Abstract
A role for auxin in the regulation of shoot branching was described originally in the Thimann and Skoog model, which proposes that apically derived auxin is transported basipetally directly into the axillary buds, where it inhibits their growth. Subsequent observations in several species have shown that auxin does not enter axillary buds directly. We have found similar results in Arabidopsis. Grafting studies indicated that auxin acts in the aerial tissue; hence, the principal site of auxin action is the shoot. To delineate the site of auxin action, the wild-type AXR1 coding sequence, which is required for normal auxin sensitivity, was expressed under the control of several tissue-specific promoters in the auxin-resistant, highly branched axr1-12 mutant background. AXR1 expression in the xylem and interfascicular schlerenchyma was found to restore the mutant branching to wild-type levels in both intact plants and isolated nodes, whereas expression in the phloem did not. Therefore, apically derived auxin can suppress branching by acting in the xylem and interfascicular schlerenchyma, or in a subset of these cells.
INTRODUCTION
The central philosophy in the study of the regulation of plant shoot architecture is the concept of apical dominance, whereby the growing apical meristem suppresses the growth of axillary meristems, lying in the axils of leaves below it. By decapitating plants and substituting various compounds for the apex, Thimann and Skoog (1934) demonstrated that apical dominance could be mediated by the plant hormone auxin. This led to the direct inhibition hypothesis, which proposes that auxin, synthesized at the shoot apex, is transported basipetally down the stem to the bud, which it enters to mediate growth inhibition (Thimann, 1937). The Thimann and Skoog model has formed the basis for many subsequent investigations using both classic physiology and molecular genetic approaches (reviewed by Cline, 1991, 1994). Although most experiments support the idea of auxin as an inhibitor of bud growth, its precise mode of action remains unresolved.
The first aspect of this model—that auxin, which in planta is predominantly indole-3-acetic acid (IAA), is produced at the growing shoot apex—has been demonstrated for a number of plant species, although the precise contribution of the meristem, leaf, and young stem tissues to the production of auxin is not known (Thimann and Skoog, 1934; White et al., 1975; Hosokawa et al., 1990; Ljung et al., 2001).
The second aspect of the Thimann and Skoog model is that auxin is transported basipetally into the axillary buds. Basipetal transport of auxin in the stem has been demonstrated to occur in a polar manner and to be required for apical dominance in a number of plant species (Morris, 1977; Everat-Bourbouloux and Bonnemain, 1980; Brown and Phillips, 1982; Lim and Tamas, 1989; Okada et al., 1991). However, auxin transport often occurs too slowly to account for the kinetics of bud repression (Hall and Hillman, 1975; Brown et al., 1979; Everat-Bourbouloux and Bonnemain, 1980). Furthermore, in many species, although basipetal auxin transport in the stem is required, the auxin does not appear to enter the bud (Hillman et al., 1977; Morris, 1977; Brown et al., 1979; Everat-Bourbouloux and Bonnemain, 1980; Prasad et al., 1993). Consistent with this finding, endogenous auxin levels in axillary buds do not correlate with the degree of bud inhibition. In some species, the level of auxin in buds released from apical dominance remains constant or even increases as they grow out (Hillman et al., 1977; Pilate et al., 1989; Gocal et al., 1991), in contrast to the predictions of the Thimann and Skoog model.
The evidence for the remote action of auxin has led to the suggestion that auxin acts by regulating the production of a second messenger that is transported into the bud (Snow, 1937). The best documented candidate for such a second messenger is cytokinin, whose biosynthesis and export from the roots is controlled by auxin and which can enter buds and stimulate their growth (Palni et al., 1988; Bangerth, 1994; Ekölf et al., 1995; Li et al., 1995). However, the kinetics of bud break are faster than those of cytokinin increase in the bud, so cytokinins may not be the primary signal for bud growth (Turnbull et al., 1997).
Recent work in pea also demonstrates that cytokinin cannot be the only second messenger for auxin. The ramosus (rms) mutants from pea show increased aerial branching, which in rms1, rms2, and rms5 appears to be attributable to the lack of a graft-transmissible factor that moves up the plant and is required for auxin-mediated repression (Beveridge et al., 1994, 1997a, 2000; Morris et al., 2001) The rms1 phenotype is not the result of increased cytokinin export from roots, because rms1 xylem exudates contain much lower levels of cytokinin than do wild-type exudates (Beveridge et al., 1997b). Hence, the analysis of these mutants suggests the existence of novel branching regulators that are required for the auxin inhibition of bud growth. The fact that these move acropetally from below the node where bud inhibition takes place has led some authors to suggest that the term “apical dominance” is inappropriate to describe the control of branching (Napoli et al., 1999).
More recently, molecular work has focused on the model plant Arabidopsis. Unlike previously examined species, the majority of the secondary nodes in Arabidopsis lie in a rosette, with only a minority of the nodes being accessible for study on the primary inflorescence (the cauline nodes). When grown in long days, Arabidopsis exhibits only weak suppression of branching with respect to the cauline nodes, with bud release occurring soon after floral transition and inflorescence elongation in a basipetal progression (Hempel and Feldman, 1994; Cline, 1996; Stirnberg et al., 1999; Grbic and Bleecker, 2000).
Phenotypic analyses of auxin response mutants have provided persuasive evidence for an in vivo role for auxin in shoot-branching control in Arabidopsis. Mutations in AXR1, which confers auxin resistance, result in increased branching, with the degree of branching correlating with the degree of insensitivity to auxin in the different alleles (Lincoln et al., 1990; Timpte et al., 1995). Similarly, mutations in the AXR3 locus, which leads to an increased amplitude in auxin response, inhibit branching even at the cauline nodes (Leyser et al., 1996; Cline et al., 2000).
Although observations from these mutants are consistent with a role for auxin in mediating apical dominance, all of the lines are phenotypically pleiotropic, so the observed patterns of branching could be the indirect consequences of other auxin-regulated phenotypes, such as reduced fertility (Hensel et al., 1994). However, analysis of the response of the mutant buds to apically applied auxin in excised node assays suggests that the branching phenotypes result directly from changes in auxin sensitivity, because the buds of axr1-12 mutants were found to be auxin resistant in this assay, whereas the buds of axr3-1 mutants showed increased inhibition in response to apical auxin (Stirnberg et al., 1999; Cline et al., 2000).
Current evidence suggests that auxin acts indirectly and that root genotype can affect the ability of apical auxin to inhibit bud growth. To improve our understanding of the site of auxin action in the suppression of branching, we have attempted to suppress the shoot-branching defect of the severe axr1-12 mutation by introducing the wild-type AXR1 cDNA sequence under the control of a range of promoters that drive restricted patterns of gene expression in the stem. Previously, the AXR1 gene was shown to be expressed in the zones of active cell division and expansion and in the vasculature of older tissue (del Pozo et al., 2002). Our data indicate that the expression of AXR1 in the xylem and interfascicular schlerenchyma tissues is sufficient to restore wild-type shoot branching.
RESULTS
Suppression of Branching Is Not Dependent on Auxin Transport into the Bud
Apically applied auxin, but not basally applied auxin, has been shown to inhibit the growth of Arabidopsis buds on isolated cauline nodes (Stirnberg et al., 1999), an effect that is dependent on polar auxin transport (Chatfield et al., 2000). To determine whether auxin is transported into Arabidopsis axillary buds, the distribution of radiolabeled IAA applied to isolated nodes was analyzed. Isolated 22-mm nodal stem sections (11 mm on each side of the node), containing an axillary bud that had not grown out, were excised from the secondary inflorescences of soil-grown plants and placed in microfuge tubes containing 30 μL of Arabidopsis thaliana salt (ATS; Lincoln et al., 1990) nutrient solution supplemented with 1 μM 14C-IAA, a level sufficient to inhibit the outgrowth of buds in isolated nodes (Chatfield et al., 2000). The nodes were incubated for 18 h, after which the amount of radiolabel in the bud and in the terminal 5 mm of stem, at the end opposite to that placed in the ATS, was measured.
Auxin was applied either apically or basally to the node and in the presence or absence of the auxin transport inhibitor 2-naphthoxyacetic acid (NPA). Apically applied IAA was transported along the stem segment significantly more than basally applied IAA, and this basipetal transport was blocked by the polar auxin transport inhibitor NPA (Table 1). In all treatments, the amount of radiolabel accumulated by the bud was extremely low, and this level was not significantly different among treatments. Therefore, the level of uptake of IAA, or metabolites of IAA, into axillary buds is independent of polar IAA transport. Because the repression of bud growth is dependent on the supply of auxin from the polar auxin stream (Chatfield et al., 2000), there is no correlation between the amount of radiolabel in the bud and the degree of inhibition of bud growth. Hence, auxin does not mediate bud repression in Arabidopsis by entering the bud.
