Abstract
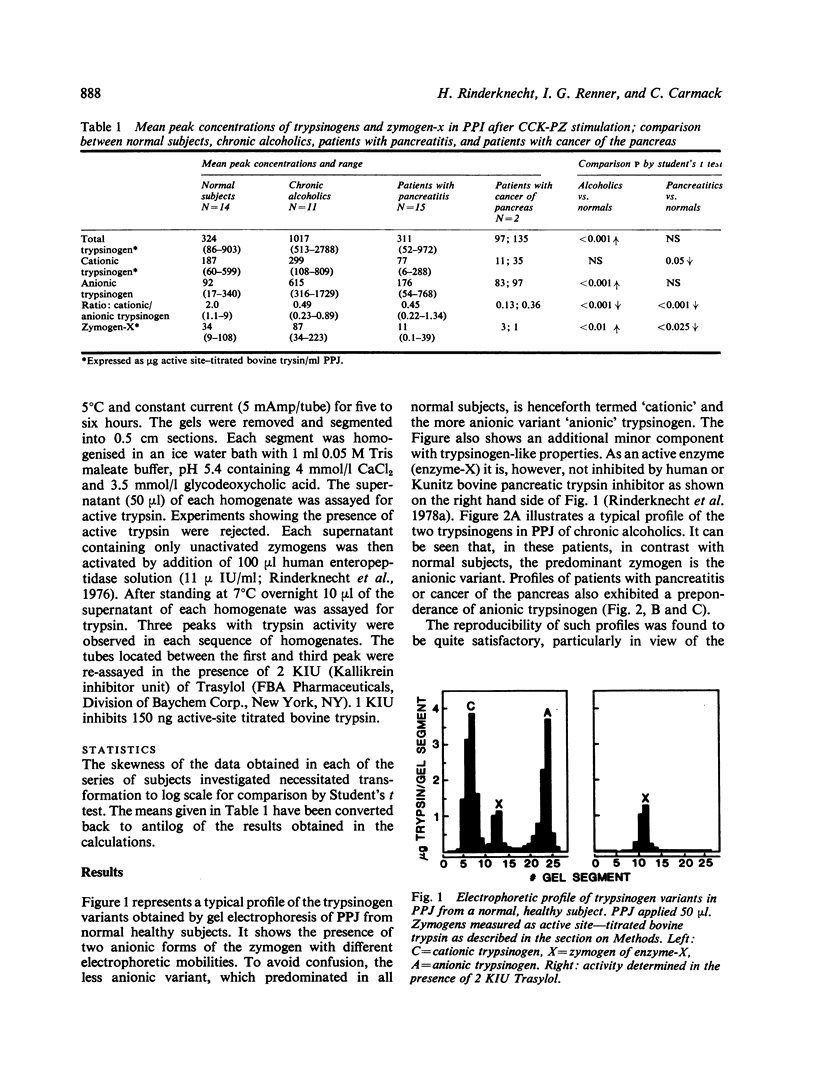
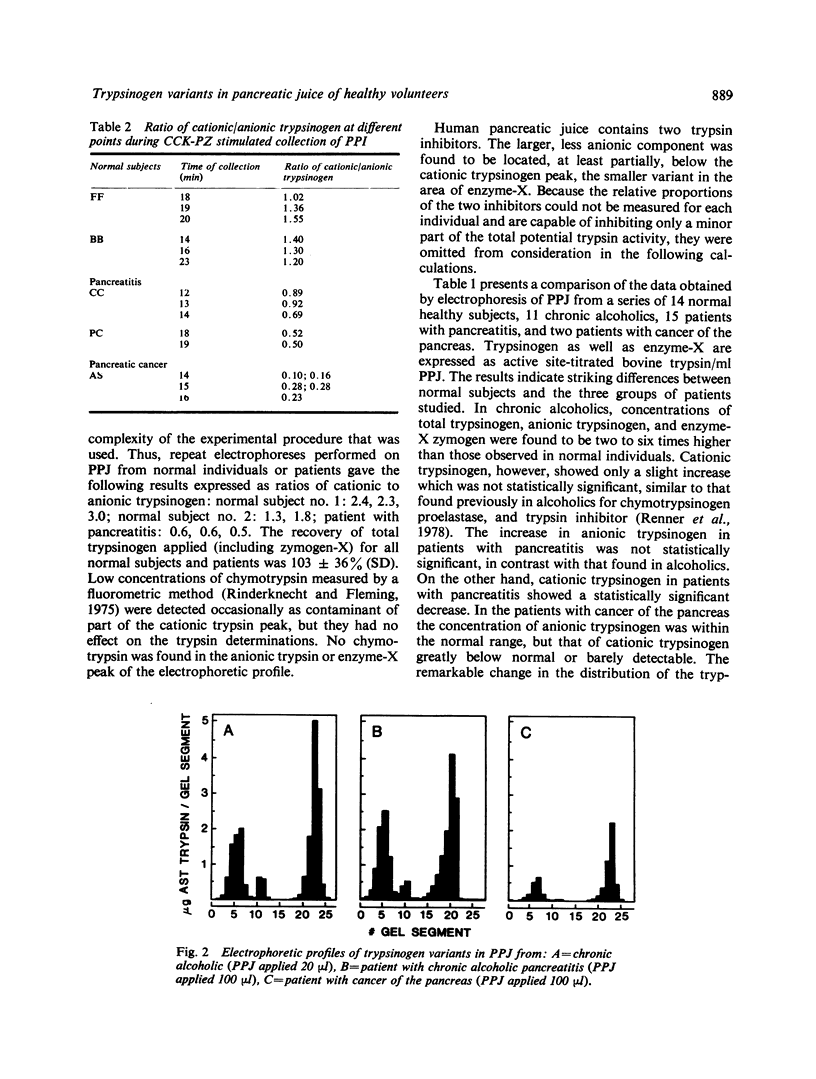
Polyacrylamide gel electrophoresis of pure pancreatic juice from 14 healthy normal subjects, 11 chronic alcoholics without detectable pancreatic disease, 15 patients with pancreatitis, and two with cancer of the pancreas consistently demonstrated the presence of two variants of trypsinogen with different electrophoretic mobilities. In healthy normal subjects the proportion of cationic to anionic trypsinogen was invariably greater than 1 and averaged about 2. In chronic alcoholics, patients with pancreatitis or cancer of the pancreas, this ratio, with a single exception, was below one and averaged about 0·45. The extraordinary consistency of these findings suggests that the quantitative relationship between cationic and anionic trypsinogen in human pancreatic juice may be a very sensitive indicator of incipient or existing pancreatic pathology. The most acceptable explanation for the reversal of the normal zymogen ratio in pancreatic disease is a selective increase in the synthesis of the anionic variant relative to that of the cationic species. Total trypsinogen concentrations differed widely from one another in the three patient groups, but the ratio of cationic to anionic trypsinogen exhibited little change and remained below 1. Our results also demonstrate for the first time a specific effect of chronic alcohol abuse on the secretory profile of a pancreatic enzyme in human subjects. A newly discovered minor, trypsinogen-like component of human pancreatic juice was found to be significantly increased in pancreatic juice of chronic alcoholics, decreased in pancreatic secretions of patients with pancreatitis, and barely detectable in those of two patients with cancer of the pancreas.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burch G. E., Ansari A. Chronic alcoholism and carcinoma of the pancreas. A correlative hypothesis. Arch Intern Med. 1968 Sep;122(3):273–275. [PubMed] [Google Scholar]
