Summary
Invasive annual grasses introduced by European settlers have largely displaced native grassland vegetation in California and now form dense stands that constrain the establishment of native perennial bunchgrass seedlings. Bunchgrass seedlings face additional pressures from both livestock grazing and barley and cereal yellow dwarf viruses (B/CYDVs), which infect both young and established grasses throughout the state.
Previous work suggested that B/CYDVs could mediate apparent competition between invasive exotic grasses and native bunchgrasses in California.
To investigate the potential significance of virus-mediated mortality for early survivorship of bunchgrass seedlings, we compared the separate and combined effects of virus infection, competition and simulated grazing in a field experiment. We infected two species of young bunchgrasses that show different sensitivity to B/CYDV infection, subjected them to competition with three different densities of exotic annuals crossed with two clipping treatments, and monitored their growth and first-year survivorship.
Although virus infection alone did not reduce first-year survivorship, it halved the survivorship of bunchgrasses competing with exotics. Within an environment in which competition strongly reduces seedling survivorship (as in natural grasslands), virus infection therefore has the power to cause additional seedling mortality and alter patterns of establishment.
Surprisingly, clipping did not reduce bunchgrass survivorship further, but rather doubled it and disproportionately increased survivorship of infected bunchgrasses.
Together with previous work, these findings show that B/CYDVs can be potentially powerful elements influencing species interactions in natural grasslands.
More generally, our findings demonstrate the potential significance of multitrophic interactions in virus ecology. Although sometimes treated collectively as plant ‘predators’, viruses and herbivores may exert influences that are distinctly different, even counteracting.
Keywords: apparent competition, barley yellow dwarf virus, grazing, invasive species, pathogen
Introduction
European settlers introduced Avena spp. (wild and slender oats), Bromus spp. (bromes) and other annual grasses into California several hundred years ago. In a dramatic example of invasion success, these exotic grasses have almost completely displaced native grassland vegetation, including native perennial bunchgrasses (Clements 1934; Heady 1977; Bartolome et al. 1986; Hamilton 1997). Currently, bunchgrass communities comprise less than 0.1% of valley grassland area (Davis et al. 1998) [estimates for Great Central Valley Region], and most bunchgrass individuals must both compete with dense stands of exotic annuals and contend with grazing by cattle and sheep.
Competition for water and light can be intense, with smaller bunchgrasses succumbing to pressure from the quicker growing annuals (Dyer & Rice 1997; Dyer & Rice 1999; Hamilton et al. 1999), and bunchgrass populations are often recruitment limited (Dyer & Rice 1997; Hamilton et al. 1999; Seabloom et al. 2003). However, the capacity of the exotics to intensify resource competition does not fully explain their success and current dominance (Seabloom et al. 2003; Corbin & D’Antonio 2004) and factors such as changes in disturbance patterns may have played influential roles in directing the trajectories of native and exotic species.
Viruses may be one such factor shaping grassland dynamics (Malmstrom 1998; Malmstrom et al. 2005a, b). Although a group of generalist aphid-vectored viruses called the barley and cereal yellow dwarf viruses (B/CYDVs; Luteoviridae) have been widespread in California grasses and cereals (Griesbach et al. 1990a; Griesbach et al. 1990b) since 1951 or earlier (Oswald & Houston 1951), they have been largely overlooked in previous studies of grassland ecology. We have established experimentally that BYDV collected from California grasslands can cause notable stunting and loss of fecundity over the short term, and mortality over the long term (several years), in a broad range of native bunchgrasses, in the absence of other environmental constraints (Malmstrom et al. 2005a). We observed similar fecundity loss in established bunchgrass populations with naturally acquired B/CYDV infection (Malmstrom et al. 2005a) and substantial infection-associated mortality in commercial bunchgrass seed production fields (C.M.M., pers. observ.). In addition, we found that exotic annuals such as Avena and Bromus can attract and enhance the fecundity of the aphid vectors for B/CYDVs and substantially increase virus incidence in young bunchgrasses nearby (Malmstrom et al. 2005b). Together, these findings suggest that B/CYDVs could influence grassland dynamics by mediating apparent competition between native and exotic species. In apparent competition, the presence of one host species alters the influence of a shared pathogen (or predator) on a second species and thus indirectly leads to the second species’ decline (Holt 1977).
