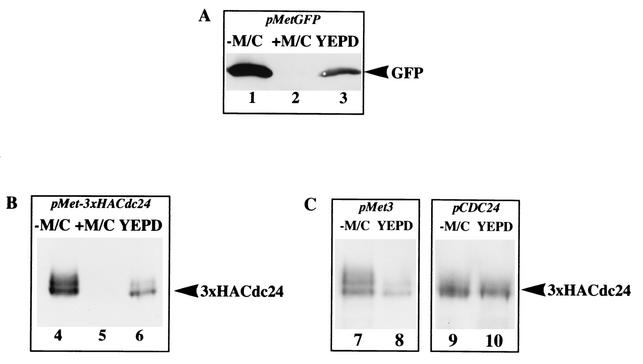
To compare the regulation of CDC24 expression via the MET3 promoter and its own promoter, we examined the levels of epitope-tagged Cdc24 in cells grown in different conditions. The MET3 promoter is fully repressed in the presence of both millimolar methionine (Met) and cysteine (Cys) and yet permits expression at lower Met and Cys levels (6) (Fig. 3A). We compared the amount of Cdc24 expressed from the MET3 promoter in permissive (−M/C), repressive (+2.5 mM M/C) and intermediate (YEPD; which contains 1 mM methionine) conditions. Using a GFPCDC24 fusion (data not shown) and a triple-HA epitope-tagged version of CDC24 behind the MET3 promoter, two to three times less Cdc24 was observed when cells were grown on YEPD medium compared with SC medium lacking Met and Cys (Fig. 3B, lane 6 versus lane 4). These results indicate that the Met (1 mM) and Cys concentrations present in YEPD medium are sufficient for partial repression of the MET3 promoter. The addition of 2.5 mM Met and Cys to SC media completely blocked CDC24 expression (lane 5). Together, these results indicate that the MET3 promoter constructs can be used to assess the function of Cdc24 in invasive hyphal growth. We next compared the expression level of CDC24 behind the MET3 and its own promoter (Fig. 3C). The level of 3xHACdc24 expressed from the MET3 promoter was ∼3-fold lower than that observed with the CDC24 promoter in cells grown in YEPD (Fig. 3C, lanes 8 and 10). However, we observed similar levels of 3xHACdc24 with the two different promoters in cells grown in synthetic medium lacking Met and Cys (Fig. 3C, lanes 7 and 9). As illustrated in Fig. 3B and C, the 3xHACdc24 fusion migrated as a double band on SDS-PAGE, suggesting that this protein is phosphorylated, similar to Cdc24 in S. cerevisiae (3, 12).
FIG. 3.
Regulated expression of the MET3 promoter. (A) Cells carrying MET3 promoter GFP grown in SC medium lacking Met and Cys (lane 1), in SC medium containing 2.5 mM Met and Cys (lane 2), or in YEPD (lane 3) were lysed by agitation with glass beads and analyzed by SDS-PAGE, followed by immunoblotting, and then probed with anti-GFP polyclonal sera (30). (B) CDC24/pMet3xHACdc24 cells were analyzed as described for panel A with anti-HA monoclonal sera (lanes 4 to 6). (C) Cells expressing 3xHACdc24 under the control of the MET3 promoter (left panel) or CaCdc24 promoter (right panel) grown in SC medium lacking Met and Cys (lanes 7 and 9) and YEPD (lanes 8 and 10) were analyzed as described for panel A. Similar amounts of protein were loaded in each lane, as judged by Ponceau red staining of the blots.
Using the same approach, we constructed strains in which one copy of C. albicans CDC42, BUD1, or BEM1 was deleted and the MET3 promoter was integrated immediately 5′ of the ORF of the sole copy of each gene.
Cdc24 is required for vegetative growth.
