Abstract
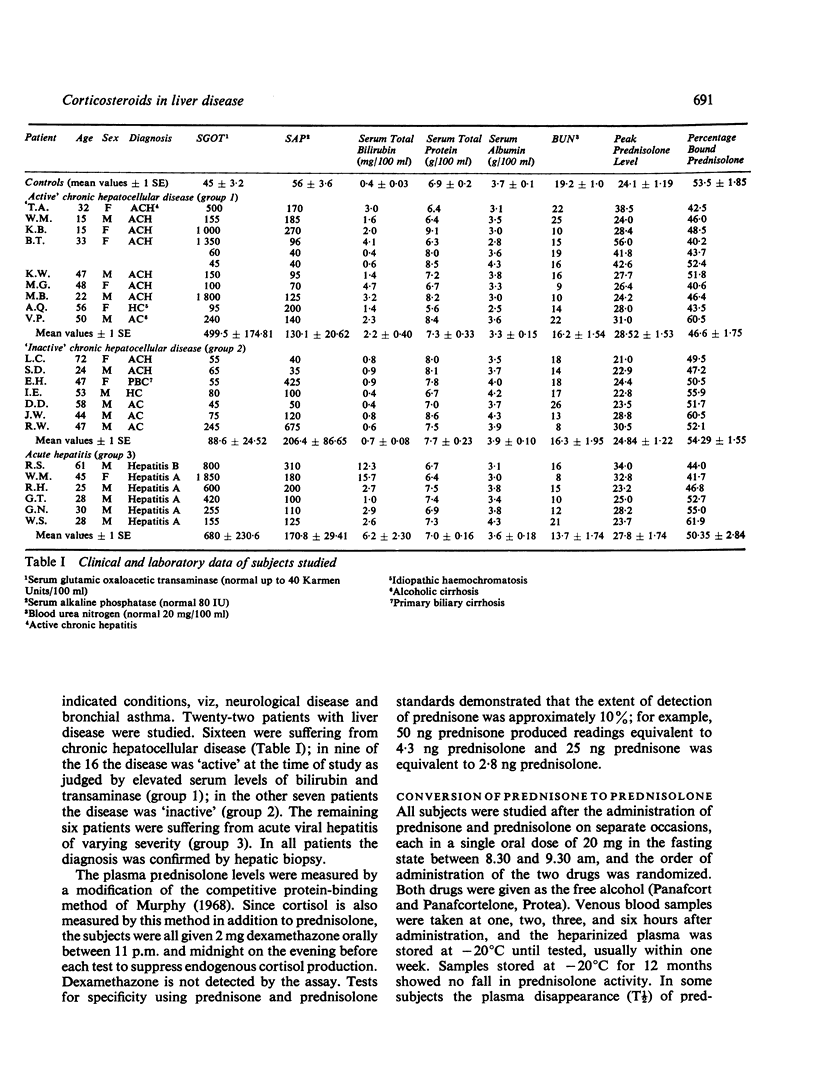
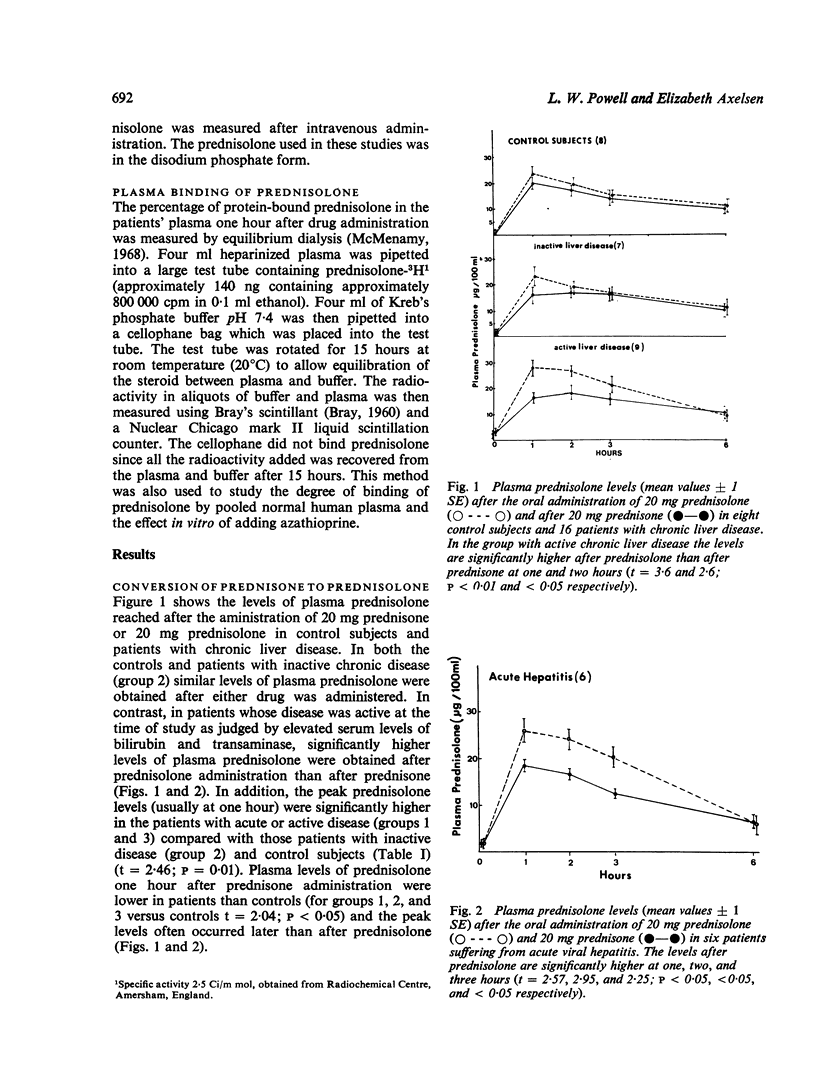
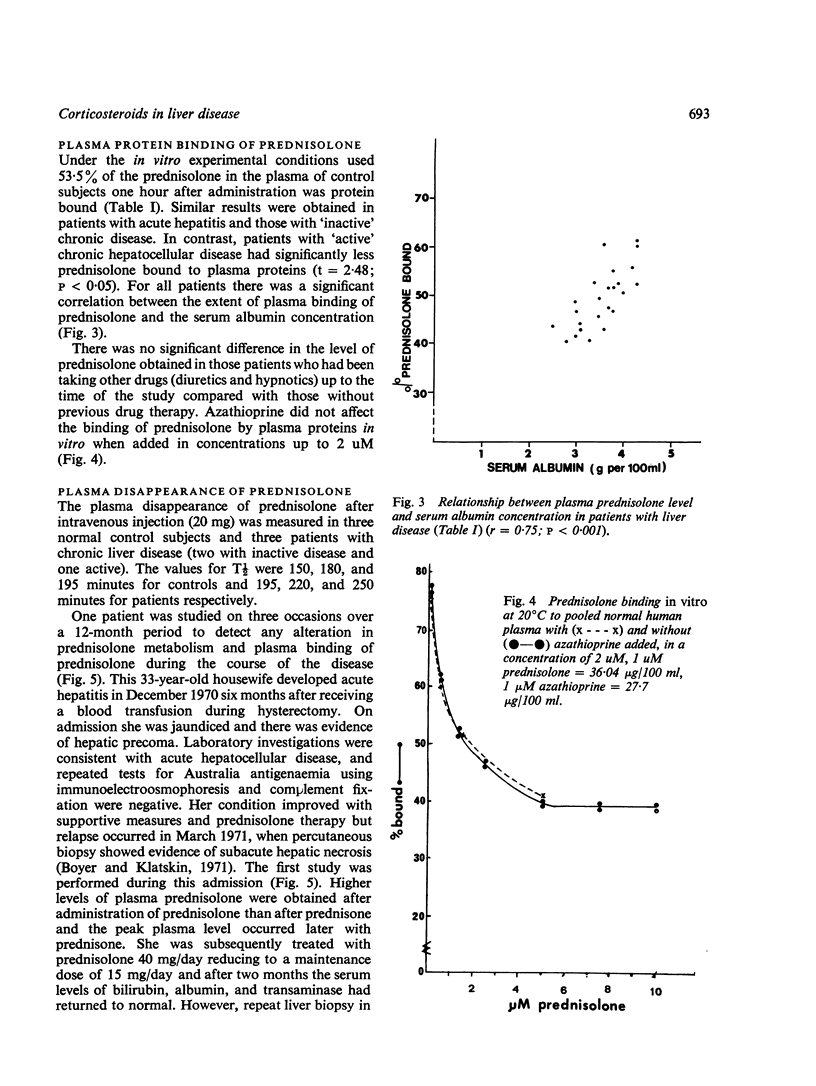
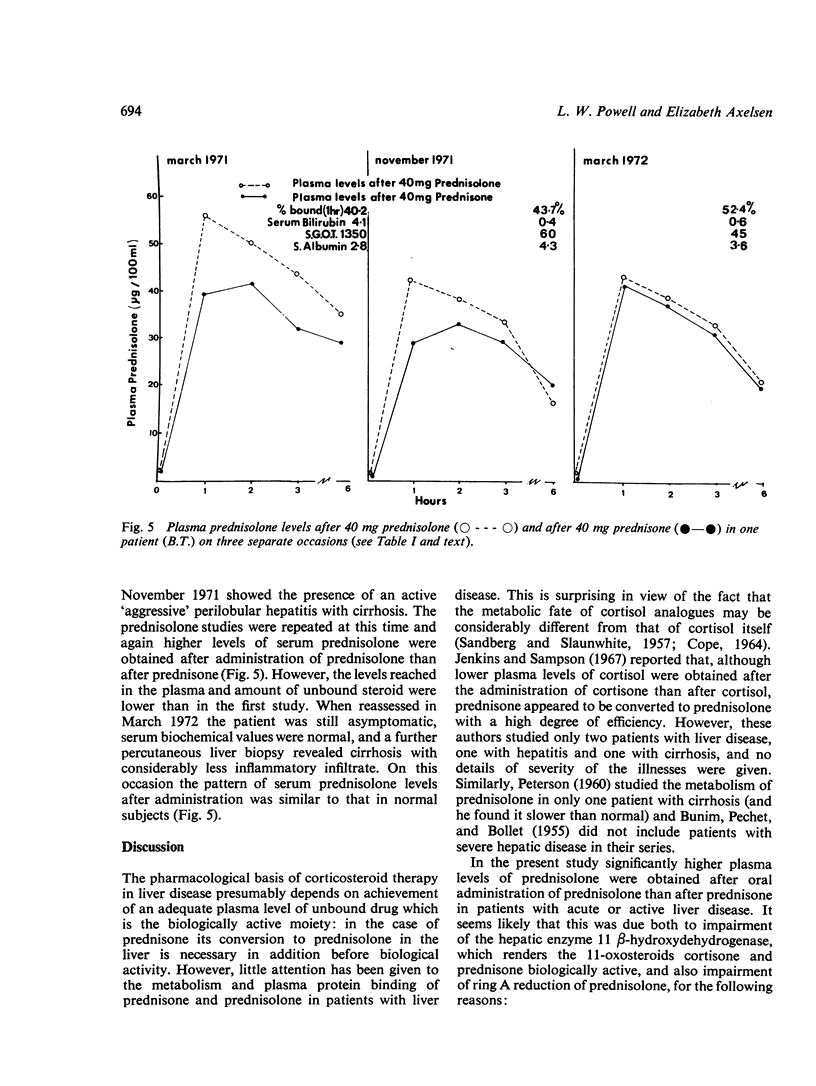
The conversion of prednisone to the biologically active corticosteroid prednisolone and the degree of plasma protein binding of prednisolone were studied in 22 patients with acute or chronic liver disease and in eight control subjects. In patients whose disease was active at the time of study, as judged by elevated serum levels of bilirubin and transaminase, significantly higher levels of plasma prednisolone were obtained after prednisolone administration than after equivalent doses of prednisone. In addition, the amount of unbound drug in the plasma was higher in patients with active disease. There was a significant correlation between the extent of plasma protein binding of prednisolone and the serum albumin concentration. Azathioprine did not affect the plasma binding of prednisolone in vitro. The plasma half-life of prednisolone was prolonged in two of three patients with chronic liver disease studied.