In general, B/CYDV pressure in California appears to be strong and widespread, with some spatial and temporal variation (Oswald & Houston 1951; Griesbach et al. 1990a; Griesbach et al. 1990b), as is typical with B/CYDVs world-wide (Irwin & Thresh 1990). Large aphid flights (Pike et al. 1989) exert broad synoptic virus pressure, while local movement of aphids and virus among neighbouring plants contributes additional variability. B/CYDV incidence can be high: infection rates among young bunchgrasses were found to exceed 70% after only 2 years of exposure to natural virus pressure (Malmstrom et al. 2005b). In established natural populations, rings of infected seedlings can sometimes be found around older, infected bunchgrasses (C.M.M., unpubl. data), suggesting that established plants might serve as reservoirs that increase virus pressure on nearby seedlings.
Given the widespread virus pressure in California, and the likelihood that seedlings experience significant exposure in some situations, the goal of this study was to compare the effects of virus infection on bunchgrass seedling with those of two other factors (interspecific competition and grazing) that are recognized as influential in grasslands. To capture the range in variability of bunchgrass response, we infected young individuals of two bunchgrasses species that show different sensitivity to B/CYDV infection (Malmstrom et al. 2005a), subjected them to competition with three different densities (0, low, high) of exotic annuals (Bromus) and to two clipping treatments (+, –) at each density, and monitored their first-year growth and survivorship in a large field experiment. In addition, because the survivorship of bunchgrass seedlings competing with exotics can be strongly influenced by water and light availability (Dyer & Rice 1999; Hamilton et al. 1999), we quantified Bromus’ influence on environmental resources by measuring soil moisture and canopy properties in subplots. We focused on first-year responses to treatments, because bunchgrass populations are often recruitment limited and bunchgrass individuals are particularly vulnerable to competition when they are small (Dyer & Rice 1997; Hamilton et al. 1999; Seabloom et al. 2003). We predicted that infected individuals would survive less well in competitive situations and regrow more poorly after grazing, because B/CYDV infection causes phloem limitations that impair carbohydrate transport (Esau 1957; Irwin & Thresh 1990) and stunt bunchgrasses (Malmstrom et al. 2005a). We thus expected that survivorship would be lowest when bunchgrasses had to contend simultaneously with infection, competition and grazing.
Materials and methods
Overview
Site
Because government regulations limit the release and manipulation of viruses outdoors, this work was conducted in a secure field facility at the University of California Davis Plant Pathology Research Facility in Yolo County in the middle of California's Central Valley (38.5° N, 121.8° W). The landscape in this region was once dominated by wild grasslands and wetlands but now largely comprises commercial agricultural fields and rangelands alongside expanding urban centres. Remnant bunchgrass populations are found scattered throughout the region, including in the drainage areas adjacent to the research facility. Over the last 10 years, restoration programs have re-established more than 100 hectares of additional bunchgrass populations in agricultural fields and rangelands in the western part of the county (http://science.calwater.ca.gov/pdf/SIA_grasslands_063005.pdf). We conducted our experiment in a 0.3-hectare research field that had been fallowed (left bare) the previous year. The soils in the field were Yolo Loam and Yolo Fine Sandy Loam (mixed, nonacid, thermic Typic Xerorthents).
The climate in Yolo County is mediterranean and annual precipitation averages 44 cm (Davis 1 WSW Western Regional Climate Center). Cool-season grasses, such as those studied here, generally initiate new growth with the onset of the rainy season in October–November and grow throughout the winter and spring. The exotic annual species typically senesce in May as the rains end and the hot summer dry period begins, while native bunchgrasses may remain green for weeks longer.
