Abstract
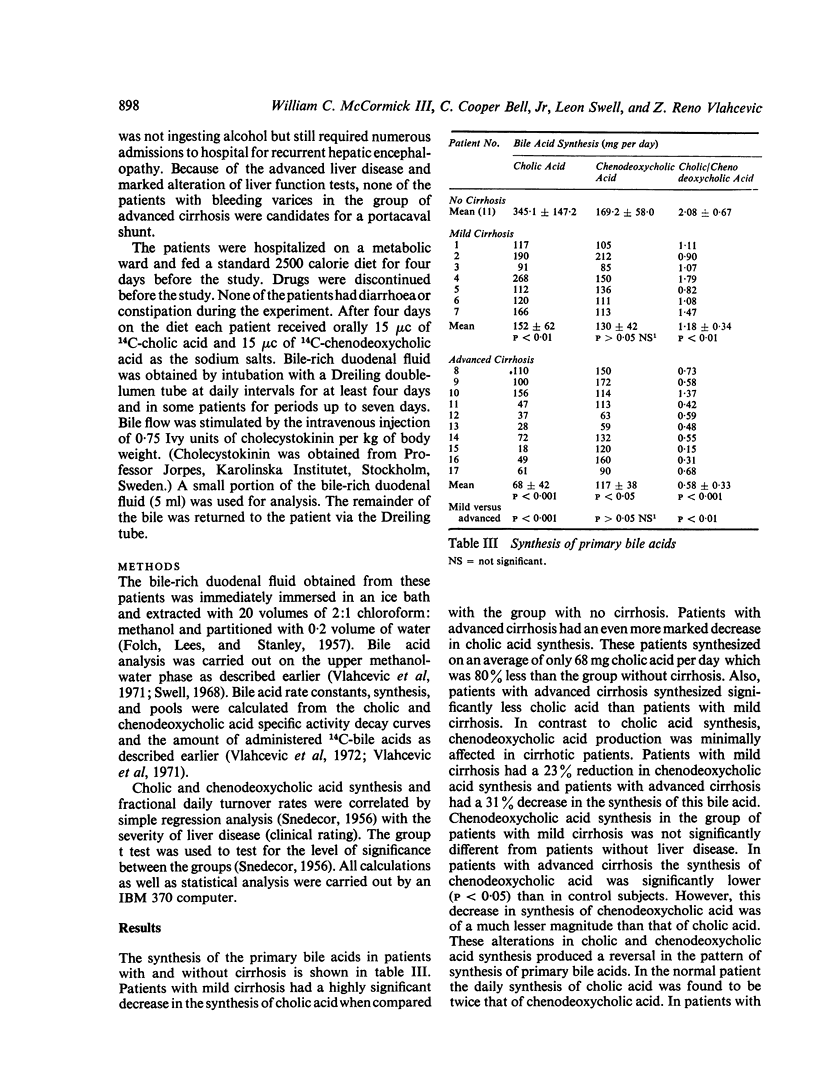
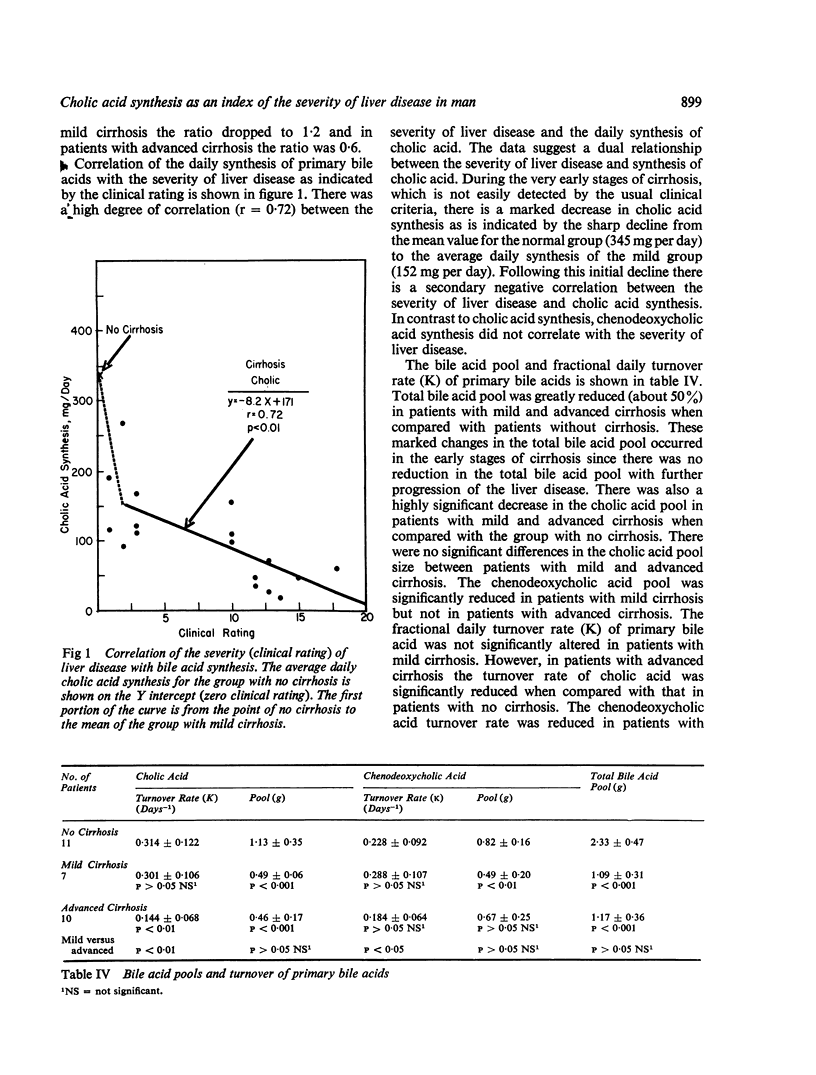
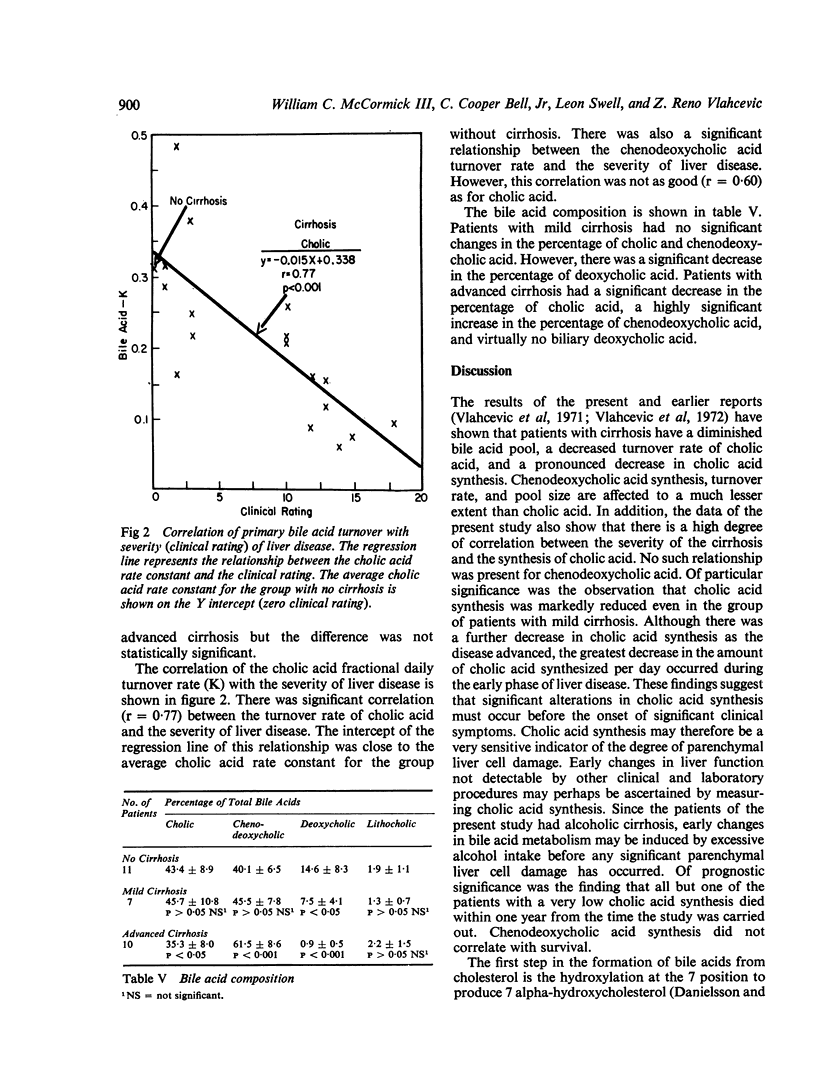
Bile acid pool size and kinetics were determined in 17 patients with cirrhosis and 11 patients without liver disease and correlated with the severity of liver disease as determined by the usual clinical and laboratory criteria. In order to assess the severity of liver disease, a grading system was devised which assigned numerical values to various clinical signs and laboratory results. The total clinical score and the patients were divided into two groups of advanced (7-18 points) or mild (1-6 points) cirrhosis. The clinical rating was then correlated with the various aspects of bile acid metabolism. Cholic acid synthesis was markedly reduced in the early stages of cirrhosis and continued to decrease with the advancement of the liver disease. There was an inverse correlation between synthesis of cholic acid and the severity of cirrhosis. Nine of the 10 patients with advanced cirrhosis and a very low cholic acid synthetic rate (average 68 mg per day) died within one to 13 months from the start of the study. Patients with mild cirrhosis also had significantly reduced cholic acid synthesis (average 152 mg per day) but they all were well and alive three to 23 months after the study. In contrast, chenodeoxycholic acid synthesis was not markedly affected in either patients with mild or advanced cirrhosis. There was also a high degree of correlation between the fractional daily turnover rate of cholic acid and the severity of liver disease. The fractional daily turnover rate of cholic acid was greatly reduced (50%) in patients with advanced cirrhosis. Deoxycholic acid was reduced in patients with mild cirrhosis and virtually absent from the bile of patients with advanced cirrhosis. The findings of the present report provide evidence that cholic acid synthesis is a sensitive indicator of the hepatocellular damage, whereas chenodeoxycholic acid synthesis is relatively unaffected by cirrhosis. The selective alteration in cholic acid synthesis probably resides in a deficiency of one or more enzymes regulating the formation of the 3-keto, 7 alpha, 12 alpha-dihydroxy precursor of cholic acid.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. E., Kok E., Javitt N. B. Bile acid synthesis in man: metabolism of 7 -hydroxycholesterol- 14 C and 26-hydroxycholesterol- 3 H. J Clin Invest. 1972 Jan;51(1):112–117. doi: 10.1172/JCI106780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum M., Spritz N. The metabolism of intravenously injected isotopic cholic acid in Laennec's cirrhosis. J Clin Invest. 1966 Feb;45(2):187–193. doi: 10.1172/JCI105331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAREY J. B., Jr The serum trihydroxy-dihydroxy bile acid ratio in liver and biliary tract disease. J Clin Invest. 1958 Nov;37(11):1494–1503. doi: 10.1172/JCI103741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- OSBORN E. C., WOOTTON I. D., da SILVA L., SHERLOCK S. Serum-bile-acid levels in liver disease. Lancet. 1959 Dec 12;2(7111):1049–1053. doi: 10.1016/s0140-6736(59)91527-2. [DOI] [PubMed] [Google Scholar]
