Abstract
Short loops of dog small intestine, filled with a buffered glucose solution, were subjected to one hour's total ischaemia by clamping the corresponding mesenteric artery and vein as well as the intestinal wall at each end of the loop. Immediately after the ischaemic period and 24 hours later, their functional capacity, together with that of neighbouring control loops, was determined by studying the absorption of phenylalanine and β-methyl-glucoside in vitro and by measuring the levels of Na+-K+-ATPase in the mucosa. The release of lysosomal enzymes after the ischaemia was studied by gauging the levels of acid phosphatase in the venous blood draining the ischaemic loop. The state of the mucosal microcirculation was investigated by injection of indian ink into the mesenteric artery removal of the loop.
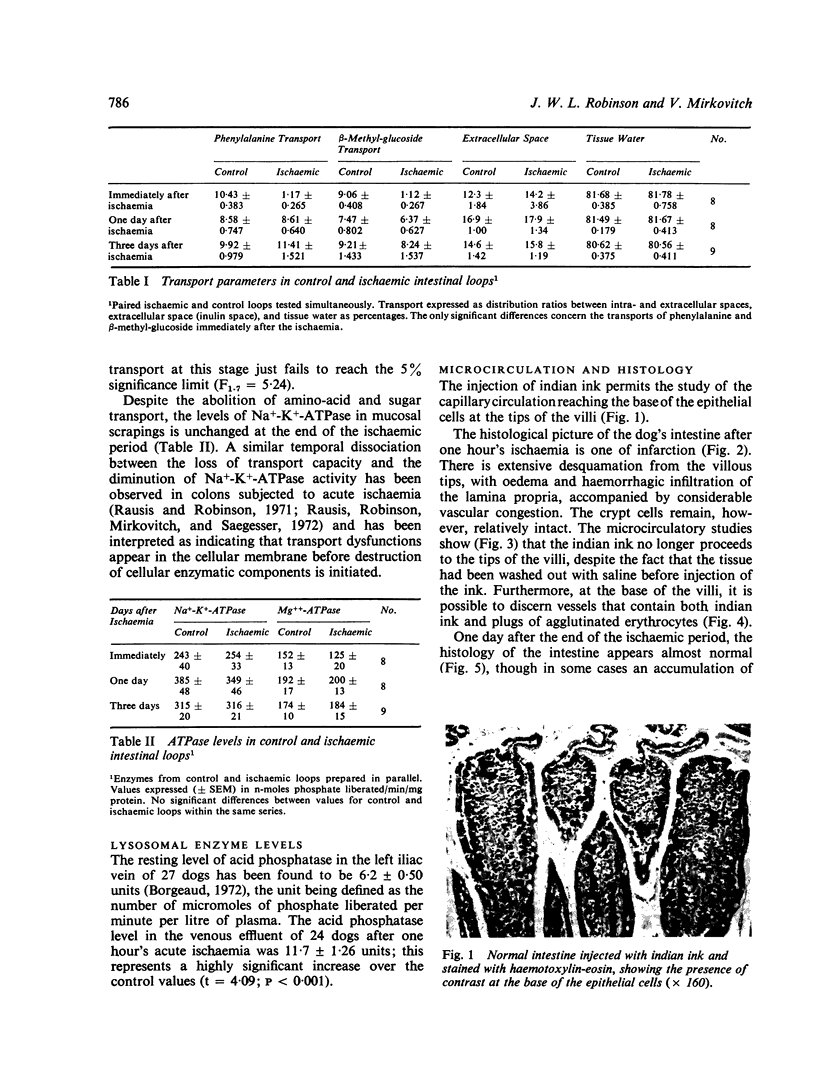
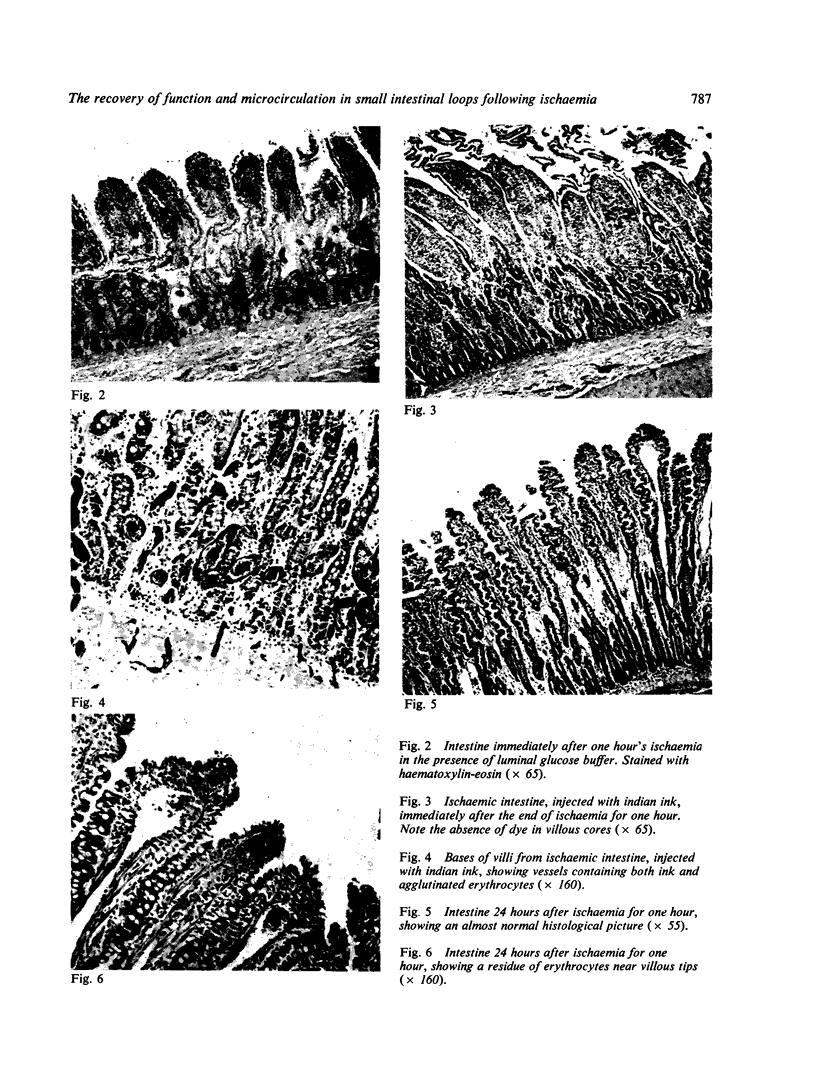
Immediately after ischaemia, considerable structural damage was observed in the intestinal mucosa, with desquamation of the villous tips, oedema, vascular stasis, and haemorrhagic infiltration in the lamina propria. No dye was observed in the mucosal capillaries. All transport capacity was abolished, but ATPase levels were unchanged. A significant release of lysosomal enzymes into the venous blood was noted.