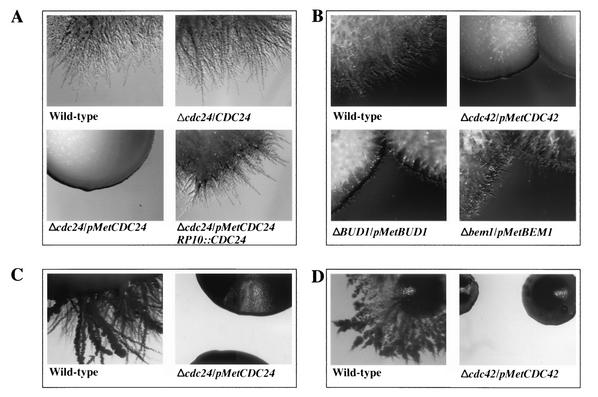
Initially, we compared the growth of wild-type, Δcdc24/CDC24 Δcdc24/pMetCDC24, and Δcdc24/pMetCDC24/RP10::CDC24 strains in repressive conditions. Although the wild-type and heterozygote strains grew normally, Δcdc24/pMetCDC24 cells were inviable (Fig. 4A). Reintroduction of a copy of CDC24 into Δcdc24/pMetCDC24 cells restored growth on synthetic medium containing 2.5 mM Met and Cys. This recovery of growth indicates that the pMetCDC24 does not behave in a dominant fashion, a finding consistent with normal growth of CDC24/pMetCDC24 strains (data not shown). Strains with regulated expression of CaCDC42, CaBUD1, and CaBEM1 were also examined (Fig. 4B). Δcdc42/pMetCDC42 cells were also inviable under repressive conditions, as previously reported (41), whereas the Δbud1/pMetBUD1 cells grew normally, similar to a homozygous disruptant (44), and Δbem1/pMetBEM1 cells grew poorly. C. albicans strains with regulated expression of CDC24, CDC42, BUD1, and BEM1 all grew on rich YEPD medium (data not shown). These results suggest that the level of Cdc24 or Cdc42 in this condition is sufficient for vegetative growth.
FIG. 4.
C. albicans Cdc24 and Cdc42 are essential for normal vegetative growth. (A and B) Indicated strains were grown to exponential phase in SC medium lacking methionine and cysteine. Serial dilutions were spotted onto SC plates lacking or containing 2.5 mM of methionine and cysteine, and the plates were incubated at 30°C for 2 to 4 days.
The Cdc24/Cdc42 GTPase module is required for invasive growth.
We next examined the ability of the various strains to invade solid agar. Strains were grown on YEPD plates containing FCS and incubated at 30°C. Under these conditions, all strains initially grew as colonies on the surface of the agar. Figure 5A illustrates that wild-type and Δcdc24/CDC24 colonies were substantially filamentous and invaded the agar substrate extensively. Strikingly, the Δcdc24/pMetCDC24 strain did not become hyphal and invade the solid surface (Fig. 5A). The invasive hyphal growth defects of this strain could be due to a suboptimal level of Cdc24 in YEPD FCS medium which, although sufficient for growth of the yeast form, is insufficient for hyphal growth. Consistent with this notion, reintroduction of a copy of CDC24 into Δcdc24/pMetCDC24 cells suppressed the hyphal growth defect to a level similar to that of the wild type or heterozygote (Fig. 5A). As illustrated in Fig. 5B, Δcdc42/pMetCDC42 cells are also unable to invade an agar substrate, in contrast to Δbem1/pMetBEM1 cells, which grow in an invasive hyphal fashion. Colonies of Δbud1/pMetBUD1 cells were intermediate between these two extremes, forming invasive filaments slower than the wild-type and Δbem1/pMetBEM1 cells, a finding consistent with a previous study (44). The invasive hyphal growth defects of both Δcdc24/pMetCDC24 and Δcdc42/pMetCDC42 were independent of the stimuli used; similar results were observed with YEPD alone (data not shown) or on medium completely lacking Met and Cys (synthetic media containing extensively dialyzed FCS) at 30°C (Fig. 5C and D), which nonetheless triggered invasive hyphal growth of wild-type strains. Together, these results suggest that Δcdc24/pMetCDC24 and Δcdc42/pMetCDC42 cells are defective in invasive hyphal growth either due to a reduced level of the respective protein or due to an inability to appropriately regulate CDC24 and CDC42 expression in response to stimuli, which trigger hyphal growth. The comparison of the expression level of CDC24 behind CDC24 promoter and Met promoter in cells grown in synthetic medium (Fig. 3C) indicates that it is unlikely that the absolute level of Cdc24 is responsible for the hyphal growth defect, suggesting that this defect is rather the result of CDC24 ectopic expression.
