Abstract
Most of the symplastic water transport in plants occurs via aquaporins, but the extent to which aquaporins contribute to plant water status under favorable growth conditions and abiotic stress is not clear. To address this issue, we constitutively overexpressed the Arabidopsis plasma membrane aquaporin, PIP1b, in transgenic tobacco plants. Under favorable growth conditions, PIP1b overexpression significantly increased plant growth rate, transpiration rate, stomatal density, and photosynthetic efficiency. By contrast, PIP1b overexpression had no beneficial effect under salt stress, whereas during drought stress it had a negative effect, causing faster wilting. Our results suggest that symplastic water transport via plasma membrane aquaporins represents a limiting factor for plant growth and vigor under favorable conditions and that even fully irrigated plants face limited water transportation. By contrast, enhanced symplastic water transport via plasma membrane aquaporins may not have any beneficial effect under salt stress, and it has a deleterious effect during drought stress.
INTRODUCTION
Plant growth depends strongly on water absorption from the soil and its movement from the roots to other plant parts and finally to the atmosphere. Moreover, plant water status is important not only for growth under favorable environmental conditions; their ability to tolerate water deficits (Bray, 1993) and high salt levels (Cheeseman, 1988; Blumwald, 2000) also depends heavily on their water status, which is altered in response to environmental conditions (Bohnert et al., 1995; Steudle and Peterson, 1998).
Water transport within and between plant tissues uses both the apoplastic and the symplastic routes; therefore, a crucial proportion of the water molecules have to cross a number of cellular membranes (Quigley et al., 2002). Transport via the apoplastic route is driven by physical forces and is regulated mostly by differences in water potentials between the soil, plant, and atmosphere (Mizrahi, 1972; Munns, 1993; Maurel, 1997). According to the “composite transport model” suggested by Steudle and Frensch (1996), this route is coupled with the transpiration rate. The radial component of the symplastic route is regulated largely by a family of water channel proteins called aquaporins (Amodeo et al., 1999). Aquaporins facilitate the efficient transport of water molecules across membranes in many plants and also in many other organisms in nature (Chrispeels and Maurel, 1994; Kirch et al., 2000; Tyerman et al., 2002). The relative contributions of the apoplastic and symplastic routes to water transport in plants under favorable growth conditions, as well as under drought and salt stresses, remain a matter of debate (Carvajal et al., 1998; Schaffner, 1998; Amodeo et al., 1999; Tyerman et al., 1999).
The role of plant aquaporins in water transport across membranes has been proven by their expression in Xenopus laevis oocytes, and the same studies also revealed that some aquaporins can transport other small molecules, such as glycerol, solutes, and ions (Quigley et al., 2002; Tyerman et al., 2002). Plants possess several large families of aquaporins, which reside in the plasma membrane, the tonoplast, or in other cellular membranes (Jauh et al., 1999; Tyerman et al., 2002). Differential expression of genes that encode different aquaporin isoforms during plant development has been shown to be associated with various physiological processes. Such processes include stomatal closure and opening, organ movement, cell elongation, and cell division (Agre et al., 1993; Chrispeels and Agre, 1994; Suleyman et al., 2000; Morillon et al., 2001; Moshelion et al., 2002).
The potential relationship between the role of aquaporins in the regulation of plant water status and the regulation of aquaporin gene expression is unclear. The expression of some genes that encode plasma membrane aquaporins, such as Arabidopsis RD28 and the NeMip2 and NeMip3 genes of Nicotiana excelsior, is stimulated under drought stress (Yamaguchi et al., 1992; Yamada et al., 1997). Conversely, expression of the Mesembryanthemum crystallinum MIPA plasma membrane aquaporin gene is downregulated under salt stress (Yamada et al., 1995), whereas expression of the Arabidopsis PIP1a gene is not altered significantly by stress conditions (Grote et al., 1998).
Reduction of PIP1b expression level in Arabidopsis, by means of an antisense construct driven by the 35S promoter, resulted in a significant change in root morphology and an increase in root mass (Kaldenhoff et al., 1998). However, water potential and plant weight remained unaffected. In a more recent study, Siefritz et al. (2002) reduced the expression level of the tobacco PIP1b homolog (NtAQP1) in transgenic tobacco by means of the same antisense approach. This resulted in a higher sensitivity to polyethylene glycol, lower root hydraulic conductivity, and reduced transpiration rate than those in nontransformed control plants under drought stress.
Despite the extensive research on aquaporin function per se, little is known about the physiological role(s) of aquaporins in plant growth under favorable conditions or during adaptation to salt and drought stresses. Two of the key questions that have not been addressed are (1) whether water movement via aquaporins represents a rate-limiting factor for plant vigor under favorable growth conditions, and (2) how aquaporins affect the responses of plants to drought and salt stresses. To address these questions, we overexpressed the Arabidopsis AthH2 gene, which encodes PIP1b, in transgenic tobacco plants. We found that the transgenic plants exhibited better growth performance under favorable conditions but not under salt or drought stress.