Experimental design
We designed the experiment to simulate natural grasslands as much as possible while quantifying three-way interactions between virus infection, competition and simulated livestock grazing on bunchgrass survivorship. Our primary aim was to examine the significance of virus infection on bunchgrasses competing with exotics, with and without grazing, because these are the conditions most bunchgrasses currently face in invaded grasslands.
The experimental field was established in a split-plot design with 60 long rectangular plots (1 × 15 m beds) each subdivided into 12 subplots (1 × 1.25 m). The plots were laid out in two immediately adjacent 15-m wide columns of 30 plots each. Within each column, plots were separated from their neighbours by 0.5-m furrows. Virus infection was the whole-plot treatment, with 30 beds randomly designated for inoculated plants (V+) and 30 for mock-inoculated ones (V–). Virus treatments were segregated by bed to reduce the chance of virus spread between plants when row covers were in place (see below). The 12 subplot treatments, which were assigned randomly among the subplots in each whole plot, represented the remaining three variables: bunchgrass species (two), density of exotic grasses (0, low, high) and clipping (+, –).
Bunchgrass species
To capture the range in variability of bunchgrass response, we chose two co-occurring bunchgrasses species –Nassella pulchra (A. Hitchc.) Barkworth (purple needlegrass) and Elymus multisetus (J. G. Smith) Burtt Davy (big squirreltail) − that were previously found to experience markedly different mortality rates when individuals were treated with virus in the absence of competition (Malmstrom et al. 2005a). The two species are of similar stature and co-occur throughout the California valley grasslands. Nassella pulchra is one of the most common native grasses remaining in invaded valley grasslands and the most frequently studied, whereas E. multisetus is less abundant, less studied and considerably more susceptible to virus-induced mortality (Malmstrom et al. 2005a). Seed was obtained from Hedgerow Farms, Winters (Yolo County), California, USA, where it was produced for use in Sacramento Valley restoration projects: N. pulchra seed was derived from a Yolo County population and E. multisetus from a Tehama County population (referred to as NPY and EMT in Malmstrom et al. 2005a). These two populations of the two species are frequently planted together in mixes and can grow well in the area, but also often acquire B/CYDV infection (C.M.M., unpubl. data).
Experimental Manipulations
Interspecific competition
To generate a competitive environment like that experienced by bunchgrass seedlings in invaded grasslands, we sowed Bromus hordeaceus L. (soft chess) in competition subplots at one of two rates − 5000 seeds m−2 (low-density Bromus, LB) or 28 000 seeds m−2 (high-density Bromus, HB) – and 2 days later planted a single 8-week-old bunchgrass seedling (previously virus-inoculated or mock-inoculated) in the centre of each subplot. To compare the effects of grazing and virus infection in the absence of competition, we also grew bunchgrasses in subplots without Bromus (0B). We chose B. hordeaceus because it is a dominant species in California grasslands and its stature allows it to grow without interfering with the row covers (see below). We used commercially available B. hordeaceus seed mix (S & S Seed, Carpenteria, CA, USA), which comprises a range of California genotypes, is widely planted throughout the state and has been used in previous work on the effects of BYDV-PAV infection (Malmstrom et al. 2005a). In the competition subplots, our aim was to replicate a common scenario for bunchgrass recruitment in contemporary grasslands: one tiny bunchgrass seedling trying to establish itself amidst thousands of exotics. Seeding rates corresponded to densities seen in natural grasslands (Biswell & Graham 1956; C.M.M., pers. observ.).