FIG. 5.
C. albicans Cdc24 and Cdc42 are essential for invasive hyphal growth in response to serum. The indicated strains were grown in SC medium lacking methionine and cysteine, and serial dilutions were spotted onto YEPD plates containing FCS (A and B) or plates with SC medium that lacked methionine and cysteine but containing DFCS (C and D). Plates were then incubated for 5 (A, C, and D) or 3 (B) days at 30°C.
Cdc24 and Cdc42 are required for initiation of hyphal growth.
A defect in invasive hyphal growth could be due to either an inability of yeast-form cells to hyperpolarize and form germ tubes, i.e., initiate hyphal growth, or to an inability to maintain this hyphal polarized growth. To distinguish between these possibilities, we analyzed the behavior of cells in liquid YEPD medium containing FCS at 37°C and counted the number of cells (n > 200) with germ tubes. After 3 h in the presence of 50% FCS, >90% of wild-type cells had elongated germ tubes (Fig. 6A and B). Strikingly, only 0.5% of Δcdc24/pMetCDC24 cells had observable germ tubes (Fig. 6A). This germ tube formation defect was not seen with Δcdc24/CDC24 or Δcdc24/pMetCDC24/RP10::CDC24 cells, which both had >90% of cells with germ tubes. Δbud1/pMetBUD1 and Δbem1/pMetBEM1 strains formed germ tubes similar to wild-type cells (40 and 70% cells with germ tubes, respectively) in contrast to Δcdc42/pMetCDC42 cells, which had the same defect than Δcdc24/pMetCDC24 cells (Fig. 6B). Despite the defect in germ tube formation, Δcdc24/pMetCDC24 and Δcdc42/pMetCDC42 cells nonetheless grew in YEPD FCS, as indicated by the presence of budded cells. However, the size of cells from each strain was larger than with the wild type, as previously reported for a PCK1-regulated cdc42 strain (41). No further increase of the percentage of Δcdc24/pMetCDC24 or Δcdc42/pMetCDC42 cells with germ tubes was observed after 5 h (data not shown), suggesting that this defect was not due to a slower morphological transition.
FIG. 6.
C. albicans Cdc24 and Cdc42 are required for germ tube formation. (A and B) The indicated strains were grown in YEPD, pelleted, and resuspended in YEPD, and then an equal volume of FCS was added. Cells were incubated at 37°C for 3 h, fixed with formaldehyde, and imaged. (C) The indicated strains were treated and analyzed as described for panels A and B, except that they were grown in SC medium lacking methionine and cysteine and then resuspended in the same medium containing an equal volume of DFCS. The percentages of cells with germ tubes were determined (n > 200).
Since the germ tube formation defect of Δcdc24/pMetCDC24 and Δcdc42/pMetCDC42 cells in YEPD FCS media could be due to a reduced level of these two proteins, the behavior of these cells was observed in medium lacking Met and Cys containing dialyzed FCS at 37°C. A similar defect in germ tubes formation was observed in this medium (Fig. 6C), with only 10 and 1% of cells with germ tubes for Δcdc24/pMetCDC24 and Δcdc42/pMetCDC42 strains, respectively, compared to 65% for the wild-type. No further increase of the percentage of Δcdc24/pMetCDC24, Δcdc42/pMetCDC42, or wild-type cells with germ tubes was observed after 5 h (data not shown). Closer examination revealed that the cell size of the two mutant strains was now similar to the wild-type cells and that a portion of the buds were elongated and had a pseudohyphal appearance. Our results suggest that Δcdc24/pMetCDC24 and Δcdc42/pMetCDC42 strains are defective in initiation of cell polarization, which precedes hyphal formation, in YEPD FCS media. However, in SC DFCS media lacking Met and Cys, in which a higher level of Cdc24 was observed, Δcdc24/pMetCDC24 cells appeared to initiate polarization but were still unable to maintain this directional growth. Both Δcdc24/pMetCDC24 and Δcdc42/pMetCDC42 strains, although able to undergo vegetative, yeast-form growth, were unable to form hyphae in response to serum.