RESULTS
Overexpression of PIP1b Enhances Plant Vigor under Favorable Growth Conditions
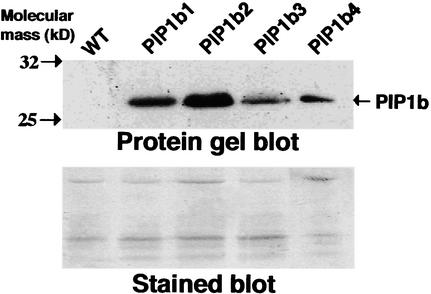
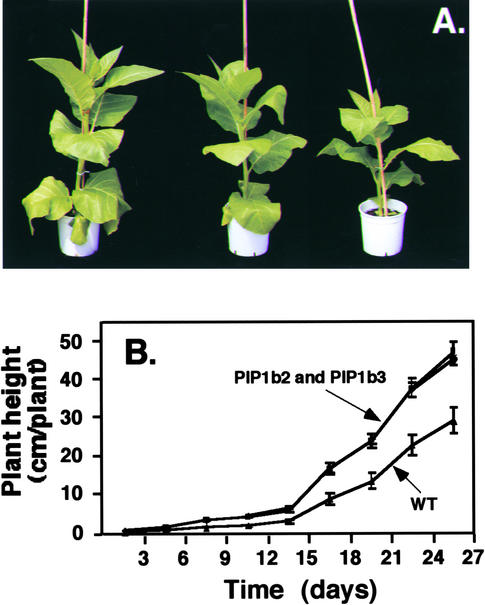
To examine the relative contributions of plasma membrane aquaporins to water status in plants, we transformed tobacco plants with a chimeric gene (35S-PIP1b-AU1) that encodes an Arabidopsis plasma membrane PIP1b aquaporin via the 35S promoter of Cauliflower mosaic virus. The encoded protein also contained an AU1 epitope tag at its C terminus. Fifteen Basta-resistant transgenic plants were regenerated and selfed to obtain T2 plants. To test for the expression of PIP1b, enriched membrane fractions were prepared from wild-type and four representative transgenic tobacco plants and reacted on a protein gel blot with anti-AU1 epitope antibodies. A cross-reacting band with the size expected of PIP1b was detected at variable intensities in all of these transgenic plants but not in the nontransformed control plants (Figure 1). Two transgenic lines, PIP1b2 and PIP1b3, were selected for further studies, and all of the analyses were performed on T2 plants. The transgenic plants expressing Arabidopsis PIP1b exhibited a significantly altered growth rate compared with that of the nontransformed plants. They were taller and their average stem diameters were ∼11 to 13% thicker than those of wild-type plants (Figure 2A). Moreover, their increased growth rate was particularly pronounced from ∼3 weeks after germination (Figure 2B). A similar altered growth pattern also was observed in many of the other independently transformed transgenic lines (data not shown). Moreover, the greater height of the transgenic plants resulted from the increased length and number of internodes, whereas the sizes of fully developed leaves in the transgenic and wild-type plants were comparable (data not shown).
Figure 1.
Overexpression of Arabidopsis PIP1b in Transgenic Tobacco Plants.
In the gel at top, proteins from membrane-enriched fractions of wild-type (WT) and representative transgenic tobacco plants independently transformed with the chimeric 35S-PIP1b-AU1 gene (PIP1b1 to PIP1b4) were reacted by protein gel blot analysis with monoclonal antibodies raised against the AU1 epitope tag. The migrations of two molecular mass markers and of PIP1b are indicated at left and right, respectively. The gel at bottom shows Coomassie blue staining of the gel at top.
Figure 2.
Morphology of the PIP1b-Overexpressing Transgenic Tobacco Plants.
(A) Phenotypes of 65-day-old wild-type (right) and transgenic PIP1b2 (left) and PIP1b3 (center) plants.
(B) Growth rates of wild-type (WT) and transgenic PIP1b2 and PIP1b3 plants. The growth rates of PIP1b2 and PIP1b3 plants were very similar; therefore, their curves overlap. Bars represent the standard error of the mean of at least five plants per genotype.
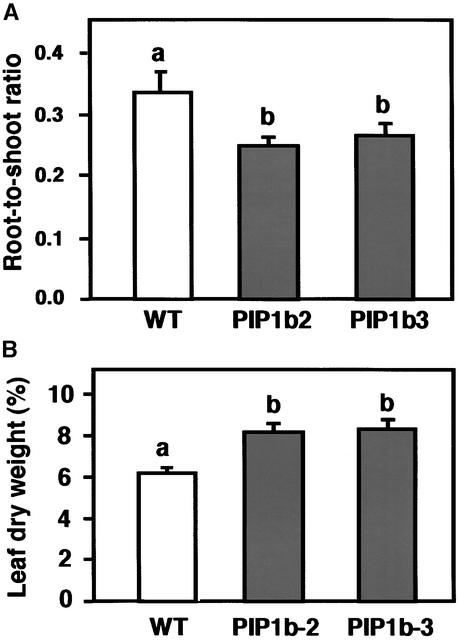
Because of the morphological difference between the wild-type and transgenic plants, we tested whether PIP1b overexpression affected the relative masses of the roots or shoots. The transgenic plants had a significantly lower root/shoot mass ratio than the wild-type plants (Figure 3A). However, this lower ratio was mostly the result of a nearly 50% increase in shoot fresh weight rather than a major decrease in root fresh weight (data not shown). This finding suggests that under favorable growth conditions, the PIP1b-overexpressing plants require a relatively lower root mass to support shoot growth and development. To determine further whether PIP1b overexpression affected leaf dry weight, the youngest fully expanded leaves were evaluated. Leaves of the transgenic plants contained nearly 30% more dry matter than leaves of the nontransformed control plants (Figure 3B).
Figure 3.
Effects of PIP1b Overexpression on Root/Shoot Ratio and Relative Water Content.
