Abstract
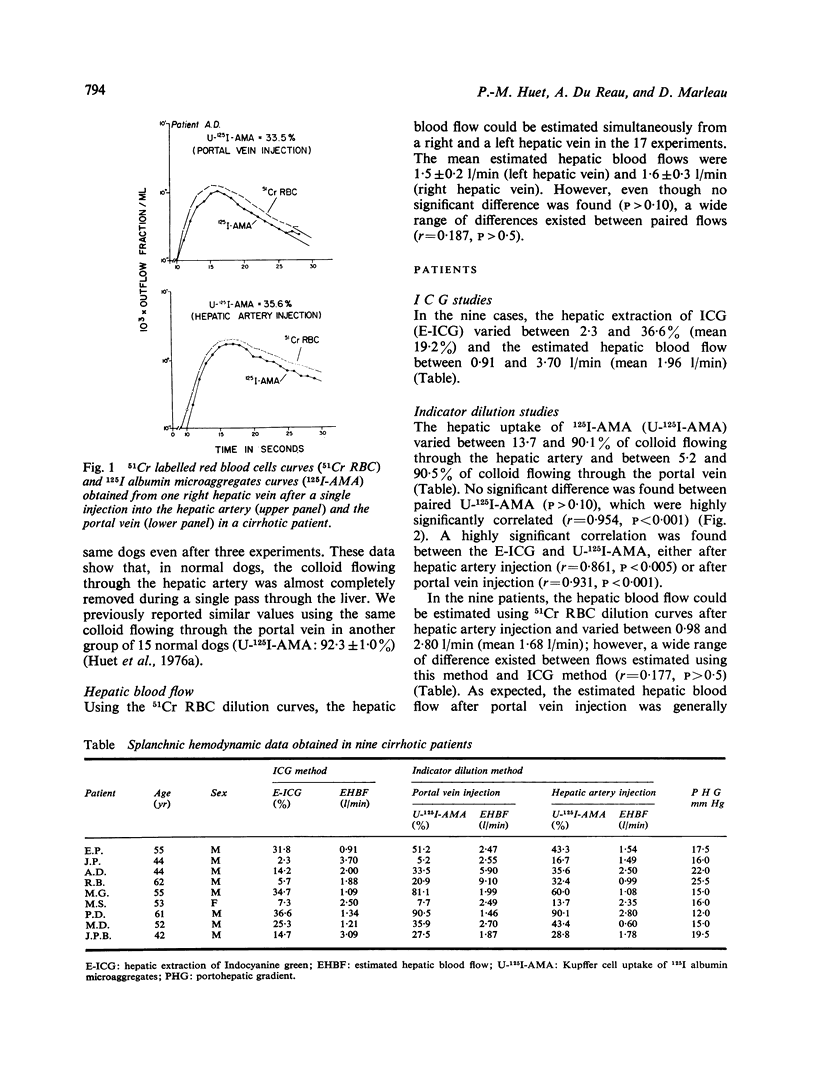
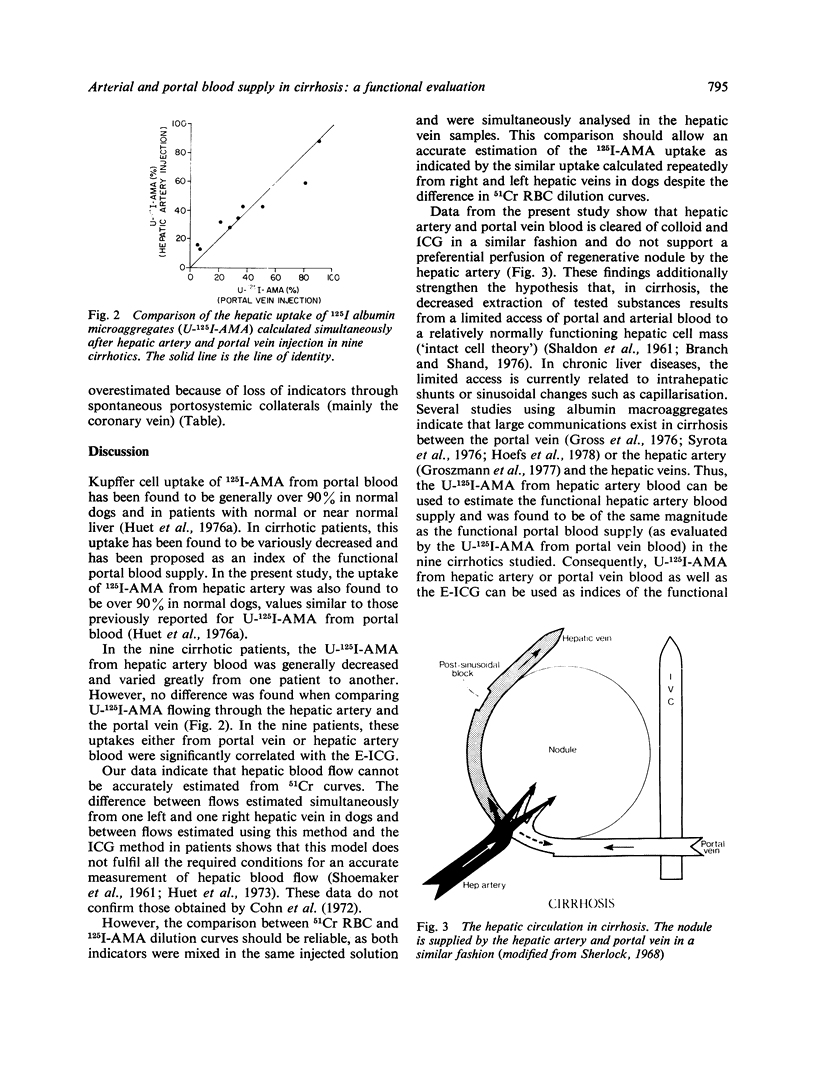
The uptake of 125I albumin microaggregates (U-125I-AMA) from portal blood, during a single passage through the hepatic reticuloendothelial system, has been found to be generally decreased in cirrhosis. To investigate if a similar phenomenon occurs for the colloid flowing through the hepatic artery, the U-125I-AMA was first calculated in normal dogs after injection of a mixture of 51Cr red blood cells (51Cr-RBC) and 125I-AMA into the hepatic artery by comparing hepatic indicator dilution curves (IDC) obtained with both indicators. In nine dogs, the U-125I-AMA from hepatic artery blood was generally over 90%, as previously reported for the same colloid flowing through the portal vein in another group of normal dogs. This approach was then applied in nine patients with alcoholic cirrhosis who underwent combined umbilicoportal vein, hepatic vein, and hepatic artery catheterisation because of severe portal hypertension. Hepatic indicator dilution curves were obtained in the nine patients after injection of a mixture of 51Cr-RBC and 125I-AMA into the portal vein and the hepatic artery. The U-125I-AMA from portal and hepatic artery blood was measured by comparing 51Cr-RBC and 125I-AMA hepatic IDC. U-125I-AMA varied between 5·2 and 90·5% after portal vein injection and between 13·7 and 90·1% after hepatic artery injection; not difference was found between paired values. In all patients the extraction of indocyanine green (E-ICG) was calculated during a continuous infusion and significant correlations were found between E-ICG and U-125I-AMA from portal blood (r=0·931; p <0·001) or from hepatic artery blood (r=0·861; p <0·005). The decreased uptakes can be related to intrahepatic shunts or sinusoidal changes responsible for ineffective phagocytosis and restricted access of dye to parenchymal cells. These data indicate that in cirrhosis the hepatic artery and portal vein blood is cleared of colloid and ICG in a similar fashion and suggest nearly identical blood supply to the regenerative nodules by the hepatic artery and portal vein. Thus U-125I-AMA from hepatic artery or portal vein blood, as well as the E-ICG, may be used to estimate the functional hepatic blood supply in cirrhosis; this may prove to be useful in the prognosis of patients before portacaval shunts.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branch R. A., Shand D. G. Propranolol disposition in chronic liver disease: a physiological approach. Clin Pharmacokinet. 1976;1(4):264–279. doi: 10.2165/00003088-197601040-00002. [DOI] [PubMed] [Google Scholar]