The Cdc24/Cdc42 GTPase module is necessary for virulence.
To assess the role of these proteins in C. albicans virulence, we tested these strains in a murine model for systemic infection. Mice were injected with a URA+ wild-type strain (15) and the four URA+ HIS+ strains Δcdc24/pMetCDC24, Δcdc42/pMetCDC42, Δbud1/pMetBUD1, and Δbem1/pMetBEM1. Mice injected with the wild-type strain resulted in 50% mortality after 5 days, whereas even after 40 days the Δcdc24/pMetCDC24 and Δcdc42/pMetCDC42 strains did not result in mouse mortality (Fig. 7A). Mice injected with either of the other two strains, Δbud1/pMetBUD1 and Δbem1/pMetBEM1, survived longer than those injected with the wild-type control; 50% of the former group died after 9 days, and 30% of the latter group died after 16 days. To rule out the possibility that the avirulence of Δcdc24/pMetCDC24 and Δcdc42/pMetCDC42 strains is due to their arginine auxotrophy, ARG4 was reintroduced into the Δcdc24/pMetCDC24 strain. As a control, a strain prototrophic for URA+, HIS+, and ARG+ was used. The results illustrated in Fig. 7B demonstrate that the HIS1 and ARG4 markers do not affect virulence in a murine model of systemic infection, since 50% of mice injected with either strain (URA+ or URA+/HIS+/ARG+) died after 6 days. The reintroduction of ARG4 into the Δcdc24/pMetCDC24 cells had no effect on the avirulence of this strain, whereas the reintroduction of CDC24, under the control of its own promoter, resulted in a strain with a virulence identical (50% mice mortality after 5 days) to that of the wild type. In addition, the virulence of the heterozygote strain was examined and was also similar to that of the wild-type strain (50% mouse mortality after 7 days), indicating that cells with only one copy of CDC24 are pathogenic. These results suggest that the Cdc24/Cdc42 GTPase module is necessary for virulence, although we cannot exclude the possibility that the reduced virulence of Δcdc24/pMetCDC24 and Δcdc42/pMetCDC42 strains is in part due to an impaired growth rate of these strains in mice.
FIG. 7.
C. albicans Δcdc24/pMetCDC24 and Δcdc42/pMetCDC42 strains are avirulent. (A) Δcdc24/pMetCDC24 (▵), Δcdc42/pMetCDC42 (▿), Δbud1/pMetBUD1 (⋄), and Δbem1/pMetBEM1 (○) strains (106 cells) were injected into the tail vein of CD1 mice (n = 10) and monitored for survival over 40 days. A URA+ wild-type (□) strain was used as a control. (B) Prototrophic (HIS+ URA+ ARG+) versions of wild-type (⋄), Δcdc24/CDC24 (○), Δcdc24/pMetCDC24 (▵), and Δcdc24/pMetCDC24/RP10:: CDC24 (▿) strains and a URA+ wild-type (□) were injected in mice as described in panel A, and mouse mortality was monitored for 20 days.
DISCUSSION
Cdc24, the Cdc42 exchange factor, is required for viability and invasive hyphal growth in C. albicans. A strain in which the only functional copy of CaCDC24 is under the control of the regulatable MET3 promoter was used to examine the consequences of complete shutoff of expression, reduced expression, and ectopic expression. Complete repression on methionine- and cysteine-containing media resulted in inviable cells, indicating that the CaCdc24 is required for vegetative growth. Under conditions of reduced CaCDC24 expression, such as in YEPD medium, cells grew vegetatively; they were, however, larger than wild-type cells. In synthetic media lacking methionine and cysteine, where little difference was observed in CaCDC24 expression under the control of the MET3 promoter or its own promoter, vegetative growth was comparable to that of a wild-type strain. C. albicans yeast-form cells undergo a transition to invasive hyphal filaments in response to serum and/or elevated temperature. Strikingly, Δcdc24/pMetCDC24 cells were defective for invasive hyphal growth and germ tube formation in a media permissive for vegetative growth that contained dialyzed serum.