One day later structural and functional recovery was complete, and vascularization of the villous core was restored.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bounous G., McArdle A. H. Release of intestinal enzymes in acute mesenteric ischemia. J Surg Res. 1969 Jun;9(6):339–346. doi: 10.1016/0022-4804(69)90078-x. [DOI] [PubMed] [Google Scholar]
- Chiu C. J., McArdle A. H., Brown R., Scott H. J., Gurd F. N. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970 Oct;101(4):478–483. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- Chiu C. J., Scott H. J., Gurd F. N. Intestinal mucosal lesion in low-flow states. II. The protective effect of intraluminal glucose as energy substrate. Arch Surg. 1970 Oct;101(4):484–488. doi: 10.1001/archsurg.1970.01340280036010. [DOI] [PubMed] [Google Scholar]
- King E. J. The colorimetric determination of phosphorus. Biochem J. 1932;26(2):292–297. doi: 10.1042/bj0260292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- PASSI R. B., LANSING A. M. EXPERIMENTAL INTESTINAL MALABSORPTION PRODUCED BY VASCULAR INSUFFICIENCY. Can J Surg. 1964 Jul;7:332–340. [PubMed] [Google Scholar]
- ROBINSON J. W., JEQUIER J. C., FELBER J. P. THE ACCUMULATION OF AMINO ACIDS BY VARIOUS PREPARATIONS OF DOG INTESTINE. Experientia. 1965 Jan 15;21:12–13. doi: 10.1007/BF02136353. [DOI] [PubMed] [Google Scholar]
- Rausis C., Robinson J. W., Mirkovitch V., Saegesser F. Ischémie colique nécrosante et gangreneuse: faits cliniques et recherche expérimentale. Helv Chir Acta. 1972 May;39(1):251–258. [PubMed] [Google Scholar]
- Rausis C., Robinson J. W. Sensibilite de la muqueuse intestinale à l'insuffisance circulatoire. Schweiz Rundsch Med Prax. 1972 Feb 15;61(7):217–219. [PubMed] [Google Scholar]
- Riecken E. O., Stewart J. S., Dowling R. H. Neuere Methoden in der Diagnostik intestinaler Störungen. Internist (Berl) 1966 May;7(5):209–217. [PubMed] [Google Scholar]
- Robinson J. W., Antonioli J. A., Mirkovitch V. The intestinal response to ischaemia. Naunyn Schmiedebergs Arch Pharmakol Exp Pathol. 1966;255(2):178–191. doi: 10.1007/BF00543211. [DOI] [PubMed] [Google Scholar]
- Robinson J. W. Experimental intestinal malabsorption states and their relation to clinical syndromes. Klin Wochenschr. 1972 Feb 15;50(4):173–185. doi: 10.1007/BF01487140. [DOI] [PubMed] [Google Scholar]
- Robinson J. W., Felber J. B. Compartments of the uptake of amino-acids by intestinal fragments during in vitro incubation. Gastroenterologia. 1965;104(6):335–342. doi: 10.1159/000202112. [DOI] [PubMed] [Google Scholar]
- Röttger P., Oran M. Histochemische und biochemische Untersuchungen zur Frage des Enzym-Gehaltes der Dünndarmschleimhaut nach temporärer Ischämie. Verh Dtsch Ges Pathol. 1969;53:189–193. [PubMed] [Google Scholar]
- Varró V. Die Beziehungen zwischen Resorption und Blutzirkulation im Dünndarm. Internist (Berl) 1966 May;7(5):250–253. [PubMed] [Google Scholar]
- Winne D. Der Einfluss der Durchblutung auf die Wasser- und Salzresorption im Jejunum der Ratte. Naunyn Schmiedebergs Arch Pharmakol. 1970;265(5):425–441. [PubMed] [Google Scholar]
- Winne D. Durchblutung und enterale Resorption. Z Gastroenterol. 1971;9(6):429–441. [PubMed] [Google Scholar]