- RUDMAN D., KENDALL F. E. Bile acid content of human serum. I. Serum bile acids in patients with hepatic disease. J Clin Invest. 1957 Apr;36(4):530–537. doi: 10.1172/JCI103450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefer S., Hauser S., Bekersky I., Mosbach E. H. Biochemical site of regulation of bile acid biosynthesis in the rat. J Lipid Res. 1970 Sep;11(5):404–411. [PubMed] [Google Scholar]
- Shefer S., Hauser S., Mosbach E. H. 7-alpha-hydroxylation of cholestanol by rat liver microsomes. J Lipid Res. 1968 May;9(3):328–333. [PubMed] [Google Scholar]
- Theodor E., Spritz N., Sleisenger M. H. Metabolism of intravenously injected isotopic cholic acid in viral hepatitis. Gastroenterology. 1968 Aug;55(2):183–190. [PubMed] [Google Scholar]
- Turnberg L. A., Grahame G. Bile salt secretion in cirrhosis of the liver. Gut. 1970 Feb;11(2):126–133. doi: 10.1136/gut.11.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahcevic Z. R., Buhac I., Bell C. C., Jr, Swell L. Abnormal metabolism of secondary bile acids in patients with cirrhosis. Gut. 1970 May;11(5):420–422. doi: 10.1136/gut.11.5.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahcevic Z. R., Buhac I., Farrar J. T., Bell C. C., Jr, Swell L. Bile acid metabolism in patients with cirrhosis. I. Kinetic aspects of cholic acid metabolism. Gastroenterology. 1971 Apr;60(4):491–498. [PubMed] [Google Scholar]
- Vlahcevic Z. R., Juttijudata P., Bell C. C., Jr, Swell L. Bile acid metabolism in patients with cirrhosis. II. Cholic and chenodeoxycholic acid metabolism. Gastroenterology. 1972 Jun;62(6):1174–1181. [PubMed] [Google Scholar]
- Vlahcevic Z. R., Miller J. R., Farrar J. T., Swell L. Kinetics and pool size of primary bile acids in man. Gastroenterology. 1971 Jul;61(1):85–90. [PubMed] [Google Scholar]


