Abstract
Intestinal monosaccharide transport was studied in a series of rats with a self-filling jejunal blind loop using 3mM arbutin (p-hydroxyphenyl-B-glucoside) or 1mM D-fructose as substrate in vitro and 10 mM arbutin or 5mM D-fructose in vivo. These results were compared with changes in the bacterial flora and state of conjugation of intraluminal bile salts in those animals. Observations were also made of the microscopic and ultrastructural appearances of the small-intestinal epithelium.
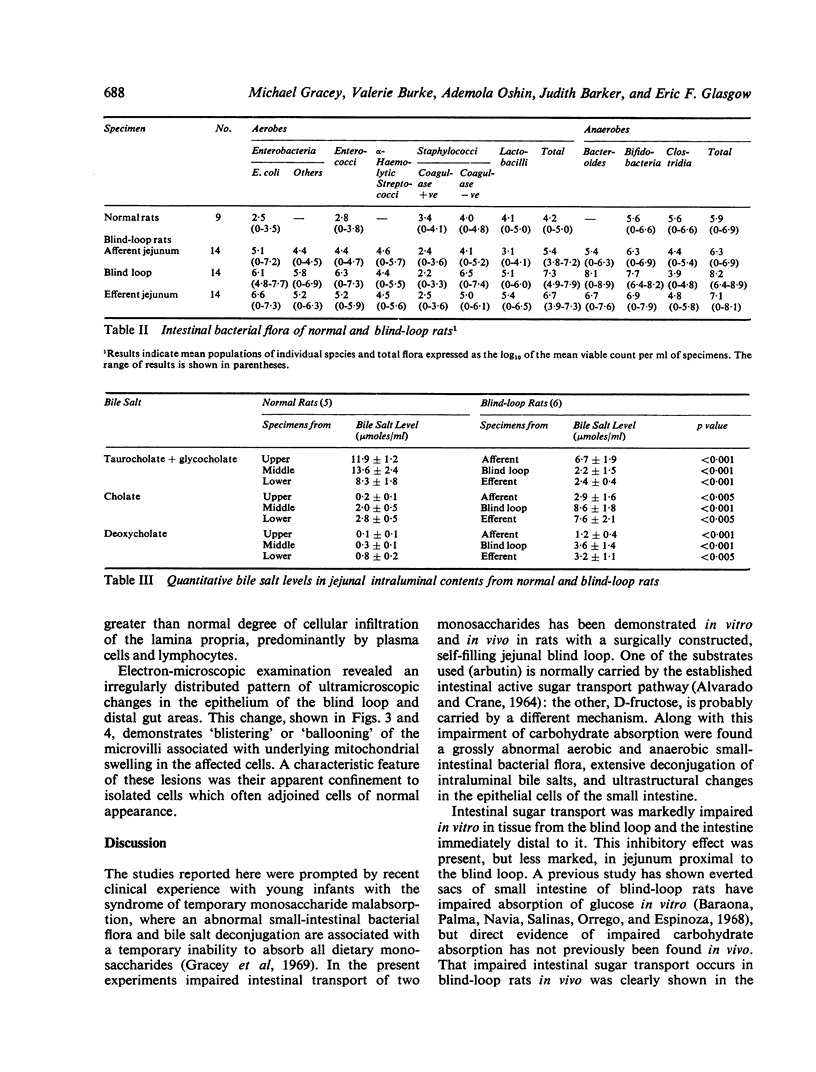
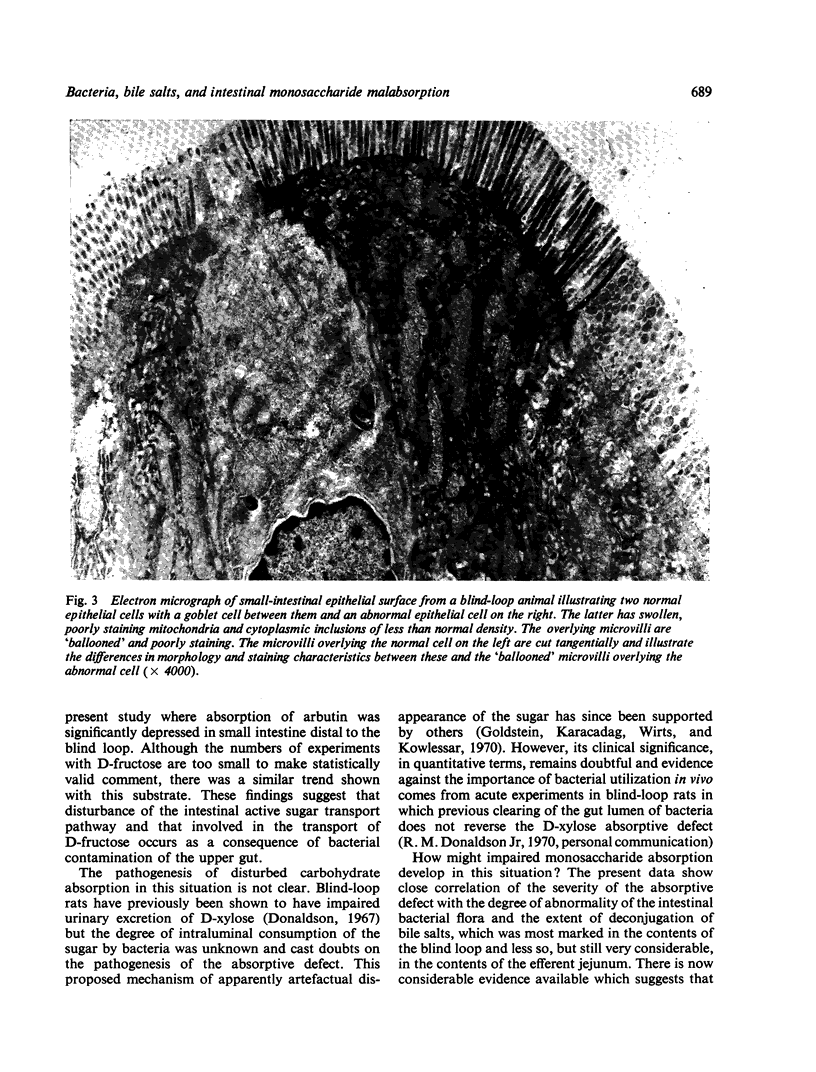
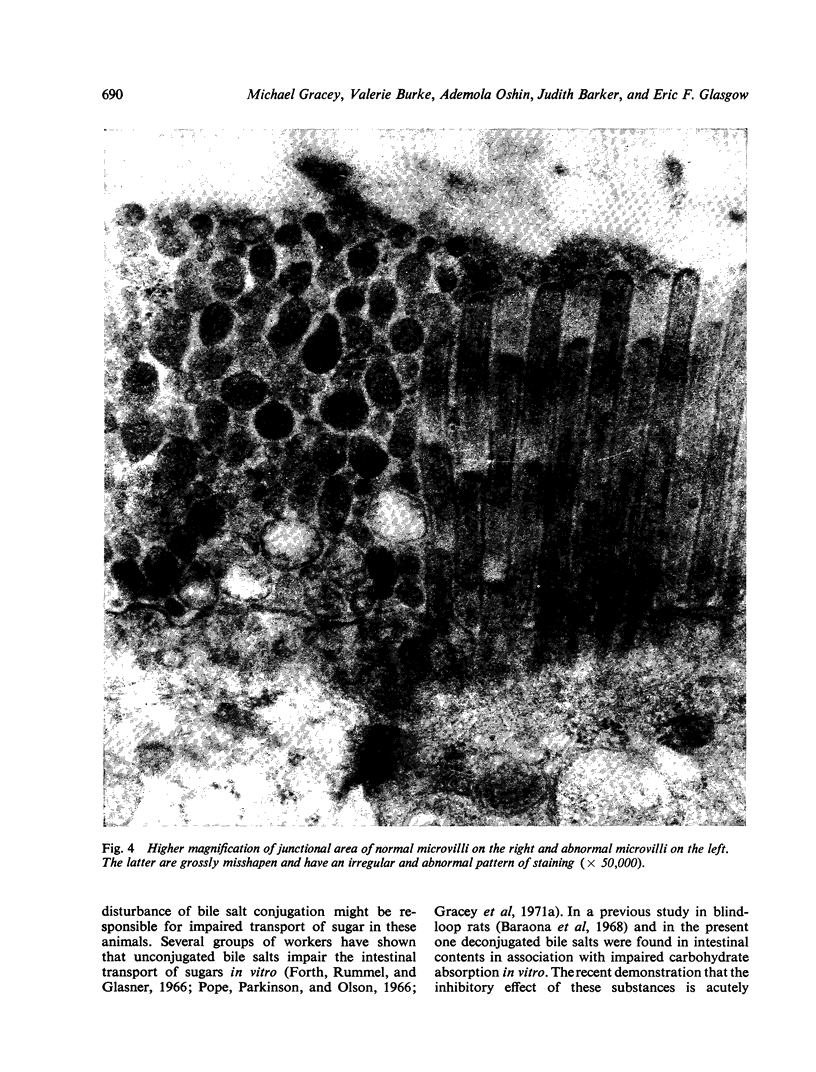
In the small intestine of blind-loop rats intestinal monosaccharide transport is impaired, and in vitro is most marked in the blind loop, less so in the efferent jejunum, and not significantly altered in the afferent jejunum. A similar pattern of disturbed monosaccharide absorption was demonstrated by perfusions in vivo. The degree of the transport defect correlates closely with the luxuriance of the anaerobic flora, which averaged 108 per millilitre in the blind loop, 107 in the efferent jejunum, and 106 in the afferent jejunum. A similar pattern of abnormality of bile salt conjugation occurred. In the blind loop the ratio of free to conjugated bile salts was grossly abnormal; this disturbance was somewhat less marked in the efferent jejunum and considerably less in the intraluminal contents of the afferent jejunum. An irregularly distributed lesion, consisting of swelling and vacuolation of microvilli and intracellular organelles, was demonstrated in the small-intestinal epithelium of blind-loop animals.
