Abstract
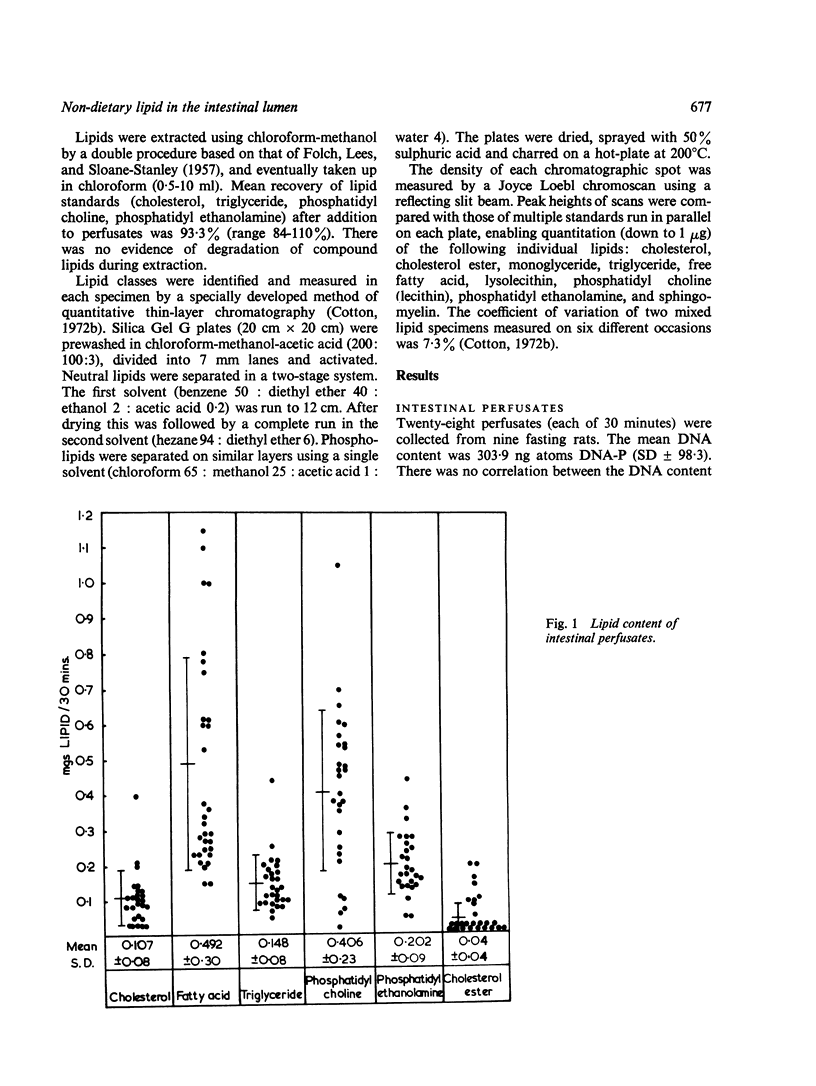
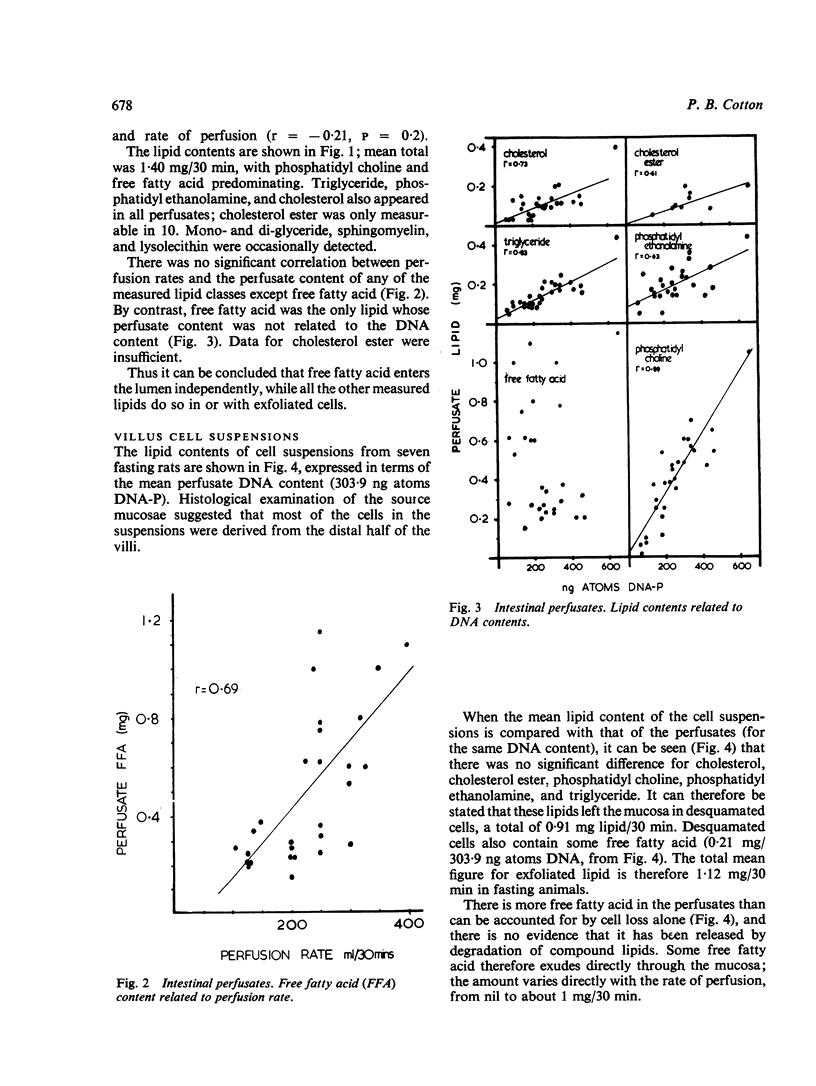
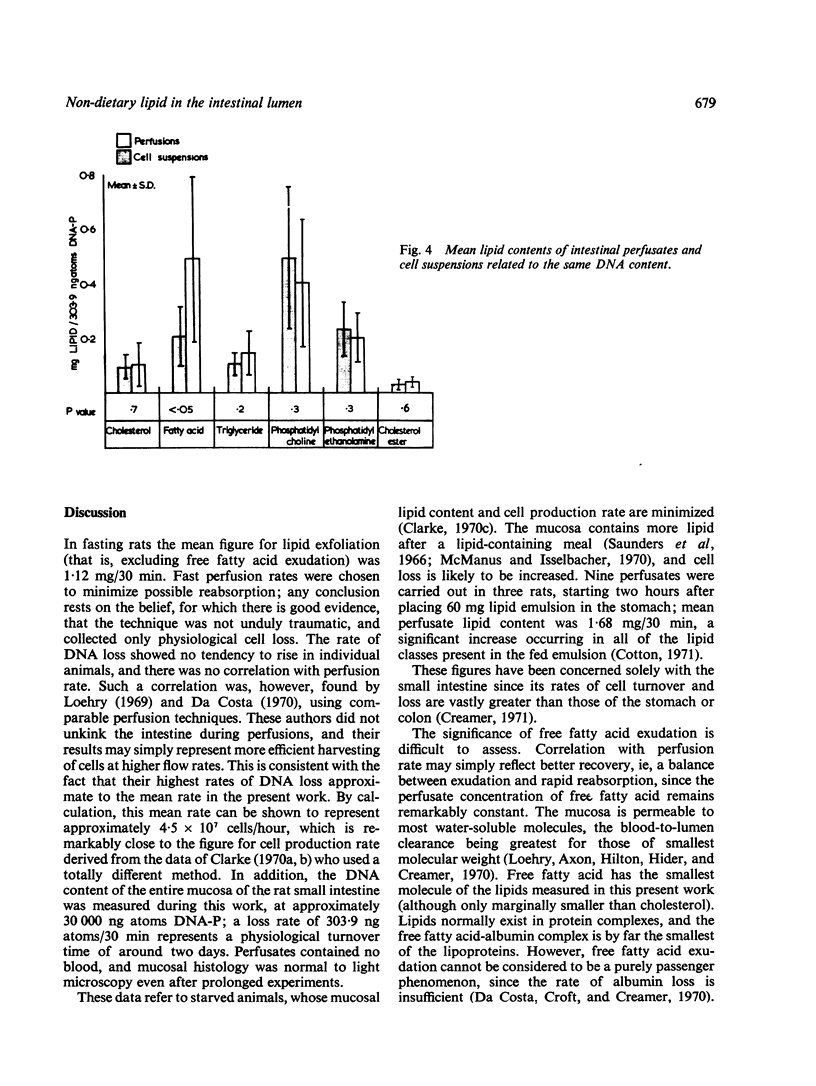
Lipid in the intestinal lumen is mainly dietary in origin, but there is also an endogenous component from bile, bacteria, and the mucosa (through exudation and cell loss). Perfusion experiments in fasting rats demonstrate that exfoliated cells carry with them into the small intestinal lumen an average of 1·12 mg lipid/30 minutes; lipid classes consisting of phosphatidyl choline (lecithin), triglyceride, cholesterol, cholesterol ester, phosphatidyl ethanolamine, and free fatty acid. Fatty acid also enters the lumen independently of cells by exudation.