Virus infection
To infect bunchgrasses, we inoculated the V+ individuals with BYDV-PAV-PH2 (GenBank accession number AY879231) collected from wild Avena fatua growing adjacent to the NPY N. pulchra in Yolo County. This virus was used in our earlier competition/grazing-free experiment (Malmstrom et al. 2005a), and we used it again here to ensure that the results would be comparable. We chose BYDV-PAV as our experimental virus species because it is the most commonly reported B/CYDV in California (Griesbach et al. 1990a). Because little is known about the molecular diversity of B/CYDVs in natural grasslands, we initially selected the BYDV-PAV-PH2 isolate at random from a set of California isolates that we had collected from wild hosts; we did not pre-screen it for aggressiveness and we did not have any data on its effects on hosts. Phylogenetic analysis of its coat protein nucleotide sequence places it within Cluster II of the cpA group of BYDV-PAV (Bisnieks et al. 2004; C. M. Malmstrom et al., unpublished work). In both the earlier study and this one, we used only this virus because the logistics of working with several different viruses in a large outdoor experiment can become unmanageable.
Bunchgrass seedlings were started in 6.4-cm plug trays in October 2001, grown for 3 months in an aphid-free glasshouse until the two- to three-leaf stage, and then inoculated or mock-inoculated with BYDV-PAV-PH2 in December 2001. For inoculations (and mock-inoculations), 10 viruliferous aphids (or 10 non-viruliferous ones) were caged on each plant and allowed to feed for 3.5 days before being killed with pesticide. For the aphid, we used local Rhopalosiphum padi L. (the birdcherry-oat aphid), which we had collected from the same site as BYDV-PAV-PH2 and then maintained virus-free in our laboratory, as per Malmstrom et al. (2005a). Rhopalosiphum padi is one of the most common B/CYDV vectors in California (Pike et al. 1989) and is found on exotics and natives alike (Malmstrom et al. 2005b; C.M.M., pers. observ.). After inoculation, the seedlings were planted in the field (see above).
To control the introduction of virus from external sources and movement of virus within treatments after planting, we protected each bed with a 1-m wide, 15-m long tunnel of Agribon-19 row cover (Peaceful Valley Farm Supply, Grass Valley, CA, USA) supported on wire hoops (peak height c. 0.66 m) and stapled to the ground, as in previous work (Malmstrom et al. 2005a). Agribon-19 is a lightweight spun polypropylene fabric that permits light penetration and gas exchange but prevents aphids from alighting on plants. Row covers such as this are commonly used to protect high-value agricultural crops, as well as plant pathology experiments. Row covers were removed 19 February 2002, before the grasses grew tall enough to touch them or the weather warmed. Aphids were controlled thereafter with c. biweekly pesticides rotating between contact foliar insecticides (pyrethroids, applied at labelled rates) and two systemic pesticides (25% thiamethoxam at 1 oz active ingredient per acre (70 g ha−1): Actara 25WG, Syngenta Crop Protection, Inc., Greensboro, NC, USA, or 50% pymetrozine at 2.75 oz per acre (192.6 g ha−1): Fulfill, Syngenta).
Grazing
To simulate herbivory resulting from non-selective rotational grazing by sheep or cattle, one-half of the subplots (both competition subplots and those with bunchgrasses alone) were clipped with shears three times in spring, beginning immediately after row covers were removed. At each clipping, all plants (Bromus and bunchgrasses) were clipped to an equal height, because sheep or cattle could not preferentially select or avoid bunchgrasses that were this small. Plots were clipped to 5 cm on 21 February and on 12–14 March, and to 7.6 cm on 4 April. We raised clipping height slightly for the last treatment to prevent extreme removal of bunchgrass meristems, which became more elevated as bunchgrasses grew. Clippings were removed with a rake or vacuum.
Quantifying Bunchgrass Response
Bunchgrass survivorship
We quantified experiment-wide bunchgrass survivorship in early May, as Bromus was senescing, and in late June, towards the end of the bunchgrass growing season.
Bunchgrass growth
To facilitate interpretation of the survivorship data, we also compared the effects of virus infection and grazing on bunchgrass growth in the no-competition subplots, measuring bunchgrass height and basal circumference at the end of the growing season (in late June and early July), and harvesting, drying and weighing their above-ground biomass. We did not take a comparable suite of measurements in the competition subplots because bunchgrass seedlings there were entangled with the Bromus canopy and we did not want to risk altering survivorship patterns by disturbing the canopy. Instead, to make a general estimate of the effects of competition on plant size, we counted tillers on all bunchgrasses once, at the beginning of May. Substantial mortality had already occurred at this point in the competition subplots, so the tiller counts in those subplots were derived only from survivors and are thus biased.