Impaired absorption of monosaccharides is a further consequence of bacterial contamination of the upper gut. It is suggested that this defect is caused by the presence of high levels of deconjugated bile salts produced by an abnormal anaerobic bacterial flora in the small intestine.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALVARADO F., CRANE R. K. STUDIES ON THE MECHANISM OF INTESTINAL ABSORPTION OF SUGARS. VII. PHENYLGLYCOSIDE TRANSPORT AND ITS POSSIBLE RELATIONSHIP TO PHLORIZIN INHIBITION OF THE ACTIVE TRANSPORT OF SUGARS BY THE SMALL INTESTINE. Biochim Biophys Acta. 1964 Oct 9;93:116–135. doi: 10.1016/0304-4165(64)90266-1. [DOI] [PubMed] [Google Scholar]
- BIHLER I., CRANE R. K. Studies on the mechanism of intestinal absorption of sugars. V. The influence of several cations and anions on the active transport of sugars, in vitro, by various preparations of hamster small intestine. Biochim Biophys Acta. 1962 May 7;59:78–93. doi: 10.1016/0006-3002(62)90699-6. [DOI] [PubMed] [Google Scholar]
- Baraona E., Palma R., Navia E., Salinas A., Orrego H., Espinoza J. The role of unconjugated bile salts in the malabsorption of glucose and tyrosine by everted sacs of jejunum of rats with the "blind-loop syndrome". Acta Physiol Lat Am. 1968;18(4):291–297. [PubMed] [Google Scholar]
- CRANE R. K., MANDELSTAM P. The active transport of sugars by various preparations of hamster intestine. Biochim Biophys Acta. 1960 Dec 18;45:460–476. doi: 10.1016/0006-3002(60)91482-7. [DOI] [PubMed] [Google Scholar]
- Coello-Ramírez P., Gutierres-Topete G., Lifshitz F. Pneumatosis intestinalis. Am J Dis Child. 1970 Jul;120(1):3–9. doi: 10.1001/archpedi.1970.02100060037002. [DOI] [PubMed] [Google Scholar]
- Dietschy J. M. Effects of bile salts on intermediate metabolism of the intestinal mucosa. Fed Proc. 1967 Nov-Dec;26(6):1589–1598. [PubMed] [Google Scholar]
- Donaldson R. M., Jr Role of enteric microorganisms in malabsorption. Fed Proc. 1967 Sep;26(5):1426–1431. [PubMed] [Google Scholar]