(A) The root/shoot mass ratio of wild-type (white column) and transgenic PIP1b2 and PIP1b3 (gray columns) lines grown hydroponically.
(B) Dry weight percentage was measured in the first fully exposed leaves of wild-type (white column) and transgenic PIP1b2 and PIP1b3 (gray columns) lines.
Bars represent the standard errors of the means of at least five plants per genotype. Different letters above the columns indicate statistically significant differences at the 0.05 level. WT, wild type.
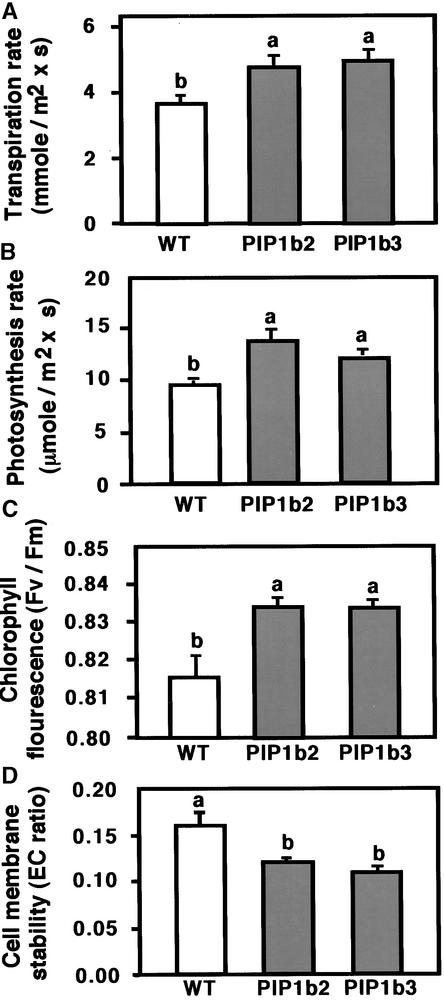
The physiological observations summarized in Figure 3 suggested that PIP1b overexpression resulted in improved plant growth under favorable conditions. Therefore, we hypothesized that PIP1b overexpression may have caused a concerted stimulation of cellular and physiological processes that regulate plant vigor. To test this hypothesis, we examined several representative physiological parameters that control plant vigor: (1) transpiration rate, a trait generally associated with the rates of water consumption and transport in the plant; (2) photosynthesis rate, a trait positively correlated with plant vitality and biomass production; (3) photochemical quantum efficiency (measured as the chlorophyll fluorescence parameter Fv/Fm [maximum photochemical efficiency of photosystem II in the dark-adapted state]), a trait positively correlated with the organization and vitality of photosystem II; and (4) cell membrane stability, a parameter indicating membrane vitality, measured by electric conductivity (EC) (see Methods). Relatively lower EC values represent lower membrane nutrient leakage and hence better membrane characters. A sharp increase in EC can be obtained under severe abiotic stress and may indicate the collapse of the membrane.
When grown under favorable growth conditions, the transgenic plants overexpressing PIP1b had significantly higher transpiration rates, photosynthesis rates, and Fv/Fm than the nontransformed control plants (Figures 4A, 4B, and 4C, respectively). In addition, the transgenic plants possessed lower cell membrane stability values than the nontransformed control plants, which correlate with better membrane vitality (Figure 4D). These results suggest that under favorable growth conditions, the transgenic plants had a more vigorous growth habit than the control plants.
Figure 4.
Effects of PIP1b Overexpression on Several Physiological Parameters of the Plants.
Transpiration rate (A), photosynthesis rate (B), the chlorophyll fluorescence parameter Fv/Fm (C), and cell membrane stability (D) (measured by an EC ratio whose relatively lower values are correlated with better membrane vitality) are shown. Measurements were performed on the first fully exposed leaves of wild-type (white columns) and transgenic PIP1b2 and PIP1b3 (gray columns) lines. Bars represent the standard errors of the means of at least five plants per genotype. Different letters above the columns indicate statistically significant differences at the 0.05 level. WT, wild type.
Overexpression of PIP1b Enhances Stomatal Density and Water Consumption under Favorable Growth Conditions
The higher transpiration rate of the PIP1b-overexpressing plants than the wild-type plants suggested that PIP1b overexpression led to higher stomatal density. To address this possibility, we measured the average stomatal density in epidermis derived from the upper and lower surfaces of leaves from wild-type, PIP1b2, and PIP1b3 plants grown under favorable growth conditions. Both PIP1b2- and PIP1b3- overexpressing plants had significantly higher stomatal density at both leaf epidermis layers (Table 1).
Table 1.
Stomatal Density in Epidermis of Fully Exposed Leaves of Wild-Type and PIP1b-Overexpressing Plants Grown under Favorable Growth Conditions
| Sample | Wild Type | PIP1b2 | PIP1b3 |
|---|---|---|---|
| Upper leaf surface | 67.2 ± 1.9 | 82.1 ± 2.2 | 82.3 ± 2.4 |
| Lower leaf surface | 145.0 ± 2.6 | 174.6 ± 3.4 | 163.5 ± 3.9 |
Average stomatal density values (number/mm2 ± se) were determined from analysis of 30 microscopic fields obtained from leaves derived from three independent plants for each genotype. All values for the PIP1b2 and PIP1b3 plants were significantly different from those for the wild-type plants at the 0.05 level.