Table 1.
Transport of Radiolabeled Auxin into Nodes of Arabidopsis
| Buds
|
Stems
|
|||
|---|---|---|---|---|
| Sample | Apical | Basal | Apical | Basal |
| −NPA | 14.2 ± 0.9 | 15.8 ± 2.4 | 2221.0 ± 232.3 | 13.4 ± 0.5 |
| +NPA | 11.9 ± 0.8 | 13.5 ± .2 | 31.5 ± 2.93 | 16.3 ± 0.7 |
Apical and basal indicate from which end of the node the auxin was supplied. All values are cpm, mean ± se, n = 8.
Auxin Acts in the Shoot to Repress Bud Growth
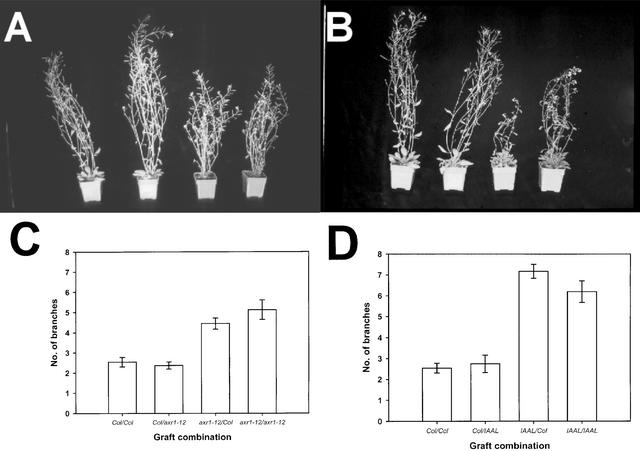
Because auxin does not accumulate in the bud, it must act remotely. One model that can explain such action involves the suppression of cytokinin biosynthesis in the root by auxin (Bangerth, 1994; Li et al., 1995). To determine if auxin acts via the root, two sets of grafts were used. The first produced chimeric plants consisting of auxin-sensitive aerial tissue with auxin-resistant roots, and vice-versa, by reciprocal grafting between axr1-12 and Columbia (Col) wild-type plants. The second set of grafts involved similar reciprocal grafting between Col and plants carrying a bacterial IAAL gene (Jensen et al., 1998), which encodes an enzyme that conjugates auxin to Lys and hence produces auxin-deficient tissue. Grafting at the hypocotyl was performed after 5 days of growth, and plants in which grafting was successful were transferred to soil after another 6 days. The degree of branching from the rosette nodes was assessed after an additional 35 days of growth under long-day conditions.
The visible phenotypes of all of the adult plants are shown in Figures 1A and 1B, and the quantitative analysis of the branching is shown in Figures 1C and 1D. Both axr1-12/axr1-12 and IAAL/IAAL controls showed significantly more branching than Col/Col control plants. Grafting Col scions onto either axr1-12 or IAAL rootstocks produced plants with a Col aerial branching phenotype. This finding indicates that auxin signaling in the root is not required for the suppression of shoot branching. Similarly, grafting of axr1-12 or IAAL scions onto Col rootstocks led to plants with mutant levels of branching. In the case of the axr1-12/Col graft, the auxin signaling in the root cannot compensate for the lack of signaling in the shoot. Interpretation of the IAAL/Col graft is not fully possible because the conjugation of auxin in the shoot could lead to the wild-type root also being auxin deficient.
Figure 1.
Effect of Reciprocal Grafting between Col and Both axr1-12 and IAAL.
Graft designation is scion/rootstock.
(A) Visible phenotypes of reciprocal grafts between Col and axr1-12. Plants are Col/Col, Col/axr1-12, axr1-12/Col, and axr1-12/axr1-12 (left to right).
(B) Visible phenotypes of reciprocal grafts between Col and IAAL. Plants are Col/Col, Col/IAAL, IAAL/Col, and IAAL/IAAL (left to right).
(C) Number of secondary rosette branches developed by grafting between Col and axr1-12 (n = 8 to 16).
(D) Number of secondary rosette branches developed by grafting between Col and IAAL (n = 5 to 17).
All values are means ± se.
Therefore, for axr1-12 mutants and IAAL transgenic plants, the shoot-branching phenotype is determined principally by the scion genotype, indicating that the shoot is a major site for auxin action in the control of shoot branching. The only observable effect of altering the root genotype was an increased rate of development of IAAL scions grafted to Col rootstocks compared with IAAL/IAAL controls (Figure 1B). This effect probably is the result of the wild-type root system being better able to supply the scion with nutrients for growth than the relatively unbranched IAAL root system.
Tissue-Specific Rescue of axr1-12
Because auxin does not act in the bud or via action in the root to inhibit Arabidopsis axillary bud growth, it must act remotely, via the tissues of the stem. To determine which of these is the site of auxin action, a genetic approach was taken using the auxin-resistant mutant axr1-12. A number of promoter-AXR1 fusions were constructed using promoters that drive restricted expression patterns among the tissues of the stem. These constructs were introduced into mutant axr1-12 plants and scored for their ability to restore a wild-type branching pattern to the mutant.
Pattern of Expression Driven by the Promoters
The promoters used, together with their predicted patterns of expression, are described in Table 2. Only one of the promoters, GLABRA2 (GL2), is native to Arabidopsis (Szymanski et al., 1998). The patterns of expression driven by two of the other promoters, Cauliflower mosaic virus 35S (CaMV35S) and 4-coumarate-CoA ligase1 (4CL1 from parsley), also have been documented in Arabidopsis (Zijlstra and Hohn, 1992; Lee et al., 1995), although a complete description of the latter in the stem has not been reported. The rolD promoter fragment (from Agrobacterium rhizogenes) has been used to drive the reporter genes in Arabidopsis (Zhang and Forde, 1998). The pattern of expression directed by the rolC promoter (from Agrobacterium tumefaciens) has been shown to be phloem specific in other plant species (Graham et al., 1997) but not in Arabidopsis. Therefore, the expression pattern driven by this promoter in Arabidopsis was determined using the β-glucuronidase (GUS) reporter gene. Expression of the other vascular promoter, 4CL1, also was analyzed to determine its precise expression in stem tissues.
Table 2.
Summary of Promoter-AXR1 Constructs and Their Predicted Patterns of Expression
| Expression
|
||||||
|---|---|---|---|---|---|---|
| Construct | Promoter (kb) | Epidermis | Cortex | Phloem | Xylem | Pith |
| pBIAn | None | |||||
| 35S-AXR1 | CAMV35S (0.8) | √ | √ | √ | √ | √ |
| rolD-AXR1 | rolD (0.3) | √ | √ | √ | √ | √ |
| GL2-AXR1 | GL2 (2.2) | √ | ||||
| rolC-AXR1 | rolC (1.1) | √ | ||||
| 4CL1-AXR1 | 4CL (1.4) | √ | ||||
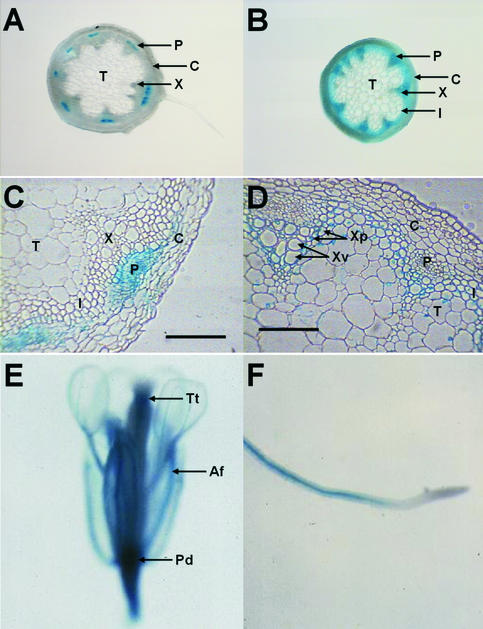
The rolC promoter was fused to the GUS reporter gene to give the construct prolC-GUS, which was introduced into wild-type Arabidopsis by A. tumefaciens–mediated transformation. Similarly, the construct p4CL1-GUS was generated by subcloning the same promoter fragment used in the AXR1 expression experiments (see below) upstream of the GUS gene. The pattern of expression driven by these promoters was determined by staining the transformants with the chromogenic GUS substrate 5-bromo-4-chloro-3-indolyl- β-d-glucuronide in at least four independent homozygous lines. Preliminary hand-cut sections indicated that the expression from the rolC promoter was restricted to the phloem, whereas expression driven by the 4CL1 promoter was confined to the xylem, as shown in Figures 2A and 2B.
