Abstract
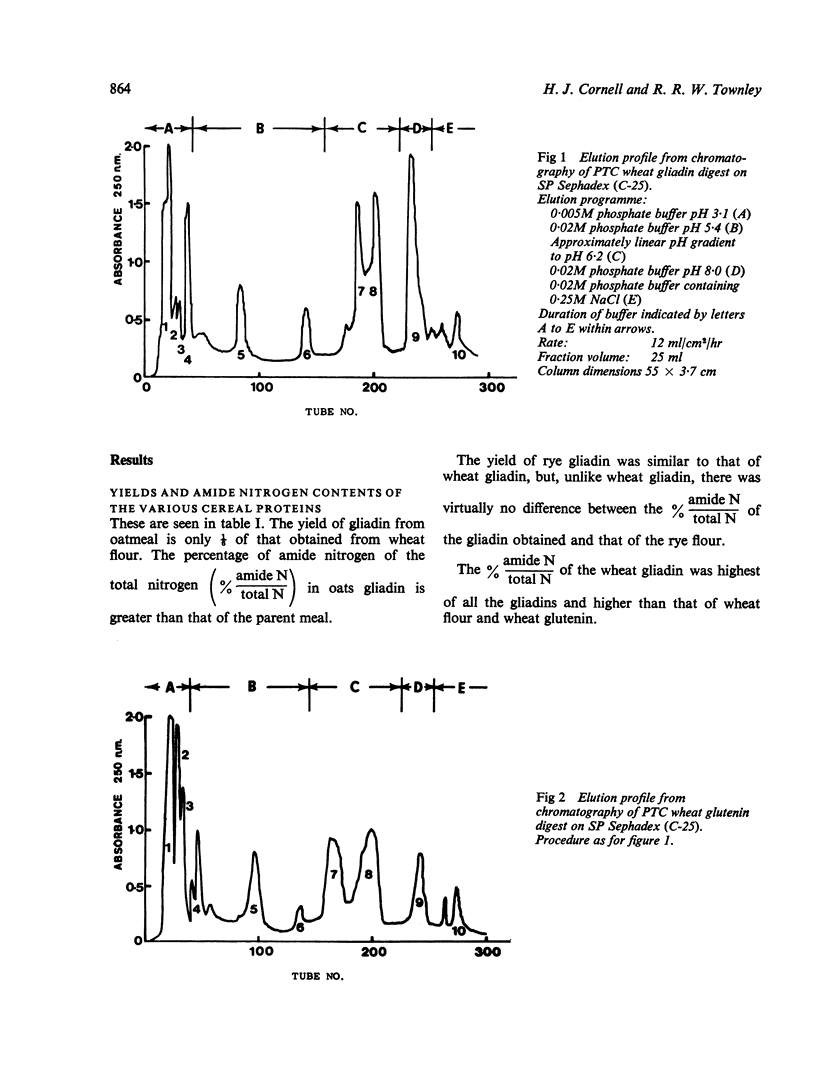
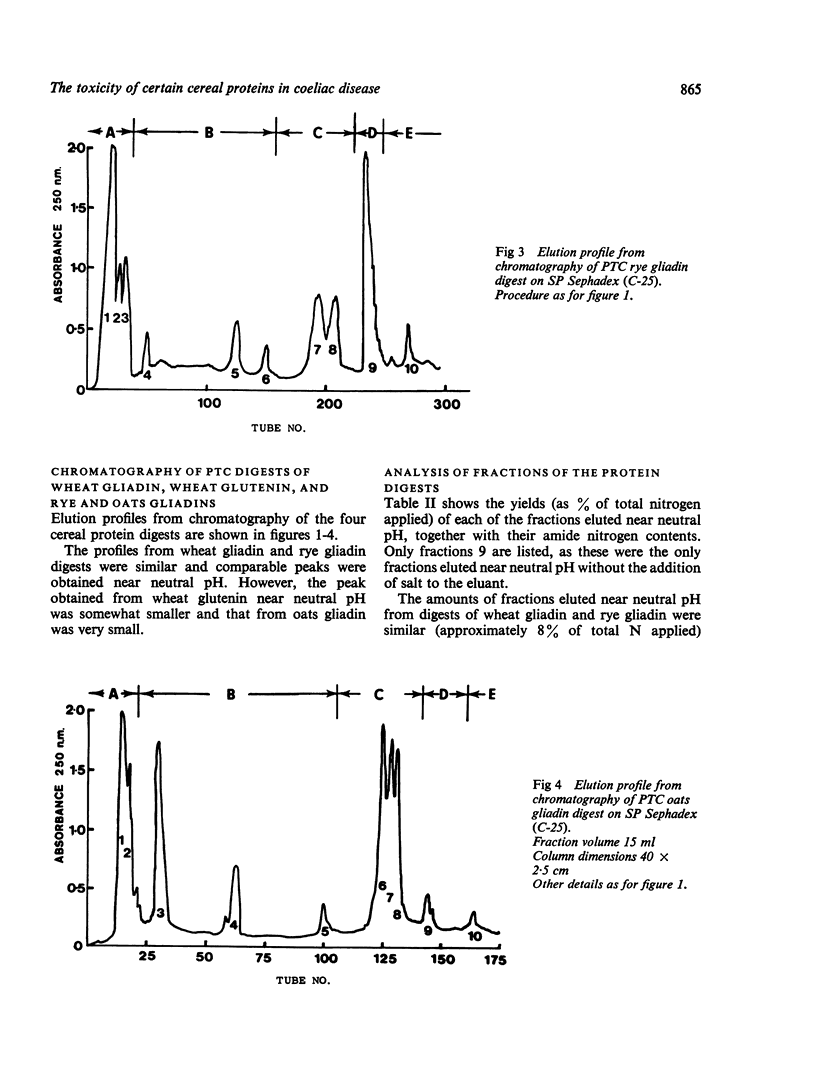
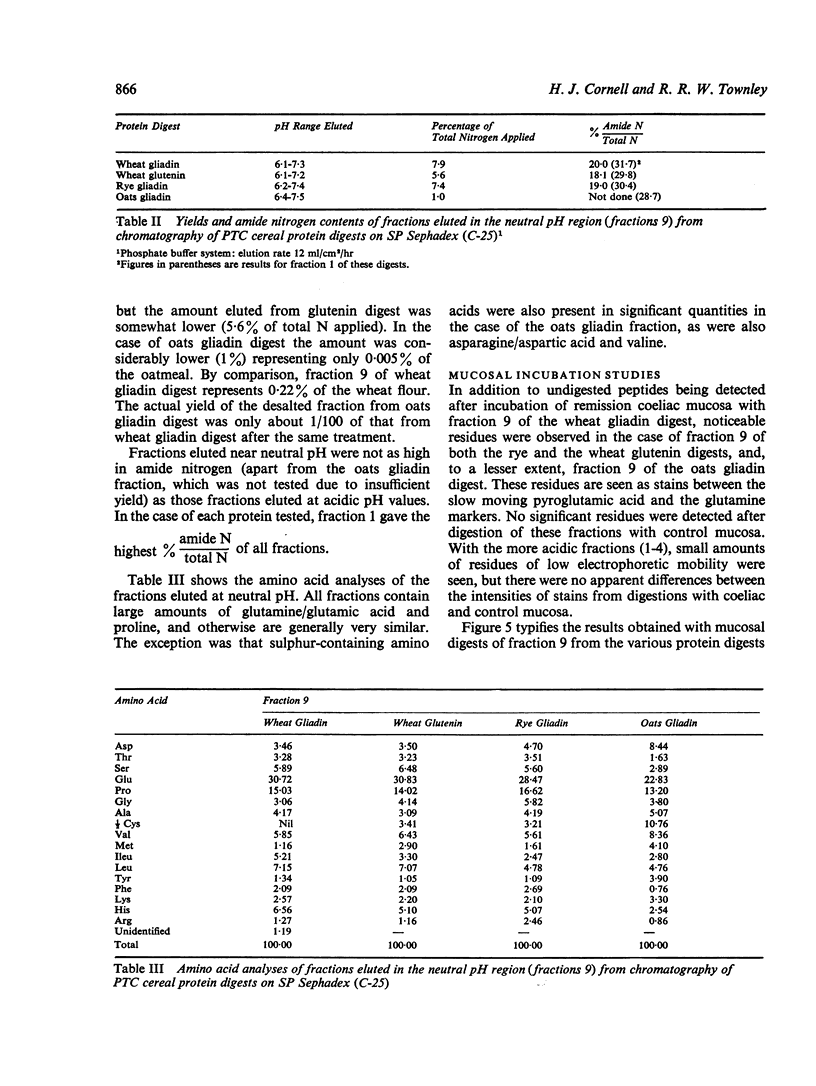