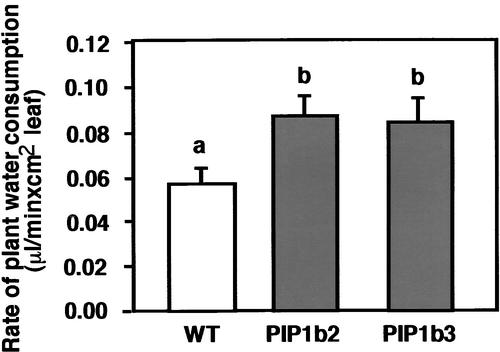
Next, we wished to determine whether the increased stomatal density in PIP1b overexpression is associated with increased water consumption. To this end, ∼30-day-old hydroponically grown plants were analyzed for water consumption using a highly accurate microelectronic potometer that was developed recently in our laboratory (R. Aharon, G. Galili, A. Blum, and Y. Kapulnik, unpublished data). Under favorable growth conditions, the PIP1b-overexpressing plants possessed a significantly higher rate of water consumption than the wild-type plants (Figure 5).
Figure 5.
Effect of PIP1b Overexpression on Plant Water Consumption.
Water consumption by 30-day-old plants grown hydroponically was analyzed using a microelectronic potometer (R. Aharon, G. Galili, A. Blum, and Y. Kapulnik, unpublished data). The rate of water consumption was measured from the linear water absorption curve and was normalized to units of leaf area. Bars represent the standard errors of the means of at least five plants per genotype. Different letters above the columns indicate statistically significant differences at the 0.05 level. WT, wild type.
Performance of PIP1b-Overexpressing Plants under Drought or Salt Stress
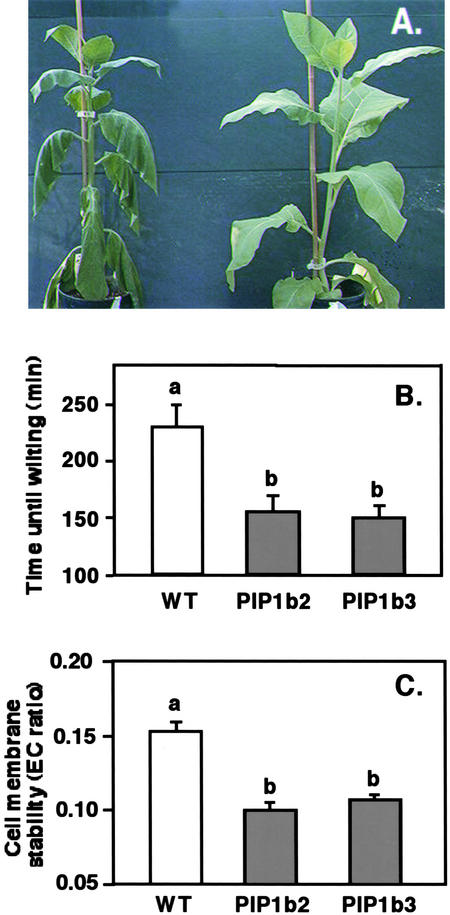
The capacity to transport water within and between plant organs plays a major role not only under favorable growth conditions but also under conditions of water scarcity. The effect of PIP1b overexpression on the response of plants to gradually imposed drought stress (by stopping irrigation) was tested using plants with equal canopy sizes (which required slightly differing growth times in the transgenic and wild-type plants). The transgenic plants started wilting, as determined by their phenotype, as soon as 19 days (PIP1b2; see phenotype in Figure 6A) or 20 days (PIP1b3) after the termination of irrigation, at which time the nontransformed control plants still had a nearly normal phenotype. It took 25 days without irrigation for the control plants to start wilting in this experiment (data not shown). The faster wilting of the transgenic plants than the wild-type plants also was associated with a faster reduction in relative leaf water content (data not shown).
Figure 6.
Response of PIP1b Overexpression to Drought Stress.
(A) Wild-type and transgenic PIP1b2 plants were subjected to drought stress as indicated in Methods. A representative PIP1b2 plant (left) and a wild-type plant (right) were photographed on the day the first plant wilted. PIP1b3 plants also wilted significantly earlier than wild-type plants, but no photograph was taken.
(B) Time for detached leaves from regularly irrigated wild-type and PIP1b2- and PIP1b3-overexpressing plants to wilt.
(C) Cell membrane stability (EC ratio) was analyzed in wild-type and PIP1b2- and PIP1b3-overexpressing plants at the time that detached leaves of the transgenic plants wilted (leaves of wild-type plants had not yet wilted).
Bars indicate the standard errors of the means of at least five plants per genotype. Different letters above the columns indicate statistically significant differences at the 0.05 level. WT, wild type.
In a different experiment, we also measured the desiccation rate of detached, first fully exposed leaves from nearly 6-week-old transgenic and control plants. Leaves of both PIP1b2 and PIP1b3 plants wilted within 150 min after cutting, when leaves of the control plants still looked nonwilted (Figure 6B). The wild-type leaves wilted only after nearly 230 min. Notably, although leaves of the transgenic plants wilted faster than those of the control plants (nearly 80 min), they still maintained significantly lower cell membrane stability values than the control plants whose leaves were not yet wilted (Figure 6C). Because similar behavior of the transgenic plants also was found under favorable growth conditions (Figure 4D), our results suggest that the better cell membrane vitality exhibited by the transgenic plants under favorable growth conditions was maintained under drought stress. These results also imply that the faster wilting of the transgenic plants under drought stress was not caused by the collapse of their cell membranes but simply by the loss of water.