Figure 2.
Patterns of Expression Driven by the rolC and 4CL1 Promoters in Arabidopsis.
GUS activity appears as a blue precipitate.
(A) Hand-cut section of stem showing rolC-GUS expression in the phloem.
(B) Hand-cut section of stem showing 4CL1-GUS expression in the xylem.
(C) Fine section of rolC-GUS stem tissue. Staining is present in the primary phloem as well as in a random selection of cortical cells.
(D) Fine section of 4CL1-GUS stem showing GUS activity in the parenchymatous cells surrounding the xylem, in the interfascicular schlerenchyma, and irregularly in the pith.
(E) rolC-GUS flower showing staining in the anther filaments, style, and pedicel.
(F) rolC-GUS root tissue showing expression in the vasculature.
Af, anther filaments; C, cortex; I, interfascicular area; P, phloem; Pd, pedicel; T, pith; Tt, transmitting tissue; X, xylem; Xp, xylem parenchymatous cell; Xv, xylem vessel element. Bars in (C) and (D) = 100 μM.
To characterize further the expression driven by the two promoters, GUS staining was monitored in fine sections of stem tissue. This analysis indicated that GUS expression in plants carrying rolC-GUS constructs occurred in the primary phloem, but staining was not always detectable in the secondary phloem fibers (Figure 2C). Expression also could be detected in some, but not all, cortical cells, with the distribution of this expression being apparently random.
In plants carrying the 4CL1-GUS construct, expression was observed in the parenchymatous cells surrounding the xylem vessel elements, whereas the vessels themselves stained infrequently (Figure 2D). Some staining also was observed in the interfascicular region between the vascular bundles. Expression in these cells has not been reported previously in tobacco, in which the 4CL1 promoter has been studied. In Arabidopsis, these cells are known to undergo sclerification, whereas they do not in tobacco (Hauffe et al., 1991; Zhong et al., 1997). Therefore, expression of a 4CL, which is involved in phenylpropanoid metabolism and hence lignification, is predicted in these tissues (Hauffe et al., 1991). As with the cortical expression observed in rolC-GUS plants, some staining in the pith was seen in 4CL1-GUS plants in an apparently random subpopulation of cells.
As well as expression in the vascular tissues of the vegetative organs, the rolC promoter also was found to drive expression in the floral organs. rolC-GUS plants displayed GUS activity in the anther filaments, pedicel, and style (Figure 2E). Expression in the latter organ occurred in a funnel-shaped set of cells, suggesting that expression was in the transmitting tissue. Expression also was observed in the vascular bundles of the root (Figure 2F).
Suppression of the axr1-12 Mutant Phenotype in T1 Plants
Preliminary experiments indicated that the phenotypes of transformants generated using many of the promoter-AXR1 fusions were unstable, with both morphological phenotypes and kanamycin resistance being lost in a high proportion of plants with each generation (data not shown). Therefore, the ability of the constructs to revert the axr1-12 branching phenotype to the wild-type phenotype was assessed using large numbers of T1 plants. After transformation by vacuum infiltration of flowers with the appropriate Agrobacterium line, T1 transgenic plants carrying each construct were selected by germination in Petri dishes on ATS medium containing kanamycin at 50 μg/mL. At least 37 T1 plants for each construct were generated. The kanamycin-resistant individuals were transferred to soil, and the branching patterns were analyzed after the primary inflorescences had ceased flowering. Plants grown in this way are in general early flowering, less robust, and have a greater number of higher order branches than those germinated directly on soil (data not shown).
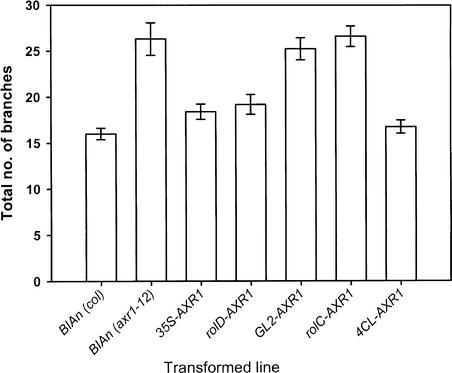
Wild-type and axr1-12 T1 plants transformed with a promoterless AXR1 cDNA were used as positive and negative controls, respectively. The outgrowth of branches differed significantly (P < 0.002) between these populations, as shown in Figure 3. Also shown are the results of the analysis of the T1 transformants generated using the promoter-AXR1 fusion constructs. Introduction of the GL2-AXR1 and rolC-AXR1 fusions into axr1-12 plants had no effect on shoot-branching habit. By contrast, the CaMV35S, rolD, and 4CL1-AXR1 fusions rescued the axr1-12 shoot-branching phenotypes.
Figure 3.
Total Number of Branches of T1 Transformants after the Termination of Flowering on the Primary Inflorescence.
All values are means ± se. For all lines, n = 40, except for BIAn (axr1-12) and GL2-AXR1, for which n = 37.
The promoters used in the three constructs that restored a wild-type branching phenotype to axr1-12 plants drove overlapping patterns of expression. The 4CL1 promoter had the most limited expression pattern, being active principally in xylem-associated cells, interfascicular schlerenchyma, and pith. Both the CaMV35S and rolD promoters also were expressed in the xylem and associated cells, but the latter promoter did not drive expression in the pith. Because the rolD-AXR1 fusion had a wild-type branching pattern, auxin sensitivity in the pith is not required for normal shoot branching. Therefore, it can be concluded from the T1 analysis that the site of auxin perception required for the inhibition of bud outgrowth is in the xylem and/or the interfascicular tissue.
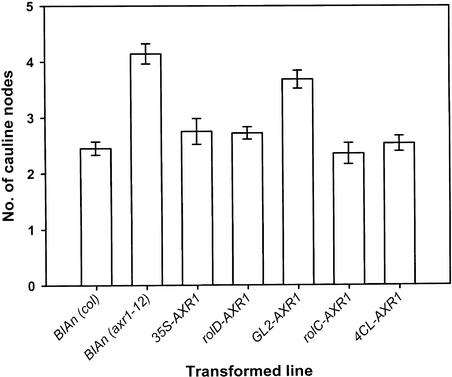
Suppression of Cauline Node Number in T1 Plants
One contribution to the increase in branching between control axr1-12 and Col plants results from an increase in the number of secondary cauline branches. However, this increased number of branches observed in axr1-12 plants does not reflect a difference in bud activity but rather reflects an increased number of cauline nodes (Figure 4), because in these growth conditions, all cauline buds on both wild-type and axr1-12 plants grow out actively (Hempel and Feldman, 1994; Stirnberg et al., 1999). When the numbers of nodes among the promoter-AXR1 lines are compared with those in the two controls, it can be seen that only GL2-AXR1 lines have a number similar to axr1-12. Therefore, the number of cauline nodes in rolC-AXR1 transformants is restored to wild-type levels, even though overall branching remains at axr1-12 levels.
Figure 4.
Number of Cauline Nodes Developed on T1 Transformants.
All values are means ± se. For all lines, n = 40, except for BIAn (axr1-12) and GL2-AXR1, for which n = 37.
Suppression of the axr1-12 Mutant Phenotype in T3 Plants
To characterize further the restoration of the wild-type branching pattern to axr1-12 plants, stable lines homozygous for the 4CL-AXR1 construct were isolated together with stable homozygous rolD-AXR1 lines. Isolation of stable homozygous 35S-AXR1 and rolC-AXR1 plants was not possible because of the loss of phenotype and kanamycin resistance in all of the subsequent generations of these transgenic lines, as described above.
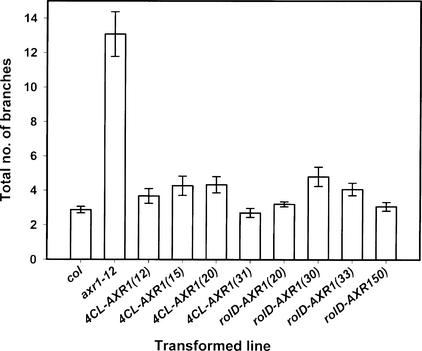
Branching was analyzed in homozygous T3 plants planted directly onto soil from four independent axr1-12 lines carrying either the 4CL-AXR1 or the rolD-AXR1 construct. Under these growth conditions, the axr1-12 mutants developed significantly more branches than wild-type plants, as illustrated in Figure 5. All of the 4CL-AXR1 and rolD-AXR1 transgenic lines developed numbers of branches that were not significantly different from wild-type levels but were significantly less than the numbers seen in the parent axr1-12 phenotype. Therefore, these data confirm the conclusions drawn from the T1 experiment.