This defect in invasive hyphal growth in conditions permissive for vegetative growth was also observed for a similarly constructed Cacdc42 strain, suggesting that regulation of Cdc24 and/or Cdc42 activity is required for invasive hyphal growth. In contrast, strains in which the only functional copy of CaBUD1 or CaBEM1 is under the control of the regulatable MET3 promoter formed invasive hyphal filaments on agar containing media and germ tubes in liquid media. These results show that the defects observed with regulated CaCDC24 or CaCDC42 strains were specific to this GTPase module and not a common feature of polarity establishment proteins. Furthermore, by using a mouse model for systemic infection, these strains were avirulent, suggesting that their defects in invasive hyphal growth result in reduced pathogenicity.
In S. cerevisiae, Bud1 is required for pseudohyphal growth (11, 40), as this G protein is necessary for unipolar budding and therefore filament formation. Although CaBUD1 is not essential for invasive hyphal growth, its reduced expression resulted in a decrease in hyphal growth and pathogenicity, a finding consistent with a previous report in which a Δbud1/Δbud1 C. albicans strain exhibited modest defects in the yeast-to-hyphal transition and virulence (44). The role of Bem1 in fungal pseudohyphal and hyphal growth is less clear. In S. cerevisiae, BEM1 is necessary for polarity establishment and has recently been shown to be required for pseudohyphal growth (22). In the dimorphic yeast Yarrowia lipolytica, cells deleted for BEM1 are viable but have cell polarity defects (13). This mutant, though unable to form hyphae in liquid and solid media, nevertheless forms pseudohyphae to a reduced extent on agar plates. In contrast, although our results indicate that Δbem1/pMetBEM1 cells grow poorly under Met and Cys repressive conditions, the cells form hyphae in solid and liquid media under conditions of partial repression (YEPD medium with FCS) or ectopic expression (SC-M/C medium with DFCS). Despite this ability to form invasive hyphal filaments, this strain displays a substantially reduced virulence in a mouse model.
Our results show that the level or activity of CaCdc42 and CaCdc24 required for yeast-form growth is likely to be different from that necessary for germ tube and subsequent filament formation. Δcdc24/CDC24 and CDC24/pMetCDC24 strains form hyphae on YEPD in response to serum, identical to a wild-type strain, indicating that a two- to threefold reduction in Cdc24 level does not affect the dimorphic transition. Strikingly, a Δcdc24/pMetCDC24 strain with ∼6-fold reduction in Cdc24 levels is defective both for germ tube formation and invasive hyphal growth. These results indicate either that a minimal level of Cdc24 is required for invasive hyphal growth or that CDC24 expression is precisely regulated via its own promoter.
Previously, it was demonstrated that transcript levels of CDC42 in C. albicans (25) and another pathogenic fungus, Penicillium marneffei (4), increased upon germ tube formation; however, it remains unclear whether such an increase is associated with the hyphal state. The function of Cdc42 in fungal hyphal growth by using dominant-active and dominant-negative mutants has been difficult to infer. For example, a dominant-active form of Cdc42 represses hyphal growth in the zoopathogenic fungus Wangiella dermatitidis; however, a cdc42 deletion strain formed hyphae normally (45). In P. marneffei, dominant-active or dominant-negative forms of Cdc42 were normal for dimorphic switching, forming hyphae similar to wild-type cells (4). In C. albicans, expression of a dominant-active or -negative form of cdc42 modifies cell morphology but, even so, hyphal structures were observed (41). Our results show that C. albicans strains ectopically expressing CDC42 are unable to form invasive hyphal filaments, suggesting a central role of the Cdc42 GTPase module in the dimorphic switch. Both a MAP kinase cascade (defined by CaCPH1 requirement) and a cAMP signaling pathway (defined by CaEFG1 requirement) are necessary for the C. albicans yeast-to-hyphal transition (2, 5, 9, 19, 21, 26, 38, 39). Recent studies suggest that both pathways require CaRAS1 (10, 18). Perhaps CaRas1 forms a complex with CaCdc24 and CaCdc42, as is the case in S. pombe (7), and this complex is required for signaling to the MAP kinase cascade via the STE20 homolog CaCST20 and the cAMP-dependent pathway via adenylyl cyclase (CaCYR1). In such a scenario, CaCdc24 and CaCdc42 might be required for the coordinated activation of MAP kinase and cAMP-dependent signaling pathways. The deleterious effects of overexpression of dominant active Cdc42 in C. albicans are suppressed by deletion of the STE20 homolog CaCST20, suggesting that this PAK family kinase is the primary effector of Cdc42 in this pathogenic fungus (41). Our results indicate that a specific regulation of Cdc24/Cdc42 activity is required for invasive hyphal growth and suggest that these proteins are required for the pathogenicity of C. albicans.