- Dani R., Nogueira C. E. Chronische kalzifizierende Pankreatitis in Brasilien -- Eine Analyse von 92 Fällen. Leber Magen Darm. 1976 Oct;6(5):272–275. [PubMed] [Google Scholar]
- Guy O., Lombardo D., Bartelt D. C., Amic J., Figarella C. Two human trypsinogens. Purification, molecular properties, and N-terminal sequences. Biochemistry. 1978 May 2;17(9):1669–1675. doi: 10.1021/bi00602a014. [DOI] [PubMed] [Google Scholar]
- Louvard M. N., Puigserver A. On bovine and porcine anionic trypsinogens. Biochim Biophys Acta. 1974 Nov 5;371(1):177–185. doi: 10.1016/0005-2795(74)90167-6. [DOI] [PubMed] [Google Scholar]
- Rinderknecht H., Fleming R. M. A new, highly sensitive and specific assay for chymotrypsin. Clin Chim Acta. 1975 Mar 10;59(2):139–146. doi: 10.1016/0009-8981(75)90021-2. [DOI] [PubMed] [Google Scholar]
- Rinderknecht H., Renner I. G., Carmack C. Activation of human pancreatic juice. Clin Chim Acta. 1976 Dec 1;73(2):369–372. doi: 10.1016/0009-8981(76)90185-6. [DOI] [PubMed] [Google Scholar]
- Rinderknecht H., Renner I. G., Douglas A. P., Adham N. F. Profiles of pure pancreatic secretions obtained by direct pancreatic duct cannulation in normal healthy human subjects. Gastroenterology. 1978 Dec;75(6):1083–1089. [PubMed] [Google Scholar]
- Robinson L. A., Kim W. J., White T. T., Hadorn B. Trypsin in human pancreatic juice--their distributions as found in 34 specimens. Two human pancreatic trypsinogens. Scand J Gastroenterol. 1972;7(1):43–45. doi: 10.3109/00365527209180737. [DOI] [PubMed] [Google Scholar]
- White T. T., Allan B. J. Intrapancreatic activation of proteases in the etiology of pancreatitis and cancer of the pancreas. Med Clin North Am. 1974 Nov;58(6):1305–1310. doi: 10.1016/s0025-7125(16)32072-7. [DOI] [PubMed] [Google Scholar]