Figure 5.
Branching in T3 Homozygous Transformants of axr1-12 Containing the 4CL-AXR1 or rolD-AXR1 Construct after 7 Weeks of Growth under Long-Day Conditions.
All values are means ± se. For all lines, n = 15, except for rolD-AXR1(50), for which n = 10.
Effect of Tissue-Specific AXR1 Expression in the Root
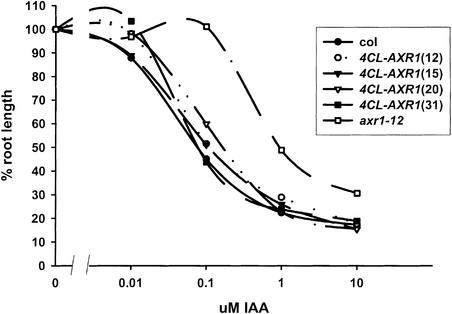
The expression driven by the 4CL1 promoter is not restricted to the stem but also occurs in the vascular system of the roots, leaves, and flowers as well as in the root cortex and sites of lateral root initiation (Lee et al., 1995). Hence, we investigated the restoration of other phenotypes of the axr1-12 mutant in the transgenic lines expressing the wild-type AXR1 cDNA from the 4CL1 promoter. Auxin resistance in the root was the criterion by which the axr1 mutations were isolated originally. When grown on medium supplemented with IAA, 4CL-AXR1 roots exhibited a similar range of sensitivity to the wild type, as shown in Figure 6. This may not be attributable to xylem expression, because the 4CL1 promoter drives less specific patterns of expression in the root than in the aerial tissue, being active in the cortex and endodermis as well as in the vascular system (Lee et al., 1995). Furthermore, 4CL1-driven expression of AXR1 restored fertility to the flowers of axr1-12 plants (data not shown).
Figure 6.
Inhibition of Root Growth in axr1-12 Plants Transformed with the 4CL-AXR1 Construct.
Plants were germinated for 3 days on ATS and then transferred to ATS supplemented with IAA and grown for another 7 days. Values are means ± se, n = 10 to 12.
Rescue of the Auxin Sensitivity of Bud Outgrowth in Vitro
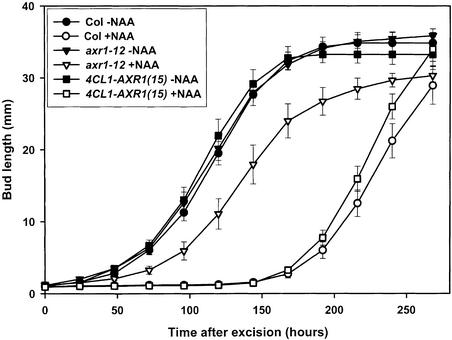
Because expression from the 4CL1 promoter restored the wild-type phenotype in several tissues, it was important to determine whether expression in the stem is sufficient to restore bud inhibition. To investigate this possibility, the responses to apically supplied auxin of isolated nodes from stable 4CL1-AXR1(15) lines, wild-type Col, and axr1-12 plants were compared in vitro using our established split-plate assay (Stirnberg et al., 1999; Chatfield et al., 2000).
The responses of axillary buds to apically applied auxin are shown in Figure 7. Outgrowth was similar for all of the lines when no auxin was present. If 1 μM 1-naphthaleneacetic acid (NAA) was supplied apically to the nodal segments, then bud outgrowth from the wild-type explants was inhibited for 6 days. In the axr1-12 mutant, this inhibition lasted <3 days. In the presence of apical NAA, the bud outgrowth of the 4CL1-AXR1(15) line was inhibited in a manner similar to that of the wild-type buds. Therefore, expression of AXR1 under the control of the 4CL1 promoter is sufficient to restore wild-type auxin-mediated inhibition of bud outgrowth to isolated nodal segments.
Figure 7.
Restoration of the in Vitro Auxin Response of Isolated axr1-12 Nodes to That of the Wild Type by the 4CL1-AXR1 Construct.
Nodes were excised from aseptically grown plants after bolting but before bud outgrowth and placed on split plates supplemented with (+NAA) or without (−NAA) 1 μM 1-NAA. For all lines, n = 12 to 15.
DISCUSSION
Since its inception >60 years ago, the central tenet of the Thimann and Skoog model, that auxin is a regulator of aerial branching, has been well supported by a wealth of experimental data. However, it is still not understood how auxin acts to inhibit the growth of axillary buds. Moreover, what little is known indicates that the mode of auxin action is not exactly that described in the original hypothesis. Evidence from a wide range of species indicates that significant levels of apically derived auxin do not enter repressed buds, and our work shows that this is true for Arabidopsis as well. These data do not preclude an auxin relay model, in which apically derived auxin stimulates de novo biosynthesis of auxin in the stem, which then enters the axillary bud. However, because in some species auxin levels remain constant or even increase in buds as they are released from apical dominance, a more likely explanation is that the site of auxin action is remote from the bud (Hillman et al., 1977; Pilate et al., 1989; Gocal et al., 1991).
To determine the site of auxin action, we made use of the axr1-12 auxin-resistant mutant of Arabidopsis. The phenotype of this mutant includes increased branching. We have demonstrated that a wild-type branching pattern is restored in plants in which the AXR1 gene is expressed in the xylem and the interfascicular sclerenchyma of the stem. This finding correlates well with the expression of the native AXR1 gene in the vascular tissues of the stem. However, these data alone do not directly implicate these tissues in the auxin response in branching. The axr1 mutation is highly pleiotropic (Lincoln et al., 1990), conferring a number of phenotypic changes that have been suggested to influence branching, such as loss of fertility (Hensel et al., 1994), reduced auxin response in the root (Bangerth, 1994; Ekölf et al., 1995), and altered cauline node number (Napoli et al., 1999). Therefore, the possibility that the rescue of branching in these experiments is an indirect effect of the restoration of one or more of these phenotypes must be considered, because the 4CL1-AXR1 construct restored all three of the phenotypes described above to wild-type levels.
By making grafts between Col and auxin-deficient/insensitive lines, we have demonstrated that auxin-mediated repression of branching occurs in the aerial tissue. Therefore, the reduced auxin response in the roots is not responsible for the profuse branching phenotype. These data support the previous work on the rms mutants (Napoli et al., 1999) and ISOPENTENYLTRANSFERASE (IPT)-expressing tobacco (Faiss et al., 1997), which suggests that auxin-mediated regulation of root-derived cytokinin may not play a significant role in the promotion of branching in the shoot in intact plants.
The restoration of fertility by the 4CL1-AXR1 construct cannot entirely explain the decreased branching. Increased activity of axillary buds in the rosette has been observed in axr1-12 mutants immediately after floral transition and before seed set (Stirnberg et al., 1999). If the 4CL1-AXR1 construct acted indirectly via fertility, this increased branching still would occur in plants carrying the construct. Hence, some of the differences in branching observed between Col and axr1-12, and their restoration by the 4CL1-AXR1 construct, are independent of fertility.
Another phenotype that could indirectly affect branching is the number of cauline nodes, and hence the number of cauline branches, which could alter branching in the rosette by altering the number of nutrients sinks. However, the cauline node number and rosette branching phenotypes are separated in rolC-AXR1 plants, in which cauline node number is restored to wild-type levels but overall branching remains at the axr1-12 level, indicating that modification in the number of cauline nodes is not the cause of increased branching.
Perhaps the strongest evidence that the inhibition of bud growth can be mediated by auxin acting in the stem comes from experiments with isolated nodes. Apically applied auxin inhibits bud outgrowth in this system, and this inhibition is dependent on polar auxin transport (Chatfield et al., 2000). Individual buds carried on isolated nodes of axr1-12 mutants are resistant to the inhibitory effects of such apically applied auxin. This finding supports the hypothesis that the branching phenotypes of axr1-12 plants are not the result of secondary effects from increased node number, reduced fertility, or differences in root development (Stirnberg et al., 1999). However, when radiolabeled auxin is fed to explants, similar very small amounts of label accumulate in the bud, regardless of whether the auxin can mediate bud inhibition; hence, auxin does not act directly in the bud. Instead, the degree of bud inhibition correlates with the amount of auxin in the polar transport stream in the stem. These data indicate that auxin, transported in the polar transport stream, can act in the stem at or near the node to regulate branching. Building on this result, the demonstration that auxin sensitivity is restored in isolated nodes of 4CL1-AXR1 plants suggests that auxin sensitivity in the xylem and the interfascicular sclerenchyma of the node and associated internodes is sufficient to suppress branching. It is not clear from our data whether expression in all of these tissues is absolutely necessary for wild-type branching, but wild-type auxin responses in the phloem and epidermis were shown not to be sufficient.