Acknowledgments
This work was supported by the Centre National de la Recherche Scientifique, the Medical Research Council, the Association pour la Recherche sur le Cancer, the Fondation pour la Recherche Médicale, and BNP-Paribas.
The cloning of CaCDC24 was initiated by Aljoscha Nern. We thank A. Mitchell and J. Berman for constructive criticism; M. Rassoulzadegan and E. Van Obbergen-Shilling for advice on animal experiments; F. Paput for aid with animal handling; Z. Amri for aid with Southern analyses; D. Owen for amino acid composition analysis; and G. Fink, A. Mitchell, and P. Sudbery for strains, plasmids, and libraries.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Bahn, Y. S., and P. Sundstrom. 2001. CAP1, an adenylate cyclase-associated protein gene, regulates bud-hypha transitions, filamentous growth, and cyclic AMP levels and is required for virulence of Candida albicans. J. Bacteriol. 183:3211-3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bose, I., J. E. Irazoqui, J. J. Moskow, E. S. Bardes, T. R. Zyla, and D. J. Lew. 2001. Assembly of scaffold-mediated complexes containing Cdc42p, the exchange factor Cdc24p, and the effector Cla4p required for cell cycle-regulated phosphorylation of Cdc24p. J. Biol. Chem. 276:7176-7186. [DOI] [PubMed] [Google Scholar]
- 4.Boyce, K. J., M. J. Hynes, and A. Andrianopoulos. 2001. The CDC42 homolog of the dimorphic fungus Penicillium marneffei is required for correct cell polarization during growth but not development. J. Bacteriol. 183:3447-3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, A. J., and N. A. Gow. 1999. Regulatory networks controlling Candida albicans morphogenesis. Trends Microbiol. 7:333-338. [DOI] [PubMed] [Google Scholar]
- 6.Care, R. S., J. Trevethick, K. M. Binley, and P. E. Sudbery. 1999. The MET3 promoter: a new tool for Candida albicans molecular genetics. Mol. Microbiol. 34:792-798. [DOI] [PubMed] [Google Scholar]
- 7.Chang, E. C., M. Barr, Y. Wang, V. Jung, H. P. Xu, and M. H. Wigler. 1994. Cooperative interaction of S. pombe proteins required for mating and morphogenesis. Cell 79:131-141. [DOI] [PubMed] [Google Scholar]
- 8.Cook, J. G., L. Bardwell, and J. Thorner. 1997. Inhibitory and activating functions for MAPK Kss1 in the S. cerevisiae filamentous growth signalling pathway. Nature 390:85-88. [DOI] [PubMed] [Google Scholar]
- 9.Ernst, J. F. 2000. Transcription factors in Candida albicans: environmental control of morphogenesis. Microbiology 146:1763-1774. [DOI] [PubMed] [Google Scholar]
- 10.Feng, Q., E. Summers, B. Guo, and G. Fink. 1999. Ras signaling is required for serum-induced hyphal differentiation in Candida albicans. J. Bacteriol. 181:6339-6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gimeno, C. J., P. O. Ljungdahl, C. A. Styles, and G. R. Fink. 1992. Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell 68:1077-1090. [DOI] [PubMed] [Google Scholar]
- 12.Gulli, M. P., M. Jaquenoud, Y. Shimada, G. Niederhauser, P. Wiget, and M. Peter. 2000. Phosphorylation of the Cdc42 exchange factor Cdc24 by the PAK-like kinase Cla4 may regulate polarized growth in yeast. Mol. Cell 6:1155-1167. [DOI] [PubMed] [Google Scholar]
- 13.Hurtado, C. A. R., and R. A. Rachubinski. 2002. Isolation and characterization of YlBEM1, a gene required for cell polarization and differentiation in the dimorphic yeast Yarrowia lipolytica. Eukaryot. Cell 1:526-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson, D. I. 1999. Cdc42: an essential Rho-type GTPase controlling eukaryotic cell polarity. Microbiol. Mol. Biol. Rev. 63:54-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirsch, D. R., and R. R. Whitney. 1991. Pathogenicity of Candida albicans auxotrophic mutants in experimental infections. Infect. Immun. 59:3297-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kubler, E., H. U. Mosch, S. Rupp, and M. P. Lisanti. 1997. Gpa2p, a G-protein alpha-subunit, regulates growth and pseudohyphal development in Saccharomyces cerevisiae via a cAMP-dependent mechanism. J. Biol. Chem. 272:20321-20323. [DOI] [PubMed] [Google Scholar]