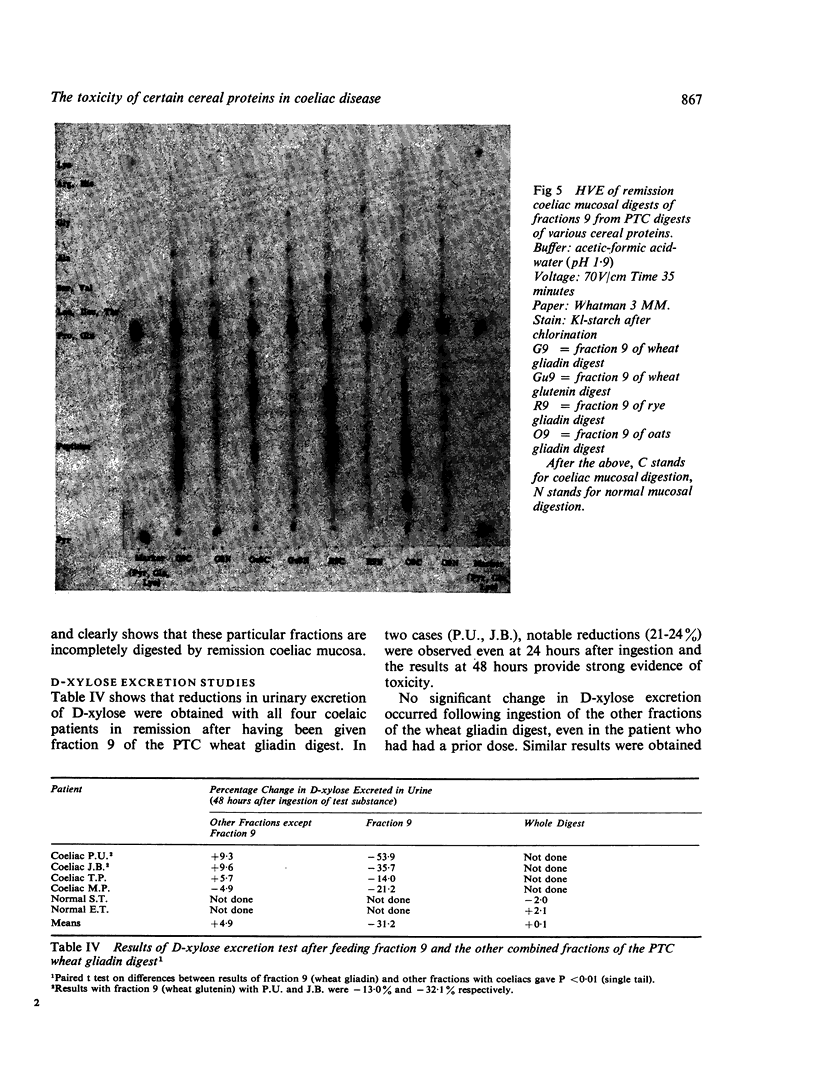
Gliadins from wheat, rye, and oats, and from wheat glutenin were digested with pepsin, trypsin, and pancreatin and the products (PTC digests) chromatographed on sulphopropyl (SP) Sephadex.