Auxin Transport Routes and the Regulation of Bud Outgrowth
The observation that auxin can act in the xylem and/or the interfascicular schlerenchyma to regulate branching has a number of implications. One striking feature is that the subset of cells in which auxin sensitivity is required includes those cells that have been implicated in the polar transport of auxin. Physiological studies suggest that polar auxin transport occurs in the vascular bundles (Wangermann, 1974; Morris and Thomas, 1978; Jacobs and Gilbert, 1983); however, the precise location cannot be determined until the isolation of components of the polar transport system. One such component, the AtPIN1 protein, which is likely to be part of an auxin efflux carrier, has been identified (Okada et al., 1991; Gälweiler et al., 1998). This protein has been shown to localize to the parenchymatous xylem and cambial cells, implicating these cells as the major conduits of polar auxin transport in the stem. Therefore, the site of auxin action in the regulation of branching may reflect the site of polar auxin transport down the stem.
The isolation of PIN1 homologs with different patterns of expression suggests that a large family of auxin efflux carriers exists in Arabidopsis that also could be involved in auxin redistribution and axillary branching (Friml et al., 1999). For example, another important correlation between auxin transport and bud growth is the lack of export of auxin from the inhibited bud. In two-branched pea plants, in which one shoot is inhibited by the other, the subordinate shoot is unable to export IAA unless the dominant shoot is decapitated (Morris, 1977; Li and Bangerth, 1999). This is the “autoinhibition at junctions effect,” whereby apically derived auxin may control the export of bud-derived auxin into the stem's polar transport stream (Li and Bangerth, 1999). However, the precise role of such an effect is unknown, and whether it is a causative agent or a symptom of apical dominance has not been demonstrated conclusively.
Relationship between Auxin and Cytokinin in the Regulation of Branching
If auxin acts in the xylem and interfascicular sclerenchyma, other plant hormones may relay the auxin signal from the stem to the bud (Snow, 1937). Some of the xylem-associated tissues in which auxin acts are adjacent to the xylem tracheary elements, in which a number of molecules are transported acropetally. Therefore, auxin could regulate the loading or unloading of xylem-transported second messengers. Cytokinin is thought to be transported mostly in the xylem, so the requirement for auxin signaling in xylem-associated cells is consistent with a role for auxin in regulating cytokinin transport to the axillary buds (Morris and Winfield, 1972; Dieleman et al., 1997).
Work on the rms mutants (Beveridge, 2000) and grafts between wild-type and cytokinin-overproducing tobacco (Faiss et al., 1997) suggest that the level of root-derived cytokinin is not a controlling factor in the regulation of branching, as had been proposed previously (Bangerth, 1994; Li et al., 1995; Blazkova et al., 1999). However, more recent studies have suggested that cytokinin synthesized locally in the node may play a role in the regulation of branching. After decapitation, a pea adenylate IPT gene was found to be upregulated in nodes from which branches would grow out (Shimizu-Sato and Mori, 2001). Therefore the increased cytokinin content of uninhibited buds could be caused by the uptake of cytokinin synthesized within the node, with auxin acting to control uptake and/or synthesis. However, because direct regulation of the IPT gene by auxin, rather than crude decapitation, has not been demonstrated, a direct link between the two cannot be made. Chen et al. (1985) have demonstrated biochemically that, in roots, cambial tissue is responsible for cytokinin synthesis, but the site of synthesis in stems has not been reported. Identification of this site by in situ localization of the putative IPT gene or biochemical assay, and correlation with the site of auxin action described here, provide further support for a role for auxin in the regulation of cytokinin biosynthesis.
Auxin and Other Hormones
Other phytohormones that have been proposed to act as relays for auxin are ethylene and abscisic acid. Abscisic acid and the ethylene precursor 1-aminocyclopropane-1-carboxylic acid both are transported in the xylem; hence, their delivery to buds could be regulated by changes in loading or unloading (Bradford and Yang, 1980; Schurr et al., 1992). Evidence for the role of these hormones in the control of bud outgrowth originally came from physiological studies correlating their absolute levels, or their rates of biosynthesis, to the state of bud inhibition (reviewed by Cline, 1991). However, such correlations do not occur for all species or under all conditions. The characterization of biosynthesis and perception mutants for these hormones recently provided new data regarding their role in branching. In Arabidopsis, ethylene does not appear to play a role in the suppression of branching, because biosynthesis/perception mutants and transgenic plants do not show altered branching patterns or block the effect of auxin-overproducing transgenes on branching (Romano et al., 1993). Furthermore, their nodes show a wild-type response to auxin in vitro (Chatfield et al., 2000). Similarly, the buds of the abscisic acid–resistant mutant abi1 do not show altered responses to auxin in vitro, although the buds are resistant to abscisic acid (Chatfield et al., 2000). Hence, although these phytohormones may regulate bud activity, apparently they are not involved in auxin-mediated apical dominance.
Auxin and Graft-Transmissible Factors
One candidate for a second messenger for auxin is the graft-transmissible signal identified from analysis of the rms mutants in pea, which has been shown to be required for auxin-mediated bud repression (Beveridge et al., 2000). Grafting studies have demonstrated that this signal can move acropetally up the plant but apparently is unable to move basipetally (Foo et al., 2001), a pattern of movement consistent with xylem transport. Therefore, the factor could be synthesized at any point along the vasculature, loaded into the xylem, and transported up the plant into the buds. If auxin controls this factor, then it must be at the level of unloading, rather than the level of synthesis or loading, because Col rootstocks are unable to restore the branching phenotype of axr1-12 scions.
Auxin and Node Number
The restoration of the wild-type branching pattern in axr1-12 plants by 4CL-AXR1, 35S-AXR1, and rolD-AXR1 was not purely by the restoration of auxin-mediated bud inhibition but also by a reduction in the number of cauline nodes. The rolC-AXR1 construct also reduced the number of nodes to wild-type levels, indicating that a wild-type auxin response in either the phloem or the xylem is sufficient to restore the number of cauline nodes in axr1-12 to wild-type levels. The role of auxin in determining the number of cauline nodes is unclear. Other mutants with modified patterns of vascular development also have been shown to influence the number of cauline nodes (Zhong et al., 1997, 1999). However, the increase in the number of nodes observed in axr1-12 may be attributable to indirect auxin effects, such as the modulation of floral transition or the elongation of the primary inflorescence.
Conclusion
Although there is strong evidence that apically derived auxin inhibits the outgrowth of axillary buds, many questions remain regarding how this effect is mediated. By defining a site of auxin action, we have created criteria with which to assess the role of putative downstream components in auxin signaling. If another signaling molecule transmits the auxin signal to the bud, then the biosynthesis of this molecule in the xylem/interfascicular tissues would be consistent with its action downstream of auxin in apical dominance. Although rigorous confirmation of this may be technically difficult, the identification of the patterns of expression of the proteins involved in the biosynthesis of such messengers would allow the relationship between the downstream messenger and auxin to be assessed. Such linkage is important in elucidating the pathways by which auxin represses axillary bud growth. Several downstream messengers may be required, with some involved in transmitting the signal into the bud and others synthesized and acting in situ in the bud.
To elucidate further the role of auxin in the regulation of apical dominance, its site of action must be defined more exactly by the use of promoters with more restricted patterns of expression than those used here. The promoters used in this work were from genes characterized previously, and this approach may produce other useful promoters, such as the AtPIN1 promoter (Gälweiler et al., 1998). Another approach would be the use of two-component enhancer-trap populations (Guyer et al., 1998). The identification of lines in which expression in restricted subsets of vascular cells occurs would allow a more precise mapping of the site of auxin action without relying on the identification of the genes controlled by these promoters. By further delimiting the subset of cells in which auxin acts in apical dominance, the relationship between the transport of auxin and its putative downstream effects and apical dominance can be analyzed.