- 17.Lamson, R. E., M. J. Winters, and P. M. Pryciak. 2002. Cdc42 regulation of kinase activity and signaling by the yeast p21-activated kinase Ste20. Mol. Cell. Biol. 22:2939-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leberer, E., D. Harcus, D. Dignard, L. Johnson, S. Ushinsky, D. Y. Thomas, and K. Schroppel. 2001. Ras links cellular morphogenesis to virulence by regulation of the MAP kinase and cAMP signalling pathways in the pathogenic fungus Candida albicans. Mol. Microbiol. 42:673-687. [DOI] [PubMed] [Google Scholar]
- 19.Leberer, E., K. Ziegelbauer, A. Schmidt, D. Harcus, D. Dignard, J. Ash, L. Johnson, and D. Y. Thomas. 1997. Virulence and hyphal formation of Candida albicans require the Ste20p-like protein kinase CaCla4p. Curr. Biol. 7:539-546. [DOI] [PubMed] [Google Scholar]
- 20.Lengeler, K. B., R. C. Davidson, C. D'Souza, T. Harashima, W. C. Shen, P. Wang, X. Pan, M. Waugh, and J. Heitman. 2000. Signal transduction cascades regulating fungal development and virulence. Microbiol. Mol. Biol. Rev. 64:746-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lo, H. J., J. R. Kohler, B. DiDomenico, D. Loebenberg, A. Cacciapuoti, and G. R. Fink. 1997. Nonfilamentous C. albicans mutants are avirulent. Cell 90:939-949. [DOI] [PubMed] [Google Scholar]
- 22.Lorenz, M. C., N. S. Cutler, and J. Heitman. 2000. Characterization of alcohol-induced filamentous growth in Saccharomyces cerevisiae. Mol. Biol. Cell 11:183-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorenz, M. C., and J. Heitman. 1997. Yeast pseudohyphal growth is regulated by GPA2, a G protein alpha homolog. EMBO J. 16:7008-7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madhani, H. D., C. A. Styles, and G. R. Fink. 1997. MAP kinases with distinct inhibitory functions impart signaling specificity during yeast differentiation. Cell 91:673-684. [DOI] [PubMed] [Google Scholar]
- 25.Mirbod, F., S. Nakashima, Y. Kitajima, R. D. Cannon, and Y. Nozawa. 1997. Molecular cloning of a Rho family, CDC42Ca gene from Candida albicans and its mRNA expression changes during morphogenesis. J. Med. Vet. Mycol. 35:173-179. [PubMed] [Google Scholar]
- 26.Mitchell, A. P. 1998. Dimorphism and virulence in Candida albicans. Curr. Opin. Microbiol. 1:687-692. [DOI] [PubMed] [Google Scholar]
- 27.Mosch, H. U., T. Kohler, and G. H. Braus. 2001. Different domains of the essential GTPase Cdc42p required for growth and development of Saccharomyces cerevisiae. Mol. Cell. Biol. 21:235-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mosch, H. U., R. L. Roberts, and G. R. Fink. 1996. Ras2 signals via the Cdc42/Ste20/mitogen-activated protein kinase module to induce filamentous growth in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 93:5352-5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murad, A. M., P. R. Lee, I. D. Broadbent, C. J. Barelle, and A. J. Brown. 2000. CIp10, an efficient and convenient integrating vector for Candida albicans. Yeast 16:325-327. [DOI] [PubMed] [Google Scholar]
- 30.Nern, A., and R. A. Arkowitz. 2000. G proteins mediate changes in cell shape by stabilizing the axis of polarity. Mol. Cell 5:853-864. [DOI] [PubMed] [Google Scholar]
- 31.Odds, F. C. 1988. Candida and candidosis, 2nd ed. Baillière-Tindall, London, United Kingdom.