- CAESAR J., SHALDON S., CHIANDUSSI L., GUEVARA L., SHERLOCK S. The use of indocyanine green in the measurement of hepatic blood flow and as a test of hepatic function. Clin Sci. 1961 Aug;21:43–57. [PubMed] [Google Scholar]
- Cohn J. N., Khatri I. M., Groszmann R. J., Kotelanski B. Hepatic blood flow in alcoholic liver disease measured by an indicator dilution technic. Am J Med. 1972 Dec;53(6):704–714. doi: 10.1016/0002-9343(72)90187-8. [DOI] [PubMed] [Google Scholar]
- GORESKY C. A. A linear method for determining liver sinusoidal and extravascular volumes. Am J Physiol. 1963 Apr;204:626–640. doi: 10.1152/ajplegacy.1963.204.4.626. [DOI] [PubMed] [Google Scholar]
- Groszmann R. J., Kravetz D., Parysow O. Intrahepatic arteriovenous shunting in cirrhosis of the liver. Gastroenterology. 1977 Jul;73(1):201–204. [PubMed] [Google Scholar]
- Huet P. M., Lavoie P., Viallet A. Simultaneous estimation of hepatic and portal blood flows by an indicator dilution technique. J Lab Clin Med. 1973 Nov;82(5):836–846. [PubMed] [Google Scholar]
- Huet P. M., Marleau D., Lavoie P., Viallet A. Extraction of 125I-albumin microaggregates from portal blood. An index of functional portal blood supply in cirrhotics. Gastroenterology. 1976 Jan;70(1):74–81. [PubMed] [Google Scholar]
- Mitra S. K. Hepatic vascular changes in human and experimental cirrhosis. J Pathol Bacteriol. 1966 Oct;92(2):405–414. doi: 10.1002/path.1700920219. [DOI] [PubMed] [Google Scholar]
- POPPER H., ELIAS H., PETTY D. E. Vascular pattern of the cirrhotic liver. Am J Clin Pathol. 1952 Aug;22(8):717–729. doi: 10.1093/ajcp/22.8.717. [DOI] [PubMed] [Google Scholar]
- Reynolds T. B. Editorial: Promises. Promises. Hemodynamics and portal-systemic shunt. N Engl J Med. 1974 Jun 27;290(26):1484–1485. doi: 10.1056/NEJM197406272902611. [DOI] [PubMed] [Google Scholar]
- SCHAFFNER F., POPER H. Capillarization of hepatic sinusoids in man. Gastroenterology. 1963 Mar;44:239–242. [PubMed] [Google Scholar]
- SHALDON S., CHIANDUSSI L., GUEVARA L., CAESAR J., SHERLOCK S. The estimation of hepatic blood flow and intrahepatic shunted blood flow by colloidal heat-denatured human serum albumin labeled with I-131. J Clin Invest. 1961 Jul;40:1346–1354. doi: 10.1172/JCI104365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syrota A., Vinot J. M., Paraf A., Roucayrol J. C. Scintillation splenoportography: hemodynamic and morphological study of the portal circulation. Gastroenterology. 1976 Oct;71(4):652–659. [PubMed] [Google Scholar]