These results suggest that in patients with acute hepatitis or active chronic liver disease there is impairment of reduction of the 11-oxo group of prednisone, and also impaired ring A reduction of prednisolone. Thus, incomplete conversion of prednisone to prednisolone occurs, which is a necessary step for biological activity; on the other hand there is also impairment of prednisolone degradation. These, together with low serum albumin concentrations which are associated with higher levels of circulating unbound prednisolone, result in quite different levels of biologically active corticosteroids compared with equivalent doses of prednisone or prednisolone in subjects without liver disease. The findings have important practical implications for the use of corticosteroids in patients with active liver disease.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUNIM J. J., PECHET M. M., BOLLET A. J. Studies on metacortandralone and metacortandracin in rheumatoid arthritis; antirheumatic potency, metabolic effects, and hormonal properties. J Am Med Assoc. 1955 Jan 22;157(4):311–318. doi: 10.1001/jama.1955.02950210007003. [DOI] [PubMed] [Google Scholar]
- Boyer J. L., Klatskin G. Pattern of necrosis in acute viral hepatitis. Prognostic value of bridging (subacute hepatic necrosis). N Engl J Med. 1970 Nov 12;283(20):1063–1071. doi: 10.1056/NEJM197011122832001. [DOI] [PubMed] [Google Scholar]
- Cook G. C., Mulligan R., Sherlock S. Controlled prospective trial of corticosteroid therapy in active chronic hepatitis. Q J Med. 1971 Apr;40(158):159–185. doi: 10.1093/oxfordjournals.qjmed.a067264. [DOI] [PubMed] [Google Scholar]
- Jenkins J. S., Sampson P. A. Conversion of cortisone to cortisol and prednisone to prednisolone. Br Med J. 1967 Apr 22;2(5546):205–207. doi: 10.1136/bmj.2.5546.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis G. P., Jusko W. J., Graves L., Burke C. W. Prednisone side-effects and serum-protein levels. A collaborative study. Lancet. 1971 Oct 9;2(7728):778–780. doi: 10.1016/s0140-6736(71)92738-3. [DOI] [PubMed] [Google Scholar]
- Mackay I. R. Chronic hepatitis: effect of prolonged suppressive treatment and comparison of azathioprine with prednisolone. Q J Med. 1968 Jul;37(147):379–392. [PubMed] [Google Scholar]
- McMenamy R. H. Binding studies by dialysis equilibrium. A description of an accurate and rapid technique. Anal Biochem. 1968 Apr;23(1):122–128. doi: 10.1016/0003-2697(68)90016-x. [DOI] [PubMed] [Google Scholar]
- Murphy B. E. Clinical evaluation of urinary cortisol determinations by competetive protein-binding radioassay. J Clin Endocrinol Metab. 1968 Mar;28(3):343–348. doi: 10.1210/jcem-28-3-343. [DOI] [PubMed] [Google Scholar]
- PETERSON R. E. Adrenocortical steroid metabolism and adrenal cortical function in liver disease. J Clin Invest. 1960 Feb;39:320–331. doi: 10.1172/JCI104043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANDBERG A. A., SLAUNWHITE W. R., Jr Differences in metabolism of prednisolone-C14 and cortisol-C14. J Clin Endocrinol Metab. 1957 Sep;17(9):1040–1050. doi: 10.1210/jcem-17-9-1040. [DOI] [PubMed] [Google Scholar]
- SCHEDL H. P., CLIFTON J. A. Small intestinal absorption of steroids. Gastroenterology. 1961 Nov;41:491–499. [PubMed] [Google Scholar]
- Sherlock S. Chronic active hepatitis. Definition, diagnosis and management. Postgrad Med. 1971 Nov;50(5):206–211. doi: 10.1080/00325481.1971.11697676. [DOI] [PubMed] [Google Scholar]
- Warmolts J. R., Engel W. K. Benefit from alternate-day prednisone in myasthenia gravis. N Engl J Med. 1972 Jan 6;286(1):17–20. doi: 10.1056/NEJM197201062860104. [DOI] [PubMed] [Google Scholar]