- 32.Pan, X., T. Harashima, and J. Heitman. 2000. Signal transduction cascades regulating pseudohyphal differentiation of Saccharomyces cerevisiae. Curr. Opin. Microbiol. 3:567-572. [DOI] [PubMed] [Google Scholar]
- 33.Pan, X., and J. Heitman. 1999. Cyclic AMP-dependent protein kinase regulates pseudohyphal differentiation in Saccharomyces cerevisiae. Mol. Cell. Biol. 19:4874-4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richards, M. J., J. R. Edwards, D. H. Culver, and R. P. Gaynes. 1999. Nosocomial infections in medical intensive care units in the United States. National Nosocomial Infections Surveillance. Syst. Crit. Care Med. 27:887-892. [DOI] [PubMed] [Google Scholar]
- 35.Roberts, R. L., and G. R. Fink. 1994. Elements of a single MAP kinase cascade in Saccharomyces cerevisiae mediate two developmental programs in the same cell type: mating and invasive growth. Genes Dev. 8:2974-2985. [DOI] [PubMed] [Google Scholar]
- 36.Robertson, L. S., and G. R. Fink. 1998. The three yeast A kinases have specific signaling functions in pseudohyphal growth. Proc. Natl. Acad. Sci. USA 95:13783-13787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sherman, F. 1991. Getting started with yeast. Methods Enzymol. 194:3-21. [DOI] [PubMed] [Google Scholar]
- 38.Sonneborn, A., D. P. Bockmuhl, M. Gerads, K. Kurpanek, D. Sanglard, and J. F. Ernst. 2000. Protein kinase A encoded by TPK2 regulates dimorphism of Candida albicans. Mol. Microbiol. 35:386-396. [DOI] [PubMed] [Google Scholar]
- 39.Stoldt, V. R., A. Sonneborn, C. E. Leuker, and J. F. Ernst. 1997. Efg1p, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J. 16:1982-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taheri, N., T. Kohler, G. H. Braus, and H. U. Mosch. 2000. Asymmetrically localized Bud8p and Bud9p proteins control yeast cell polarity and development. EMBO J. 19:6686-6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ushinsky, S., D. Harcus, J. Ash, D. Dignard, A. Marcil, J. Morchhauser, D. Y. Thomas, M. Whiteway, and E. Leberer. 2002. CDC42 is required for polarized growth in human pathogen Candida albicans. Eukaryot. Cell 1:95-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wendland, J., and P. Philippsen. 2001. Cell polarity and hyphal morphogenesis are controlled by multiple rho-protein modules in the filamentous ascomycete Ashbya gossypii. Genetics 157:601-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson, R. B., D. Davis, and A. P. Mitchell. 1999. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J. Bacteriol. 181:1868-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yaar, L., M. Mevarech, and Y. Koltin. 1997. A Candida albicans RAS-related gene (CaRSR1) is involved in budding, cell morphogenesis and hypha development. Microbiology 143:3033-3044. [DOI] [PubMed] [Google Scholar]
- 45.Ye, X., and P. J. Szaniszlo. 2000. Expression of a constitutively active Cdc42 homologue promotes development of sclerotic bodies but represses hyphal growth in the zoopathogenic fungus Wangiella (Exophiala) dermatitidis. J. Bacteriol. 182:4941-4950. [DOI] [PMC free article] [PubMed] [Google Scholar]