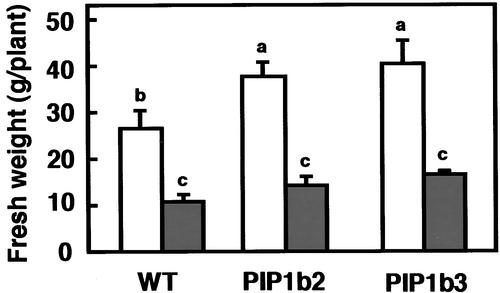
The effects of PIP1b overexpression on the performance of the control and transgenic tobacco plants under moderate salt stress also were tested. Salt stress was applied by irrigating 4-week-old plants with tap water, without supplementation or supplemented with 90 mM NaCl, for a period of 40 days. The extent of plant growth then was determined by measuring fresh weight. Under nonsaline growth conditions (no addition of NaCl), the transgenic plants developed significantly higher fresh weights than the nontransformed control plants (Figure 7, open columns). However, under saline growth conditions, both the transgenic and control plants had similar fresh weights (Figure 7, closed columns).
Figure 7.
Effect of PIP1b Overexpression on Plant Fresh Weight under Salt Stress.
Wild-type and transgenic PIP1b2 and PIP1b3 plants were irrigated for 40 days with either tap water (white columns) or tap water supplemented with 90 mM NaCl (gray columns), and total plant fresh weights were measured. Bars represent the standard errors of the means of at least five plants per genotype. Different letters above the columns indicate statistically significant differences at the 0.05 level. WT, wild type.
DISCUSSION
Overexpression of a Plasma Membrane Aquaporin Increases Plant Vigor under Favorable Growth Conditions
In the present study, we demonstrated that constitutive overexpression of a plasma membrane aquaporin enhanced plant growth and transpiration under favorable growth conditions. This finding implies that symplastic water transport via plasma membrane aquaporins represents a rate-limiting factor for water transport in plants even under favorable growth conditions. Our results also suggest that the symplastic route is dominant over the apoplastic route with regard to plant water transport. Similar findings on the dominance of symplastic water transport were obtained using transgenic tobacco plants expressing an antisense construct of the tobacco NtAQP1 gene that encodes another PIP1b isoform (Siefritz et al., 2002).
Besides the contribution of these results to our knowledge of the function of aquaporins, they also contribute to the understanding of the factors that regulate or govern plant vigor. In fact, we have shown here that water transport per se represents a rate-limiting step for plant vigor under favorable growth conditions. This was inferred from two different lines of evidence. First, the transgenic plants overexpressing PIP1b transferred more water, as deduced from their higher transpiration and water consumption rates, and also grew faster than the control nontransformed plants. Second, the transgenic plants overexpressing PIP1b exhibited more efficient photosynthesis and also accumulated more dry matter per unit of leaf area than the nontransformed control plants.
Why does PIP1b overexpression cause more efficient photosynthesis under favorable growth conditions? Mild water deficit is known to inhibit photosynthesis, particularly that controlled by photosystem II, mostly because partial stomatal closure leads to reduced CO2 penetration (Lawlor, 2002). It is possible that wild-type tobacco plants are always exposed to mild water stress because of suboptimal symplastic water transport via the aquaporins. Moreover, such stress also might result in suboptimal photosynthesis activity as a result of suboptimal stomatal opening or stomatal density. Our observation that PIP1b overexpression also enhanced the transpiration rate under favorable growth conditions supports this hypothesis.
Another interesting observation was the lower root/shoot mass ratio in the transgenic plants than in the nontransformed control plants. This observation supports and extends a previous report (Kaldenhoff et al., 1998) showing that reduction of PIP1b levels by antisense expression increased the root/shoot ratio in Arabidopsis plants. These results also suggest that root volume is regulated in accordance with the hydraulic conductivity and efficiency of water uptake, optimizing water transport into the aboveground tissues.
Because PIP1b is a member of a multigene family, how its overexpression can significantly affect plant growth remains an open question. Similarly, it also is an open question how the inhibition of expression of only a fraction of the aquaporins (Kaldenhoff et al., 1998) significantly affects plant growth. One possible explanation is that local changes in plant water status in specific cells or tissues may act as signals to alter, in a concerted and/or a hierarchic manner, an array of mechanisms that control processes associated with plant growth. Stomatal density is a complex trait that is regulated strongly by genetic factors as well as by environmental factors such as humidity, light, and CO2 partial pressure (Berger and Altmann, 2000). In addition, changes in stomatal density also are associated with changes in photosynthesis rate, which in turn is important for plant vigor (Cheeseman, 1991; Kundu and Tigerstedt, 1999). Thus, it is possible that increased water flux in the PIP1b-overexpressing plants regulated the production of higher stomatal density, which in turn caused an increased photosynthesis rate and enhanced plant vigor. However, we cannot exclude the possibility that photosynthesis also is regulated directly by sensing the water status.
Overexpression of a Plasma Membrane Aquaporin Has No Beneficial Effects under Drought or Salt Stress
In contrast to the positive effect of PIP1b overexpression under favorable growth conditions, this overexpression was not beneficial to plant growth under salt stress and was even deleterious during drought stress, in which it accelerated wilting. Thus, our results suggest that a general increase in water transport in most plant tissues and cells (as expected from the expression of the 35S promoter) is “harmful” under drought stress and perhaps under salt stress as well. Therefore, it is tempting to hypothesize that plants limit their symplastic water transport via aquaporins, and hence their transpiration rate, under favorable growth conditions as a defensive mechanism to prevent fast wilting under transient or prolonged water stress.