Fractions eluted near neutral pH from wheat, rye, and oats gliadin digests all had very similar amino acid composition, although the oats fraction was higher in sulphur-containing amino acids. The major amino acids present in all were glutamine/glutamic acid and proline.
The amount of fraction eluted near neutral pH from oats gliadin digest was about 13% of that eluted from digests of wheat and rye gliadins. Moreover, the yield of gliadin from oatmeal was only 0·5% compared with 2·4% and 2·8% from rye and wheat flours respectively.
The amount of fraction eluted from wheat glutenin digest was about 70% of that obtained from wheat and rye gliadin digests.
The fractions eluted near neutral pH from all protein digests were defectively digested by remission coeliac mucosa, and D-xylose excretion tests with the fraction from the wheat gliadin digest (fraction 9) indicated that it is harmful to subjects with coeliac disease, whereas the other fractions of this digest gave no such evidence.
The results of the present work indicate that counterpart fractions to fraction 9 obtained from wheat glutenin and rye and oats gliadins may also be important in the aetiology of coeliac disease.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bronstein H. D., Haeffner L. J., Kowlessar O. D. Enzymatic digestion of gliadin: the effect of the resultant peptides in adult celiac disease. Clin Chim Acta. 1966 Aug;14(2):141–155. doi: 10.1016/0009-8981(66)90080-5. [DOI] [PubMed] [Google Scholar]
- Cornell H. J., Townley R. R. Investigation of possible intestinal peptidase deficiency in coeliac disease. Clin Chim Acta. 1973 Jan 10;43(1):113–125. doi: 10.1016/0009-8981(73)90126-5. [DOI] [PubMed] [Google Scholar]
- FLETCHER R. F., McCRIRICK M. Y. Gluten-free diets. Br Med J. 1958 Aug 2;2(5091):299–301. doi: 10.1136/bmj.2.5091.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammons H. G., Morgan D. B., Frazer A. C., Montgomery R. D., Philip W. M., Phillips M. J. Modification in the xylose absorption test as an index of intestinal function. Gut. 1967 Aug;8(4):348–353. doi: 10.1136/gut.8.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townley R. R., Barnes G. L. Intestinal biopsy in childhood. Arch Dis Child. 1973 Jun;48(6):480–482. doi: 10.1136/adc.48.6.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townley R. R., Cornell H. J., Bhathal P. S., Mitchell J. D. Toxicity of wheat gliadin fractions in coeliac disease. Lancet. 1973 Jun 16;1(7816):1363–1364. doi: 10.1016/s0140-6736(73)91679-6. [DOI] [PubMed] [Google Scholar]
- VAN DE KAMER J. H., WEIJERS H. A. Coeliac disease. V. Some experiments on the cause of the harmful effect of wheat gliadin. Acta Paediatr. 1955 Sep;44(5):465–469. doi: 10.1111/j.1651-2227.1955.tb04269.x. [DOI] [PubMed] [Google Scholar]
- WEIJERS H. A., va de KAMER J. H. Some biochemical investigations into the cause of wheat sensitivity in celiac disease. Gastroenterology. 1960 Apr;38:587–591. [PubMed] [Google Scholar]
- Woodley J. F. Pyrrolidonecarboxylyl peptidase activity in normal intestinal biopsies and those from coeliac patients. Clin Chim Acta. 1972 Nov;42(1):211–213. doi: 10.1016/0009-8981(72)90400-7. [DOI] [PubMed] [Google Scholar]