METHODS
Plant Growth
Arabidopsis thaliana plants were grown in Klasman Substrate No. 1 compost (Klasmann-Deilmann, Geestz, Germany). Plants for morphological and physiological study were sown in shallow 35- × 23-cm trays, 4 cm apart. Plants for transformation (wild-type Columbia and axr1-12) were sown in 8-cm pots, three to four plants per pot. Seeds planted directly into compost were cold treated for 2 to 5 days before transferring to a growth chamber at 22°C under a 16-h-light/8-h-dark photoperiod (120 μmol·m−2·s−1).
Plants for hormone response assays or kanamycin selection were sterilized and sown onto Arabidopsis thaliana salts (ATS) as described by Lincoln et al. (1990). Selection for transgenic plants was by addition of 50 μg/mL kanamycin to the ATS. To kill any agrobacteria carried over in the seed, 40 μg/mL cefotaxime also was added to the ATS. The plants were given 2 to 4 days of cold treatment to synchronize germination before incubation at 22 to 27°C under a 16-h-light/8-h-dark photoperiod (50 μmol·m−2·s−1). Transformants were selected after 7 to 14 days of growth. Plants whose growth was initiated in sterile conditions were replanted into compost and placed in the growth chamber.
Auxin Distribution Experiments
Radiolabeled auxin was supplied to isolated nodes using a modification of the method described by Okada et al. (1991). The nodes were selected from the secondary inflorescences of soil-grown plants. Twenty-two-millimeter sections (11 mm on each side of the node) were excised using a razor blade and placed either upside down or rightside up in 1.5-ml Eppendorf tubes containing 30 μL of Suc-free ATS supplemented with 1 μM 2-14C–indole-3-acetic acid (American Radiolabeled Chemicals, St. Louis, MO). The nodes were incubated for 18 h under continuous illumination (30 μmol·m−2·s−1) at 22°C. Tissue for analysis was excised and extracted directly with scintillant (Microscint 20; Canberra-Packard, Pangbourne, UK) for 48 h before counting.
Grafting of Arabidopsis
Grafting of Arabidopsis seedlings was performed essentially as described by Turnbull et al. (2002). Seeds were sown on ATS medium and allowed to grow for 5 days. Grafting then was performed by cutting the seedlings at the hypocotyl and using silicon collars to maintain close contact between the scion and the rootstock. After another 6 days, successfully grafted plants were transferred to compost and allowed to grow to maturity without removal of the collar. After phenotypic scoring, the graft junctions were excised and scored for the presence of adventitious root growth from the scion. Plants that had developed adventitious roots had mixed root genotypes, so data derived from them were excluded from the final analysis.
Plasmid Constructs
DNA manipulations were performed essentially as described by Sambrook et al. (1989). All constructs were transcriptional fusions, and their structures were confirmed by sequencing.
Promoter–β-glucuronidase (GUS) fusions were generated by cloning a HindIII-BamHI fragment from the plasmid Bin19-RolC (Lerchl et al., 1995) and a SalI-BamHI fragment from the plasmid 99-G1-800 (Hauffe et al., 1991) into the corresponding polylinker sites in the binary vector pBI101 (Jefferson et al., 1987) to give the plasmids pRCG and pCLG, respectively.
The plasmid pBIAn was generated by removing the GUS coding sequence from pBI101 by digestion with BamHI and SacI and replacing it with a BamHI-SacI fragment containing the AXR1 cDNA (Leyser et al., 1993). p4CL-AXR1 was generated by cloning a fragment identical to that used for the promoter-GUS fusion into the SalI-BamHI polylinker sites in pBIAn. pRolD-AXR1 was generated by initially cloning a HindIII-BamHI 300-bp rolD promoter fragment that was supplied in plasmid PUC19 into the corresponding sites in pBI101 and then substituting the AXR1 coding region for that of GUS, as for pBIAn.
pRolC-AXR1 was generated by substituting the BamHI-SacI AXR1 coding region for that of GUS in the plasmid pRCG. pGl2-AXR1 was generated by subcloning a 2200-bp SalI-BamHI promoter fragment from the plasmid pWP362.5 (W. Paul, unpublished data) into the corresponding polylinker sites of pBIAn. p35S-AXR1 was generated by substituting the green fluorescent protein coding sequence from the plasmid pBIN35S-mGFP4 with the previously described BamHI-SacI AXR1 cDNA.
Plant Transformation
Plants were transformed by a modification of the method of Bechtold et al. (1993). Constructs were transformed into Agrobacterium tumefaciens strain GV3101 by electroporation. For plant transformation, Agrobacterium was grown to mid-log phase in Luria-Bertani medium (Sambrook et al., 1989), pelleted (2000g for 15 min), and resuspended in 0.3 to 0.5 volumes of vacuum buffer (0.22% [w/v] Murashige and Skoog [1962] salts, 2.3 mM Mes, and 0.02% [v/v] Triton X-100, pH 5.7).
T0 plants for transformation were selected at 5 to 6 weeks old when the first siliques on the primary inflorescences were expanding. Plant pots were inverted and placed in sufficient Agrobacterium-containing vacuum buffer so that the inflorescences, but not the rosettes, were covered. The plants then were subjected to a vacuum (5.7 bar) for 5 to 10 min before the vacuum was released slowly. Plant pots were returned to the upright orientation and returned to the growth cabinet, where the plants were allowed to set seed.
Histochemical Localization of GUS Activity
Histochemical localization of GUS activity was determined using material from 5- to 7-week-old plants. Tissue from several T3 plants, homozygous for the construct, for at least three independently transformed lines was analyzed for each construct.
Floral tissue was placed whole in X-Gluc staining solution [0.5 mg/mL 5-bromo-4-chloro-3-indolyl-β-d-glucuronide, 50 mM sodium phosphate, pH 7.0, 0.05% Triton X-100, 0.1 mM K4Fe(CN)6, and 0.1 mM K3Fe(CN)6] and incubated for 16 h at 37°C. Tissue then was destained in 70% (w/v) ethanol. Hand sections of stem tissue were prepared by slicing with a razor blade before staining and destaining as described above.
Stem tissue for embedding was prepared initially by slicing into 2- to 4-mm sections and then prefixing in 0.3% paraformaldehyde in 100 mM sodium phosphate, pH 7.0. Tissue was washed three times in 100 mM sodium phosphate, pH 7.0, and then stained as described above. The tissue was postfixed with 3% paraformaldehyde and 1.25% glutaraldehyde in 100 mM sodium phosphate, pH 7.0, and washed six times as described above. Tissue was dehydrated in a series of aqueous ethanol solutions as follows: 12.5, 25, 50, 75, and 100%. The tissue then was infiltrated with each of the following: ethanol:Histoclear (1:1); Histoclear; Histoclear:Paraplast (1:1); and Paraplast Plus (Sigma-Aldrich, Poole, UK). Embedded tissue was sectioned at 7 to 15 μm. Sections were fixed to glass slides covered with adhesive (1% [w/v] gelatin and 13% [v/v] glycerol) and dewaxed in xylene. Cover slips were mounted with distyrene, plasticizer, xylene mountant (British Drug House Laboratory Supplies, Poole, UK).
Analysis of Branching
For comparisons of aerial branching, plants were grown simultaneously in the same growth cabinet. For analysis of T3 plants homozygous for a transformed construct, seeds were planted directly in soil and treated as described above. Branching was assessed after 7 weeks, when the number of each type of branch was counted for each plant. For analysis of T1 plants, seeds were sown and germinated as described above. After 11 days, kanamycin-resistant seedlings were pulled and transplanted to soil. The number of branches was counted for each plant when the primary inflorescence of that plant had ceased flowering. The statistical significance of branching differences was calculated using Student's t test, whereby the number of branches in the transformants was tested for significant differences against both wild-type and axr1-12 controls.
Split-Plate Assay
The response of isolated cauline nodes to auxin was determined using the split-plate assay as described by Chatfield et al. (2000). The synthetic auxin 1-naphthyleneacetic acid was applied apically at a concentration of 1 μM, and outgrowth was measured every 24 h for 11 days.
Upon request, all novel materials described in this article will be made available in a timely manner for noncommercial research purposes.
Acknowledgments
We thank the following individuals for supplying promoters for this work: Carl Douglas (Department of Botany, University of British Columbia; 4CL); Brian Ford (Institute for Arable Crop Research, Rothamstead; rolD); Marion Kwart (Max-Planck-Institut fur Molekulare Pflanzenphysiologie; rolC); and Wyatt Paul (Biogemma UK; GL2). We also thank Pamela Mackay for technical advice concerning plant transformation and Stephen Day for critical reading of the manuscript. J.B. was supported by a European Union Framework IV grant and by a Biotechnology and Biological Science Research Council grant. S.C. was supported by a Biotechnology and Biological Science Research Council studentship with Institute for Arable Crop Research, Rothamstead.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.007542.