The relationship between the deleterious effect of PIP1b overexpression on plants under drought stress and the natural function of this protein is not simple, because expression of the native Arabidopsis PIP1b gene is stimulated by the stress-related hormone abscisic acid (Kaldenhoff et al., 1996; Siefritz et al., 2002). How can these two seemingly conflicting observations be reconciled? It is possible that the Arabidopsis PIP1b gene has special spatial and temporal expression patterns and that its induced expression under abiotic stress is important specifically in the cells and developmental stages in which it is expressed naturally. The chimeric 35S-PIP1b gene that was used in the present study is likely to be expressed ectopically with respect to the natural expression of PIP1b, both spatially and temporally. Hence, enhanced water transport via the plasma membranes of cells that do not naturally contain PIP1b may be deleterious under water stress. The role of aquaporins in plant water status under water stress is a complex issue, because the expression of different aquaporin genes may be stimulated, reduced, or unchanged under abiotic stress (Yamaguchi et al., 1992; Bohnert et al., 1995; Maurel, 1997; Kirch et al., 2000; Kawasaki et al., 2001). Indeed, this complex expression pattern suggests that maintenance of a reasonable water status under salt and drought stresses requires both increased symplastic water transport via aqua-porins in some cells and tissues and reduced water transport via aquaporins in other cells and tissues (Comparot et al., 2000).
Our observation that PIP1b overexpression accelerated wilting under natural drought stress also is opposite to the observation of Siefritz et al. (2002), who reported that reduction of PIP expression accelerated wilting upon exposure to a drought stress imposed by polyethylene glycol. Although the reason for these contradictory results is not yet solved, we believe them to be attributable to the fact that polyethylene glycol treatment imposes an unnatural osmotic drought stress that is different from a natural drought stress. A natural drought stress (i.e., termination of water availability), like that imposed in the present study, is a “long-term” process that allows the plant to adapt by stimulating a pyramid of interacting mechanisms. Thus, even though a single gene is tested in transgenic plants, its effect on drought stress will be the result of its concerted interaction with many other innate processes. Polyethylene glycol is an artificial treatment that induces the osmotic drought stress very rapidly, so wilting may occur before the plant's innate adaptation response has been stimulated. Nevertheless, we hypothesize that plants can tolerate salt and drought stresses better if the total integrated amount of symplastic water transport via plasma membrane aquaporins is reduced during the stress.
METHODS
Plant Material
Tobacco (Nicotiana tabacum cv Samsun NN) plants were grown in 3-L pots with ready-mixed soil, except for in the experiments described in Figures 3 and 5, for which plants were grown in hydroponic tanks containing half-strength Hoagland nutrient solution (Ostrem et al., 1987). Growth conditions were 25/22°C (night/day) under a 12-h photoperiod with full light intensity of 250 μE·m−2·s−1.
Cloning and Plant Transformation
The coding DNA sequence of Arabidopsis PIP1b was cloned by PCR using the following forward and reverse primers: 5′-CACACGTTACACACAGGTAGTAG-3′ and 5′-CCCACTCACAGAAAAACCTAG-AAAGCTCTAG-3′. These primers also introduced an in-frame AU1 epitope tag (5′-GATGTATCTGTACGTATC-3′) at the 3′ end of the PIP1b coding sequence before the TGA stop codon. The PCR product was subcloned into the pBluescript SK+ plasmid and then subcloned into a cassette containing a 35S promoter and an octopine synthase terminator in a binary PZP111-derived (Hajdukiewicz et al., 1994) Ti vector that contained both kanamycin and Basta resistance genes. This plasmid then was immobilized into Agrobacterium tumefaciens strain EHA105 (Hood et al., 1986), which was used to transform tobacco plants by means of the leaf disc protocol (Horsch et al., 1985). Transgenic plants were selected by spraying with 0.1% Basta. Transformation also was confirmed by PCR with the primers described above.
Isolation of Cell Membranes and Protein Gel Blot Analysis
Cell membranes were isolated as described previously (Shaul et al., 1999). Protein gel blot analysis was performed according to Tang et al. (1997) with commercial anti-AU1 monoclonal antibodies (Berkeley Antibody, Berkeley, CA).
Plant Growth under Salt Stress and Drought Stress
To initiate the salt stress, nearly 3-week-old plants were irrigated for 40 days with either tap water or tap water containing 90 mM NaCl. To initiate the drought stress, the irrigation of nearly 3-week-old plants was stopped. For both stresses, efforts were made to select plants of approximately equal size at the initiation of the stress (which required slightly different growth times for the transgenic and wild-type plants).
Measurements of Physiological Parameters and Leaf Stomata Density
The chlorophyll fluorescence parameter Fv/Fm (maximum photochemical efficiency of photosystem II in the dark-adapted state) was measured as described previously (Bolhar-Nordenkampf et al., 1989) with a portable chlorophyll fluorometer (PAM 2000; Heinz, Walz, Germany). The measurements were performed on eight mature (fully exposed) leaves from each of four plants per variant at 8:00 am (to avoid the involvement of daily photoinhibition) after 15 min of dark adaptation by dark leaf clips. Both transpiration and photosynthesis (CO2 uptake) rates were measured with a hand-held photosynthesis system CI-510 (CID, Camas, WA) in the morning (10:00 to 11:00 am) under natural sunlight (∼1200 μE·m−2·s−1) in the greenhouse. Cell nutrient leakage was measured as described previously (Blum and Adelina, 1979). Ten leaf discs (1 cm in diameter) from 8-week-old tobacco plants were measured after 24 h in the dark (at 4°C), and their nutrient leakage was measured after autoclave treatment.