Quantifying bromus response
Bromus survivorship
To quantify how Bromus survivorship in competition subplots was influenced by clipping and by intraspecific competition among Bromus, we compared seeding rates and Bromus densities on 4 March and 25 May in low- and high-density Bromus stands. Direct counts of germination rates and early seedling densities were precluded by the presence of row covers, but it was evident from visual inspection that initial seedling densities in high-density stands were substantially greater than in low-density ones. We measured Bromus density by removing and counting all Bromus individuals falling within a 6.6-cm diameter ring dropped in the north-east quadrant of each plot in all eight Bromus subplots of eight randomly chosen plots. We did not measure Bromus density in plots containing TDR rods (see below) to avoid altering soil moisture values.
Properties of Bromus canopies
To quantify properties of Bromus canopies influencing light availability for bunchgrasses in competition subplots, we measured Bromus height on 5 March and 26 April and leaf area index (LAI) on 27 March and 26 April in a subsample of Bromus subplots with a 0.8-m linear sun fleck ceptometer (Accupar, Decagon Devices, Pullman, WA, USA). We also measured the fraction of photosynthetically active radiation absorbed by the canopy (FPAR) with the same instrument on 26 April.
Quantifying changes in soil moisture
To assess whether effects of Bromus and clipping on bunchgrass survivorship might be related to changes in water availability, we used time domain reflectometry (TDR) to measure soil moisture (%) in the top 30 cm of soil (the zone most influenced by roots of exotic annuals (Holmes & Rice 1996)) in 60 Nassella subplots in the later part of the growing season after rain had stopped. We used a commercially available soil moisture system with a removable single diode probe head and 30-cm stainless steel rods (MoisturePoint Model MP-917 with SDR Type 13 probe and Type C rods, Environmental Sensors Inc., Victoria, British Columbia, Canada). We monitored soil moisture among all six Nassella subplots (two clipping treatments × three Bromus densities) within 12 randomly chosen non-virus plots. To make measurements, we sank a pair of rods 10 cm east of the bunchgrass in the middle of each stand in early April while the ground was still moist. Rods were left in place throughout the experiment. For measurements, the removable probe head was clipped onto each pair of rods. A full set of measurements was completed within one to two days. Beginning on 11 April, soil moisture was measured five times as precipitation ceased and soils dried. Only 1.8 cm of rain was recorded during the measurement period, 1.7 cm of which fell during an unusual (for the season) rain event on 20–21 May (NOAA Reference Climatological Station, Department of Land, Air and Water Resources, University of California, Davis, CA, USA).
Analysis
We used generalized estimating equation (GEE) in logistic regression with a type 3 model to analyse the binary survivorship data, using a split-plot mixed model with virus inoculation as a whole-plot effect, and species, grazing and Bromus density as subplot effects (PROC GENMOD in SAS Version 8.2, SAS Institute Inc., Cary, North Carolina, USA) (Stokes et al. 2000). To analyse continuous plant characteristics, we used a similar split-plot mixed model in PROC MIXED (Littell et al. 1996). In analysing Bromus characteristics and TDR measurements, we did not examine virus effects (because Bromus was not inoculated), and therefore used a randomized complete block mixed model with density and grazing as fixed effects and plot as a random effect (PROC MIXED).