References
- Bangerth, F. (1994). Response of cytokinin concentration in the xylem exudate of bean (Phaseolus vulgaris L.) plants to decapitation and auxin treatment and relationship to apical dominance. Planta 194, 439–442. [Google Scholar]
- Bechtold, N., Ellis, J., and Pelletier, G. (1993). In planta Agrobacterium-mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C. R. Acad. Sci. Paris 316, 1194–1199. [Google Scholar]
- Beveridge, C.A. (2000). Long-distance signalling and a mutational analysis of branching in pea. Plant Growth Regul. 32, 193–203. [Google Scholar]
- Beveridge, C.A., Murfet, I.C., Kerhoas, L., Sotta, B., Miginiac, E., and Rameau, C. (1997. a). The shoot controls zeatin riboside export from pea roots: Evidence from the branching mutant rms4. Plant J. 11, 339–345. [Google Scholar]
- Beveridge, C.A., Ross, J.J., and Murfet, I.C. (1994). Branching mutant rms2-I in Pisum sativum: Grafting studies and endogenous indole-3-acetic acid levels. Plant Physiol. 104, 953–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge, C.A., Symons, G.M., Murfet, I.C., Ross, J.J., Miginiac, E., and Rameau, C. (1997. b). The rms1 mutant of pea has elevated indole-3-acetic acid levels and reduced root sap zeatinriboside content but increased branching controlled by graft transmissible signal(s). Plant Physiol. 115, 1251–1258. [Google Scholar]
- Beveridge, C.A., Symons, G.M., and Turnbull, C.G.N. (2000). Auxin inhibition of decapitation-induced branching is dependent on graft-transmissible signals regulated by genes Rms1 and Rms2. Plant Physiol. 123, 689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazkova, J., Krekule, J., Machackova, I., and Prochazka, S. (1999). Auxin and cytokinins in the control of apical dominance in pea: A differential response due to bud position. J. Plant Physiol. 154, 691–696. [Google Scholar]
- Bradford, K.J., and Yang, S.F. (1980). Xylem transport of 1-aminocyclopropane-1-carboxylic acid, an ethylene precursor, in water-logged tomato plants. Plant Physiol. 65, 322–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, B.T., Foster, C., Phillips, J.N., and Rattigann, B.M. (1979). The indirect role of 2,4-D in the maintenance of apical dominance in decapitated sunflower seedlings (Helianthus annuus L.). Planta 146, 475–480. [DOI] [PubMed] [Google Scholar]
- Brown, B.T., and Phillips, J.N. (1982). The transport behaviour of the synthetic auxin 2,4-dichlorphenoxyacetic acid in decapitated seedlings of sunflower (Helianthus annuus L.). Aust. J. Plant Physiol. 9, 5–13. [Google Scholar]
- Chatfield, S.P., Stirnberg, P., Forde, B.G., and Leyser, O. (2000). The hormonal regulation of axillary bud growth in Arabidopsis. Plant J. 24, 159–169. [DOI] [PubMed] [Google Scholar]
- Chen, C., Ertl, J.L., Leisner, S.M., and Chang, C. (1985). Localisation of cytokinin biosynthetic site in pea plants and carrot roots. Plant Physiol. 78, 510–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline, M.G. (1991). Apical dominance. Bot. Rev. 57, 318–358. [Google Scholar]
- Cline, M.G. (1994). The role of hormones in apical dominance: New approaches to an old problem in plant development. Physiol. Plant. 90, 230–237. [Google Scholar]
- Cline, M.G. (1996). Exogenous auxin effects on lateral bud outgrowth in decapitated shoots. Ann. Bot. 78, 255–266. [Google Scholar]
- Cline, M.G., Chatfield, S.P., and Leyser, O. (2000). NAA restores apical dominance in the axr3-1 mutant of Arabidopsis thaliana. Ann. Bot. 87, 61–65. [Google Scholar]
- del Pozo, J.C., Dharmasiri, S., Hellman, H., Walker, L., Gray, W.M., and Estelle, M. (2002). AXR1-ECR1–dependant conjugation of RUB1 to the Arabidopsis cullin AtCUL1 is required for auxin response. Plant Cell 14, 421–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieleman, J.A., Verstappen, F.W.A., Nicander, B., Kuiper, D., Tillberg, E., and Tromp, J. (1997). Cytokinins in Rosa hybrida in relation to bud break. Physiol. Plant. 99, 456–464. [Google Scholar]
- Ekölf, S., Astot, C., Blackwell, J., Moritz, T., Olsson, O., and Sandberg, G. (1995). Auxin/cytokinin interactions in wild-type and transgenic tobacco. Plant Cell Physiol. 38, 225–235. [Google Scholar]
- Everat-Bourbouloux, A., and Bonnemain, J.-L. (1980). Distribution of labelled auxin and derivatives in stem tissues of intact and decapitated broad-bean plants in relation to apical dominance. Physiol. Plant. 50, 145–152. [Google Scholar]
- Faiss, M., Zalubìlovà, J., Strnad, M., and Schmulling, T. (1997). Conditional transgenic expression of the ipt gene indicates a function for cytokinins in paracrine signalling in whole tobacco plants. Plant J. 12, 401–415. [DOI] [PubMed] [Google Scholar]
- Foo, E., Turnbull, C.G.N., and Beveridge, C.A. (2001). Long-distance signaling and the control of branching in the rms1 mutant of pea. Plant Physiol. 126, 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml, J., Wisniewska, J., Schelhaas, M., Tanzler, P., Tretyn, A., and Palme, K. (1999). Analysis of the AtPIN3 gene from Arabidopsis thaliana. Biol. Plant. 42, S20. [Google Scholar]
- Gälweiler, L., Guan, C., Müller, A., Wisman, E., Mendgen, K., Yephremov, A., and Palme, K. (1998). Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282, 2226–2230. [DOI] [PubMed] [Google Scholar]
- Gocal, G.F.W., Pharis, R.P., Young, E.C., and Pearce, D. (1991). Changes after decapitation of indole-3-acetic acid and abscisic acid in the larger axillary bud of Phaseolus vulgaris L. cv Tender Green. Plant Physiol. 95, 344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, M.W., Craig, S., and Waterhouse, P.M. (1997). Expression patterns of vascular-specific promoters RolC and Sh in transgenic potatoes and their use in engineering PLRV-resistant plants. Plant Mol. Biol. 33, 729–735. [DOI] [PubMed] [Google Scholar]
- Grbic, V., and Bleecker, A.B. (2000). Axillary meristem development in Arabidopsis thaliana. Plant J. 21, 215–223. [DOI] [PubMed] [Google Scholar]
- Guyer, D., Tuttle, A., Rouse, S., Volrath, S., Johnson, M., Potter, S., Gorlach, J., Crossland, L., and Ward, E. (1998). Activation of latent transgenes in Arabidopsis using a hybrid transcription factor. Genetics 149, 633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, S.M., and Hillman, J.R. (1975). Correlative inhibition of lateral bud growth in Phaseolus vulgaris L.: Timing of bud growth following decapitation. Planta 123, 137–143. [DOI] [PubMed] [Google Scholar]
- Hauffe, K.D., Paszkowski, U., Schulzelefert, P., Hahlbrock, K., Dangl, J., and Douglas, C.J. (1991). A parsley 4CL-1 promoter fragment specifies complex expression patterns in transgenic tobacco. Plant Cell 3, 435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel, F.D., and Feldman, L.J. (1994). Bi-directional inflorescence development in Arabidopsis thaliana: Acropetal initiation of flowers and basipetal initiation of paraclades. Planta 192, 276–286. [Google Scholar]
- Hensel, L.L., Nelson, M.A., Richmond, T., and Bleecker, A.B. (1994). The fate of inflorescence meristems is controlled by developing fruits in Arabidopsis. Plant Physiol. 106, 863–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman, J.R., Math, V.B., and Medlow, G.C. (1977). Apical dominance and the levels of indole acetic acid in Phaseolus lateral buds. Planta 134, 191–193. [DOI] [PubMed] [Google Scholar]
- Hosokawa, Z., Shi, L., Prasad, T.K., and Cline, M.G. (1990). Apical dominance control in Ipomoea nil: The influence of the shoot apex, leaves and stem. Ann. Bot. 65, 547–556. [Google Scholar]
- Jacobs, M., and Gilbert, S.F. (1983). Basal localisation of the presumptive auxin transport carrier in pea stem cells. Science 220, 1297–1300. [DOI] [PubMed] [Google Scholar]