All experiments were repeated at least twice, and each experimental treatment was performed with five replications, unless stated otherwise. Data were analyzed with the JMP statistics package (version 3.1.5; SAS Institute, Cary, NC). Stomatal density was determined essentially as described previously (Berger and Altmann, 2000) by light microscopy of nail polish imprints taken from the top and bottom surfaces of the leaves.
Upon request, all novel materials described in this article will be made available in a timely manner for noncommercial research purposes.
Accession Number
The GenBank accession number for PIP1b is Z17399.
Acknowledgments
We thank Orit Shaul for her help in the isolation of cell membranes and Kira Ratner for her help in the analysis of photosynthesis and chlorophyll fluorescence. This study was supported by a grant from the Chief Scientist of the Ministry of Agriculture, Israel. G.G. is an incumbent of the Bronfman Chair of Plant Science.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.009225.
References
- Agre, P., Sasaki, S., and Chrispeels, M.J. (1993). Aquaporins: A family of water channels proteins. Am. J. Physiol. 265, F461. [DOI] [PubMed] [Google Scholar]
- Amodeo, G., Dorr, R., Vallejo, A., Stuka, M., and Parisi, M. (1999). Radial and axial water transport in the sugar beet storage root. J. Exp. Bot. 50, 509–516. [Google Scholar]
- Berger, D., and Altmann, T. (2000). A subtilisin-like serine protease involved in the regulation of stomatal density and distribution in Arabidopsis thaliana. Genes Dev. 14, 1119–1131. [PMC free article] [PubMed] [Google Scholar]
- Blum, A., and Adelina, E. (1979). Cell membrane stability as measure of drought and heat tolerance in wheat. Crop Sci. 21, 43–47. [Google Scholar]
- Blumwald, E. (2000). Sodium transport and salt tolerance in plants. Curr. Opin. Cell Biol. 12, 431–434. [DOI] [PubMed] [Google Scholar]
- Bohnert, H.J., Nelson, D.E., and Jensen, R.G. (1995). Adaptation to environment stress. Plant Cell 7, 1099–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolhar-Nordenkampf, H.R., Long, S.P., Baker, N.R., Oquist, G., Schreiber, U., and Lechner, E. (1989). Chlorophyll fluorescence as a probe of the photosynthetic competence of leaves in the field: A review of current instrumentation. Funct. Ecol. 3, 497–514. [Google Scholar]
- Bray, E.A. (1993). Molecular responses to water deficit. Plant Physiol. 103, 1035–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvajal, M., Cooke, D.T., and Clarkson, D.T. (1998). The lipid bilayer and aquaporins: Parallel pathways for water movement into plant cells. Plant Growth Regul. 25, 89–95. [Google Scholar]
- Cheeseman, J.M. (1988). Mechanisms of salinity tolerance in plants. Plant Physiol. 87, 547–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman, J.M. (1991). Patchy: Simulating and visualizing the effects of stomatal patchiness on photosynthetic CO2 exchange studies. Plant Cell Environ. 14, 593–599. [Google Scholar]
- Chrispeels, M.J., and Agre, P. (1994). Aquaporins: Water channel proteins of plant and animal cells. Trends Biochem. Sci. 19, 421–425. [DOI] [PubMed] [Google Scholar]
- Chrispeels, M.J., and Maurel, C. (1994). Aquaporins: The molecular basis of facilitated water movement through living cells. Plant Physiol. 105, 9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comparot, S., Morilon, R., and Badot, P.M. (2000). Water permeability and revolving movement in Phaseolus vulgaris L. Plant Cell Physiol. 41, 114–118. [DOI] [PubMed] [Google Scholar]
- Grote, K., Trzebiatovski, P., and Kaldenhoff, R. (1998). RNA levels of plasma membrane aquaporins in Arabidopsis thaliana. Protoplasma 204, 139–144. [Google Scholar]
- Hajdukiewicz, P., Svab, Z., and Maliga, P. (1994). The small versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol. Biol. 25, 989–994. [DOI] [PubMed] [Google Scholar]
- Hood, E.E., Helmer, G.L., Fraley, R.T., and Chilton, M. (1986). The hypervirulence of Agrobacterium tumefaciens A281 is encoded in a region of pTiBo542 outside of T-DNA. J. Bacteriol. 168, 1291–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsch, R.B., Fry, J.E., Hoffmann, N.L., Eichholtz, D., Rogers, S.G., and Fraley, R.T. (1985). A simple and general method for transferring genes into plants. Science 227, 1229–1231. [DOI] [PubMed] [Google Scholar]
- Jauh, G.Y., Thomas, E.P., and Rogers, J.C. (1999). Tonoplast intrinsic protein isoforms as markers for vacuolar functions. Plant Cell 11, 1867–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldenhoff, R., Grote, K., Zhu, J.J., and Zimmermann, U. (1998). Significance of plasmalemma aquaporins for water transport in Arabidopsis thaliana. Plant J. 14, 121–128. [DOI] [PubMed] [Google Scholar]
- Kaldenhoff, R., Kolling, A., and Richter, G. (1996). Regulation of the Arabidopsis thaliana aquaporin gene AthH2 (PIP1b). J. Photochem. Photobiol. 36, 351–354. [DOI] [PubMed] [Google Scholar]
- Kawasaki, S., Borchert, C., Deyholos, M., Wang, H., Brazille, S., Kawai, K., Galbraith, D., and Bohnert, H.J. (2001). Gene expression profiles during the initial phase of salt stress in rice. Plant Cell 13, 889–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirch, H.H., Vera-Estrella, R., Golldack, D., Quigley, F., Michalowski, C.B., Barkla, B.J., and Bohnert, H.J. (2000). Expression of water channel proteins in Mesembryanthemum crystallinum. Plant Physiol. 123, 111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu, S.K., and Tigerstedt, P.M.A. (1999). Variation in net photosynthesis, stomatal characteristics, leaf area and whole-plant phytomass production among ten provenances of neem (Azadirachta indica). Tree Physiol. 19, 47–52. [DOI] [PubMed] [Google Scholar]
- Lawlor, D.W. (2002). Limitation to photosynthesis in water-stressed leaves: Stomata vs. metabolism and the role of ATP. Ann. Bot. 89, 871–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel, C. (1997). Aquaporins and water permeability of plant membrane. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 399–429. [DOI] [PubMed] [Google Scholar]
- Mizrahi, Y. (1972). Mechanisms Which Relate to Adaptations of Plant Leaves to Root Stress. PhD dissertation (Jerusalem: Hebrew University).