Some degree of error in applying virus treatments in large experiments is common because of the nature of working with aphids (some plants may not be inoculated, for example, if an inoculation cage is disturbed during watering and the aphids escape). In analysing data sets containing values solely from subplots without Bromus (biomass, basal area, height and fecundity), we evaluated values only from individuals with appropriate infection status confirmed by RT-PCR analysis (Malmstrom & Shu 2004), which was conducted on c. 50 mg of foliar tissue harvested in March after row cover removal, i.e. when these plants were large enough to be sampled. Among Elymus, we used values from 92% of the inoculated and 87% of the mock-inoculated individuals, and among Nassella, from 74% and 97%, respectively. In data sets containing values from subplots with Bromus (survivorship and tiller counts), we used all values because we could not directly analyse bunchgrass virus status, since foliage sampling of these small plants would have influenced their survivorship, and clippings from clipping treatments were too macerated and muddy for reliable RNA extraction and analysis. However, inoculation rates in Bromus subplots can be assumed to approximate those in no-Bromus subplots because we planted the same bunchgrass batches in both. Thus, our estimates of virus effect on bunchgrass survivorship and tiller number are conservative (underestimated).
To facilitate comparisons of the magnitude of the experimental treatment effects, we calculated relative virus effect, relative competition effect and relative clipping effect in a manner analogous to that of relative yield reduction:
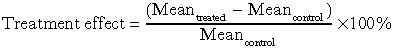 |
Results
Bunchgrass survivorship
The first-year survivorship of Elymus and Nassella seedlings did not differ significantly (species effect, P = 0.7520), contrary to our prediction. We thus removed the species term from our model, effectively combining data from the two species, which allowed us to explore other interactions further.
The only isolated treatment factor that substantially reduced first-year bunchgrass survivorship was competition with Bromus. Survivorship fell with increasing Bromus density (density effect, d.f. = 1, χ2 = 16.83, P < 0.0001). Neither virus infection nor clipping alone significantly reduced first-year survivorship (virus effect, d.f. = 1, χ2 = 0.00, P = 0.9928; clipping effect, d.f. = 1, χ2 = 1.01, P = 0.3142) (Fig. 1), nor did virus infection and clipping together, contradicting our prediction that these two factors in combination would intensify mortality.
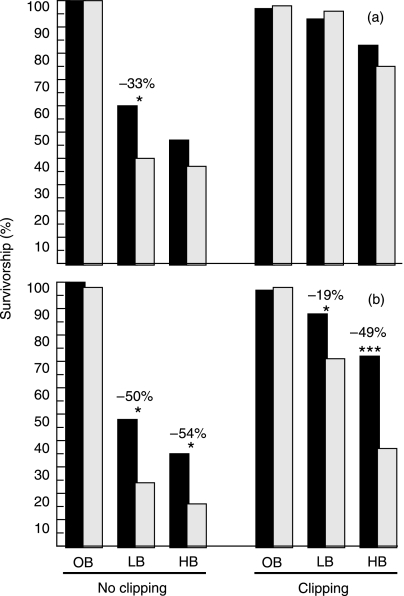
Fig. 1.
Influence of virus infection and clipping on survivorship of native bunchgrass seedlings, as a function of Bromus density. (a) Early May. (b) Late June. Black bars indicate mock-inoculated bunchgrasses, grey bars indicate virus-inoculated ones. LB and HB indicate low- and high-density Bromus subplots, respectively. Percentages above bars indicate relative virus effects (see Methods). *P < 0.05, ***P < 0.001.
The survivorship of bunchgrasses that experienced both competition with Bromus and virus infection was markedly different from that of bunchgrasses treated with a combination of competition and clipping. Virus infection intensified the consequences of competition and reduced bunchgrass survivorship by an additional c. 50% in unclipped Bromus stands of both densities (virus effect, d.f. = 1, χ2 = 15.57, P < 0.0001). The influence of infection became evident more slowly compared to the effect of competition and was most notable at the end of the season (Fig. 1).
In contrast, clipping markedly alleviated the negative influence of competition and increased the survivorship of all bunchgrasses (virus-treated and untreated) in Bromus stands by 50–300% (Fig. 1a: d.f. = 1, χ2 = 35.37, P < 0.0001; Fig. 1b: d.f. = 1, χ2 = 28.30, P < 0.0001). In low-density Bromus stands, clipping disproportionately increased the first-year survivorship of virus-treated bunchgrasses, eliminating the virus effect in May (Fig. 1a) and reducing its magnitude from −50% to −19% in June (Fig. 1b).