Since the rate of lipid exfoliation and exudation considerably exceeds the faecal lipid excretion in fasting rats, efficient reabsorption must normally occur. Calculations based on published data suggest the daily exfoliation of 12 to 30 g lipid into the small intestinal lumen of fasting man. When reabsorption is impaired, especially in states of increased cell turnover, endogenous mucosal lipid may account for a significant proportion of faecal lipid, perhaps sufficient to constitute a state of fat-losing enteropathy.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLOMSTRAND R. A study on the intestinal absorption of fat in normal adults and in non-tropical sprue with carbon-labelled oleic acid and palmitic acid. Acta Med Scand. 1955 Aug 30;152(2):129–138. doi: 10.1111/j.0954-6820.1955.tb03470.x. [DOI] [PubMed] [Google Scholar]
- BURR W. W., Jr, McPHERSON J. C., TIDWELL H. C. Secretion of labeled blood lipids into the intestine. J Nutr. 1960 Feb;70:171–175. doi: 10.1093/jn/70.2.171. [DOI] [PubMed] [Google Scholar]
- Baxter J. Origin and characteristics of endogenous lipid in thoracic duct lymph in rat. J Lipid Res. 1966 Jan;7(1):158–166. [PubMed] [Google Scholar]
- COMFORT M. W., WOLLAEGER E. E., TAYLOR A. B. Nontropical sprue; observations on absorption and metabolism. Gastroenterology. 1953 Feb;23(2):155–178. [PubMed] [Google Scholar]
- CROFT D. N., LUBRAN M. THE ESTIMATION OF DEOXYRIBONUCLEIC ACID IN THE PRESENCE OF SIALIC ACID: APPLICATION TO ANALYSIS OF HUMAN GASTRIC WASHINGS. Biochem J. 1965 Jun;95:612–620. doi: 10.1042/bj0950612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke R. M. A new method of measuring the rate of shedding of epithelial cells from the intestinal villus of the rat. Gut. 1970 Dec;11(12):1015–1019. doi: 10.1136/gut.11.12.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke R. M. Mucosal architecture and epithelial cell production rate in the small intestine of the albino rat. J Anat. 1970 Nov;107(Pt 3):519–529. [PMC free article] [PubMed] [Google Scholar]
- Creamer B. Loss from the small intestine. J R Coll Physicians Lond. 1971 Jul;5(4):323–332. [PMC free article] [PubMed] [Google Scholar]
- Croft D. N. Body iron loss and cell loss from epithelia. Proc R Soc Med. 1970 Dec;63(12):1221–1224. [PMC free article] [PubMed] [Google Scholar]
- DOUBILET H., FISHMAN L. Human biliary-pancreatic secretion. Am J Gastroenterol. 1961 May;35:499–512. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- KIM K. S., BOLLMAN J. L. Absorption of fat in the absence of both bile and pancreatic juice. AMA Arch Surg. 1954 Aug;69(2):247–254. doi: 10.1001/archsurg.1954.01270020113013. [DOI] [PubMed] [Google Scholar]
- KIM K. S., BOLLMAN J. L., GRINDLAY J. H. Metabolism of endogenous lipid of the intestine. Am J Physiol. 1956 Mar;184(3):445–448. doi: 10.1152/ajplegacy.1956.184.3.445. [DOI] [PubMed] [Google Scholar]
- Loehry C. A., Axon A. T., Hilton P. J., Hider R. C., Creamer B. Permeability of the small intestine to substances of different molecular weight. Gut. 1970 Jun;11(6):466–470. doi: 10.1136/gut.11.6.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loehry C. A., Croft D. N., Singh A. K., Creamer B. Cell turnover in the rat small intestinal mucosa: an appraisal of cell loss. II. Cell loss in rats with an abnormal mucosa. Gut. 1969 Jan;10(1):16–18. doi: 10.1136/gut.10.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus J. P., Isselbacher K. J. Effect of fasting versus feeding on the rat small intestine. Morphological, biochemical, and functional differences. Gastroenterology. 1970 Aug;59(2):214–221. [PubMed] [Google Scholar]