- Morillon, R., Lienard, D., Chrispeels, M.J., and Lassalles, J.P. (2001). Rapid movements of plant organs require solute-water cotransporters or contractile proteins. Plant Physiol. 127, 720–723. [PMC free article] [PubMed] [Google Scholar]
- Moshelion, M., Becker, D., Biela, A., Uehlein, N., Hedrich, R., Otto, B., Levi, H., Moran, N., and Kaldenhoff, R. (2002). Plasma membrane aquaporins in the motor cells of Samanea saman: Diurnal and circadian regulation. Plant Cell 14, 727–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns, R. (1993). Physiological processes limiting plant growth in saline soils: Some dogmas and hypotheses. Plant Cell Environ. 16, 15–24. [Google Scholar]
- Ostrem, J.A., Olson, S.W., Schmitt, J.M., and Bohnert, H.J. (1987). Salt stress increases the level of translatable mRNA for phosphoenolpyruvate carboxylase in Mesembryanthemum crystallinum. Plant Physiol. 84, 1270–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley, F., Rosenberg, J.M., Shahar-Hill, Y., and Bonhert, H.J. (2002). From genome to function: The Arabidopsis aquaporins. Genome Biol. 3, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner, A.R. (1998). Aquaporin function, structure, and expression: Are there more surprises to surface in water relations? Planta 204, 131–139. [DOI] [PubMed] [Google Scholar]
- Shaul, O., Hilgemann, D.W., de-Almeida-Engler, J., Van Montagu, M., Inze, D., and Galili, G. (1999). Cloning and characterization of a novel Mg2+/H+ exchanger. EMBO J. 18, 3973–3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siefritz, F., Tyree, M.T., Lovisolo, C., Schubert, A., and Kaldenhoff, R. (2002). PIP1 plasma membrane aquaporins in tobacco: From cellular effects to function in plants. Plant Cell 14, 869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steudle, E., and Frensch, J. (1996). Water transport in plants: Role of the apoplast. Plant Soil 187, 67–79. [Google Scholar]
- Steudle, E., and Peterson, C.A. (1998). How does water get through roots? J. Exp. Bot. 49, 775–788. [Google Scholar]
- Suleyman, I.A., Atsushi, A., Yoshitaka, N., and Murata, N. (2000). Inactivation of photosystems I and II in response to osmotic stress in Synechococcus: Contribution of water channels. Plant Physiol. 122, 1201–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, G., Miron, D., Zhu-Shimoni, J.X., and Galili, G. (1997). Regulation of lysine catabolism through lysine-ketoglutarate reductase and saccharopine dehydrogenase in Arabidopsis. Plant Cell 9, 1305–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyerman, S., Bohnert, H., Maurel, C., Steudle, E., and Smith, J.A.C. (1999). Plant aquaporins: Their molecular biology, biophysics and significance for plant water relations. J. Exp. Bot. 50, 1055–1071. [Google Scholar]
- Tyerman, S.D., Niemietz, C.M., and Bramley, H. (2002). Plant aquaporins: Multifunctional water and solute channels with expanding roles. Plant Cell Environ. 25, 173–194. [DOI] [PubMed] [Google Scholar]
- Yamada, S., Katsuhiro, M., Kelly, W.B., Michalowski, C.B., and Bohnert, H.J. (1995). A family of transcripts encoding water channel protein: Tissue-specific expression in the common ice plant. Plant Cell 7, 1129–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, S., Komory, T., Myers, P.M., Kuwata, S., and Imaseki, H. (1997). Expression of plasma membrane water channel genes under water stress in Nicotiana excelsior. Plant Cell Physiol. 38, 1226–1231. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, K., Koizumi, M., Urao, S., and Shinozaki, K. (1992). Molecular cloning and characterization of 9 cDNAs for genes that are responsive to desiccation in Arabidopsis thaliana: Sequence analysis of one cDNA clone encodes a putative transmembrane channel protein. Plant Cell Physiol. 33, 217–224. [Google Scholar]