- Donaldson R. M., Jr Studies on the pathogenesis of steatorrhea in the blind loop syndrome. J Clin Invest. 1965 Nov;44(11):1815–1825. doi: 10.1172/JCI105289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forth W., Rummel W., Glasner H. Zur resorptionshemmenden Wirkung von Gallensäuren. Naunyn Schmiedebergs Arch Pharmakol Exp Pathol. 1966;254(4):364–380. [PubMed] [Google Scholar]
- Goldstein F., Karacadag S., Wirts C. W., Kowlessar O. D. Intraluminal small-intestinal utilization of d-xylose by bacteria. A limitation of the d-xylose absorption test. Gastroenterology. 1970 Sep;59(3):380–386. [PubMed] [Google Scholar]
- Gracey M., Burke V., Anderson C. A. Association of monosaccharide malabsorption with abnormal small-intestinal flora. Lancet. 1969 Aug 16;2(7616):384–385. doi: 10.1016/s0140-6736(69)92734-2. [DOI] [PubMed] [Google Scholar]
- Gracey M., Burke V., Oshin A. Influence of bile salts on intestinal sugar transport in vivo. Scand J Gastroenterol. 1971;6(3):273–276. doi: 10.3109/00365527109180707. [DOI] [PubMed] [Google Scholar]
- Gracey M., Burke V., Oshin A. Intestinal transport of fructose. Lancet. 1970 Oct 17;2(7677):827–828. doi: 10.1016/s0140-6736(70)91496-0. [DOI] [PubMed] [Google Scholar]
- Gracey M., Burke V., Oshin A. Reversible inhibition of intestinal active sugar transport by deconjugated bile salt in vitro. Biochim Biophys Acta. 1971 Feb 2;225(2):308–314. doi: 10.1016/0005-2736(71)90224-0. [DOI] [PubMed] [Google Scholar]
- Harries J. T., Francis D. E. Temporary monosaccharide intolerance. Acta Paediatr Scand. 1968 Nov;57(6):505–511. doi: 10.1111/j.1651-2227.1968.tb06970.x. [DOI] [PubMed] [Google Scholar]