- Jefferson, R.A., Kavanagh, T.A., and Bevan, M.W. (1987). GUS fusions: β-Glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6, 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, P.J., Hangarter, R.P., and Estelle, M. (1998). Auxin transport is required for hypocotyl elongation in light-grown but not dark-grown Arabidopsis. Plant Physiol. 116, 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, D., Ellard, M., Wanner, L.A., Davis, K.R., and Douglas, C.J. (1995). The Arabidopsis thaliana 4-coumarate-coA ligase (4CL) gene: Stress and developmentally regulated expression and nucleotide sequence of its cDNA. Plant Mol. Biol. 28, 871–884. [DOI] [PubMed] [Google Scholar]
- Lerchl, J., Geigenberger, P., Stitt, M., and Sonnewald, U. (1995). Impaired photoassimilate partitioning caused by phloem-specific removal of pyrophosphate can be complemented by a phloem-specific cytosolic yeast-derived invertase in transgenic plants. Plant Cell 7, 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyser, H.M.O., Lincoln, C.A., Timpte, T., Lammer, D., Turner, J., and Estelle, M. (1993). Arabidopsis auxin-resistance gene-AXR1 encodes a protein related to ubiquitin-activating enzyme E1. Nature 364, 161–164. [DOI] [PubMed] [Google Scholar]
- Leyser, H.M.O., Pickett, F.B., Dharmasiri, S., and Estelle, M. (1996). Mutations in the AXR3 gene of Arabidopsis result in altered auxin response including ectopic expression from the SAUR-AC1 promoter. Plant J. 10, 403–413. [DOI] [PubMed] [Google Scholar]
- Li, C.-J., and Bangerth, F. (1999). Autoinhibition of indoleacetic acid transport in the shoots of two-branched pea (Pisum sativum) plants and its relationship to correlative dominance. Physiol. Plant. 106, 415–420. [Google Scholar]
- Li, C.-J., Guevara, E., Herrera, J., and Bangerth, F. (1995). Effect of apex excision and replacement by 1-naphthylacetic acid on cytokinin concentration and apical dominance in pea plants. Physiol. Plant. 94, 465–469. [Google Scholar]
- Lim, R., and Tamas, I.A. (1989). The transport of radiolabelled indoleacetic acid and its conjugates in nodal stem segments of Phaseolus vulgaris L. Plant Growth Regul. 8, 151–164. [Google Scholar]
- Lincoln, C., Britton, J.H., and Estelle, M. (1990). Growth and development of the axr1 mutant of Arabidopsis. Plant Cell 2, 1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljung, K., Bhalerao, R.P., and Sandberg, G. (2001). Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. Plant J. 28, 465–474. [DOI] [PubMed] [Google Scholar]
- Morris, D.A. (1977). Transport of exogenous auxin in two-branched dwarf pea seedlings (Pisum sativum L.). Planta 136, 91–96. [DOI] [PubMed] [Google Scholar]
- Morris, D.A., and Thomas, A.G. (1978). A microautoradiographic study of auxin transport in the stem of intact pea seedlings (Pisum sativum L.). J. Exp. Bot. 29, 147–157. [Google Scholar]
- Morris, D.A., and Winfield, P.J. (1972). Kinetin transport to axillary buds of dwarf pea (Pisum sativum L.). J. Exp. Bot. 23, 346–355. [Google Scholar]
- Morris, S.E., Turnbull, C.G.N., Murfet, I.C., and Beveridge, C.A. (2001). Mutational analysis of branching in pea (Pisum sativum L.): Evidence that Rms1 and Rms5 regulate the same novel signal. Plant Physiol. 126, 1205–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15, 473.–497. [Google Scholar]
- Napoli, C.A., Beveridge, C.A., and Snowden, K.C. (1999). Reevaluating concepts of apical dominance and the control of axillary bud outgrowth. Curr. Top. Dev. Biol. 44, 127–169. [DOI] [PubMed] [Google Scholar]
- Okada, K., Veda, J., Komaki, M.J., Bell, C.J., and Shimura, Y. (1991). Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell 3, 677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palni, L.M.S., Burch, L., and Horgan, R. (1988). The effect of auxin concentration on cytokinin stability and metabolism. Planta 174, 231–234. [DOI] [PubMed] [Google Scholar]
- Pilate, G., Sossountzov, L., and Miginiac, E. (1989). Hormone levels and apical dominance in the aquatic fern Marsilea drummondii A. Plant Physiol. 90, 907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad, T.K., Li, X., Abdel-Rahman, A.M., Hosokawa, Z., Cloud, N.P., LaMotte, C.E., and Cline, M.G. (1993). Does auxin play a role in the release of apical dominance by shoot inversion in Ipomoea nil? Ann. Bot. 71, 223–229. [Google Scholar]
- Romano, C.P., Cooper, M.L., and Klee, H.J. (1993). Uncoupling auxin and ethylene effects in transgenic tobacco and Arabidopsis. Plant Cell 5, 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J., Maniatis, T., and Fritsch, E.F. (1989). Molecular Cloning: A Laboratory Manual, 2nd ed. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Schurr, U., Gollan, T., and Schultze, E.D. (1992). Stomatal response to drying soil in relation to changes in xylem sap composition in Helianthus annuus. 2. Stomatal sensitivity to abscisic acid imported from the xylem sap. Plant Cell Environ. 15, 561–567. [Google Scholar]
- Shimizu-Sato, S., and Mori, H. (2001). Control of outgrowth and dormancy in axillary buds. Plant Physiol. 127, 1405–1413. [PMC free article] [PubMed] [Google Scholar]
- Snow, R. (1937). On the nature of correlative inhibition. New Phytol. 36, 283–300. [Google Scholar]
- Stirnberg, P., Chatfield, S.P., and Leyser, H.M.O. (1999). AXR1 acts after lateral bud formation to inhibit lateral bud growth in Arabidopsis. Plant Physiol. 121, 839–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymanski, D.B., Jilk, R., Pollock, S.M., and Marks, M.D. (1998). Control of GL2 expression in Arabidopsis leaves and trichomes. Development 125, 1161–1171. [DOI] [PubMed] [Google Scholar]
- Thimann, K.V. (1937). On the nature of inhibitions caused by auxin. Am. J. Bot. 24, 407–412. [Google Scholar]
- Thimann, K.V., and Skoog, F. (1934). On the inhibition of bud development and other functions of growth substance in Vicia faba. Proc. R. Soc. Lond. B Biol. Sci. 114, 317–339. [Google Scholar]
- Timpte, C., Lincoln, C., Pickett, F.B., Turner, J., and Estelle, M. (1995). The AXR1 and AUX1 genes of Arabidopsis function in separate auxin-response pathways. Plant J. 8, 561–569. [DOI] [PubMed] [Google Scholar]
- Turnbull, C., Booker, J., and Leyser, O. (2002). Micrografting techniques for testing long-distance signalling in Arabidopsis. Plant J. 32, 255–262. [DOI] [PubMed] [Google Scholar]
- Turnbull, C.G.N., Myriam, A.A., Dodd, I.C., and Morris, S.E. (1997). Rapid increases in cytokinin concentration in lateral buds of chickpea (Cicer arietinum L.) during release of apical dominance. Planta 202, 271–276. [Google Scholar]
- Wangermann, E. (1974). The pathway of transport of applied indoylacetic acid through internode segments. New Phytol. 73, 623–636. [Google Scholar]
- White, G.C., Medlow, J.R., Hillman, J.R., and Wilkins, M.B. (1975). Correlative inhibition of lateral bud growth in Phaseolus vulgaris L.: Isolation of indoleacetic acid from inhibitory region. J. Exp. Bot. 26, 419–424. [Google Scholar]
- Zhang, H.M., and Forde, B.G. (1998). An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science 279, 407–409. [DOI] [PubMed] [Google Scholar]
- Zhong, R., Jennifer, J.T., and Ye, Z.H. (1997). Disruption of interfascicular fiber differentiation in an Arabidopsis mutant. Plant Cell 9, 2159–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, R., Jennifer, J.T., and Ye, Z.H. (1999). Transformation of the collateral vascular bundles into amphivasal vascular bundles in an Arabidopsis mutant. Plant Physiol. 120, 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra, C., and Hohn, T. (1992). Cauliflower mosaic virus gene VI controls translation from dicistronic expression units in transgenic Arabidopsis plants. Plant Cell 4, 1471–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]