- Melvin K. E., Hepner G. W., Bordier P., Neale G., Joplin G. F. Calcium metabolism and bone pathology in adult coeliac disease. Q J Med. 1970 Jan;39(153):83–113. [PubMed] [Google Scholar]
- NORCIA L. N., LUNDBERG W. O. Fat excretion: the influence of dietary fat on fecal fat excretion. J Nutr. 1954 Dec 10;54(4):491–508. doi: 10.1093/jn/54.4.491. [DOI] [PubMed] [Google Scholar]
- PESSOA V. C., KIM K. S., IVY A. C. Fat absorption in absence of bile and pancreatic juice. Am J Physiol. 1953 Aug;174(2):209–218. doi: 10.1152/ajplegacy.1953.174.2.209. [DOI] [PubMed] [Google Scholar]
- Partin J. C., Schubert W. K. Small intestinal mucosa in cholesterol ester storage disease. A light and electron microscope study. Gastroenterology. 1969 Nov;57(5):542–558. [PubMed] [Google Scholar]
- Pink I. J., Croft D. N., Creamer B. Cell loss from small intestinal mucosa: a morphological study. Gut. 1970 Mar;11(3):217–222. doi: 10.1136/gut.11.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid D. J. Intravenous fat therapy. I. Nitrogen balance studies. Br J Surg. 1967 Mar;54(3):198–204. doi: 10.1002/bjs.1800540311. [DOI] [PubMed] [Google Scholar]
- Saunders D. R., Ways P. O., Parmentier C. M., Rubin C. E. Studies on the lipid composition of human small bowel mucosa. J Clin Invest. 1966 Sep;45(9):1516–1525. doi: 10.1172/JCI105458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A. K. Excretion of endogenous non-haem iron by the gastrointestinal tract in human subjects and its influence on the size of body store of iron with particular reference to coeliac disease. Br J Haematol. 1970 Jun;18(6):597–609. doi: 10.1111/j.1365-2141.1970.tb01584.x. [DOI] [PubMed] [Google Scholar]
- Tabaqchali S., Booth C. C. Jejunal bacteriology and bile-salt metabolism in patients with intestinal malabsorption. Lancet. 1966 Jul 2;2(7453):12–15. doi: 10.1016/s0140-6736(66)91744-2. [DOI] [PubMed] [Google Scholar]
- VERDINO B., BLANK M. L., PRIVETT O. S. ENDOGENOUS LIPID COMPOSITION OF THE INTESTINAL LYMPH OF RATS RAISED ON FAT-FREE, LARD, OR CORN OIL DIETS. J Lipid Res. 1965 Jul;6:356–362. [PubMed] [Google Scholar]
- WATKIN D. M. FECAL EXCRETION OF LIPIDS BEFORE, DURING AND AFTER HYPERALIMENTATION WITH FAT ADMINISTERED INTRAVENOUSLY. Am J Clin Nutr. 1965 Jan;16:213–223. doi: 10.1093/ajcn/16.1.213. [DOI] [PubMed] [Google Scholar]
- WEBB J. P., JAMES A. T., KELLOCK T. D. The influence of diet on the quality of faecal fat in patients with and without steatorrhoea. Gut. 1963 Mar;4:37–41. doi: 10.1136/gut.4.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins H. S., Howell K. E., Kellock T. D., Stalder J. The origin of faecal fat. Gut. 1969 May;10(5):400–403. doi: 10.1136/gut.10.5.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. D., Reinke R. T. Transfer of locally synthesized cholesterol from intestinal wall to intestinal lymph. J Lipid Res. 1968 Jan;9(1):85–92. [PubMed] [Google Scholar]
- da Costa L. R., Croft D. N., Creamer B. Protein loss and cell loss from the small-intestinal mucosa. Gut. 1971 Mar;12(3):179–183. doi: 10.1136/gut.12.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]