- Hill M. J., Drasar B. S. Degradation of bile salts by human intestinal bacteria. Gut. 1968 Feb;9(1):22–27. doi: 10.1136/gut.9.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaerheim A., Nygaard K. Fat absorption in rats with an intestinal blind segment: an electron microscopic study. Scand J Gastroenterol. 1968;3(3):225–233. doi: 10.3109/00365526809180594. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lifshitz F., Coello-Ramírez P., Gutiérrez-Topete G. Monosaccharide intolerance and hypoglycemia in infants with diarrhea. I. Clinical course of 23 infants. J Pediatr. 1970 Oct;77(4):595–603. doi: 10.1016/s0022-3476(70)80200-1. [DOI] [PubMed] [Google Scholar]
- Lifshitz F., Coello-Ramírez P., Gutiérrez-Topete G. Monosaccharide intolerance and hypoglycemia in infants with diarrhea. II. Metabolic studies in 23 infants. J Pediatr. 1970 Oct;77(4):604–612. doi: 10.1016/s0022-3476(70)80201-3. [DOI] [PubMed] [Google Scholar]
- POLEY J. R., DOWER J. C., OWEN C. A., Jr, STICKLER G. B. BILE ACIDS IN INFANTS AND CHILDREN. J Lab Clin Med. 1964 May;63:838–846. [PubMed] [Google Scholar]
- Semenza G., Mülhaupt E. Studies on intestinal sucrase and sugar transport. VII. A method for measuring intestinal uptake. The absorption of the anomeric forms of some monosaccharides. Biochim Biophys Acta. 1969 Jan 28;173(1):104–112. doi: 10.1016/0005-2736(69)90041-8. [DOI] [PubMed] [Google Scholar]
- WARAVDEKAR V. S., SASLAW L. D. A method of estimation of 2-deoxyribose. Biochim Biophys Acta. 1957 May;24(2):439–439. doi: 10.1016/0006-3002(57)90224-x. [DOI] [PubMed] [Google Scholar]
- Wharton B., Howells G., Phillips I. Diarrhoea in kwashiorkor. Br Med J. 1968 Dec 7;4(5631):608–611. doi: 10.1136/bmj.4.5631.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterström R., Waldenström J. Familial monosaccharide malabsorption. Bibl Paediatr. 1968;87:101–112. [PubMed] [Google Scholar]