Size and fecundity of bunchgrass plants
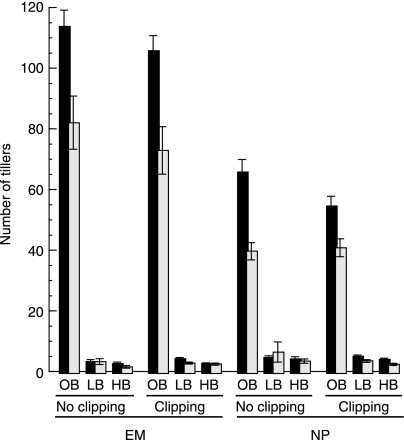
When treatment factors were considered in isolation, competition had the greatest influence on plant size as indicated by tiller counts (a size measure available from all treatments), followed by virus infection and then clipping (Fig. 2). Competition with Bromus reduced tiller counts by several orders of magnitude (density effect F2,447 = 881.40, P < 0.0001). Virus infection reduced tiller counts by 28–40% (P < 0.0001), a substantial but lesser amount. In contrast, clipping had little influence (Fig. 2) and caused only a marginal reduction in Nassella tiller number (Table 1).
Fig. 2.
Influence of interspecific competition on the size (mean ± SEM of survivors) of mock-inoculated (black bars) and virus-inoculated (grey bars), Elymus multisetus (EM) and Nassella pulchra (NP), as indicated by tiller number in May, with and without clipping. 0B indicates Bromus-free treatments. LB and HB indicate low- and high-density Bromus treatments, respectively.
Table 1.
Comparison of relative virus effects and relative clipping effects on properties of Elymus multisetus (EM) and Nassella pulchra (NP) gn without competition (see Figs 2 and 3). Individual effects listed are statistically significant at P < 0.05 level. M: marginal significance, 0.05 < P ≤ 0.10. NS: not significant. See text for discussion of interaction terms
| Virus effect (%) | Clipping effect (%) | ||||
|---|---|---|---|---|---|
| Species | On unclipped plants | On clipped plants | On uninfected plants | On infected plants | |
| Biomass | EM | −46 | −54 | NS | NS |
| NP | −61 | −53 | −31 | NS | |
| Basal area | EM | −37 | −47 | NS | NS |
| NP | −53 | −46 | −26 | NS | |
| Height | EM | −23 | −23 | NS | NS |
| NP | −26 | −22 | NS | NS | |
| Tiller no* | EM | −28 | −31 | NS | NS |
| NP | −40 | −25M | −17M | NS | |
| Seed mass | EM | −44 | −55 | NS† | −33M |
| NP | −50 | −47 | −26 | NS | |
Virus effect is underestimated for this measure because V+ treatment includes values from individuals that were not successfully inoculated (see Methods).
Overall grazing effect was significant at P = 0.0206.
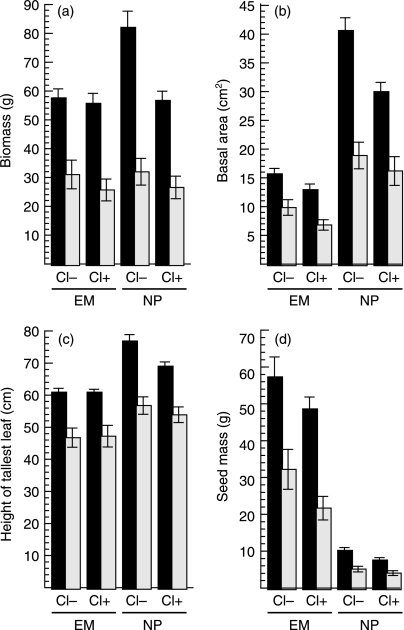
Further comparison of the influence of virus infection and clipping as single factors indicated that, in general, virus infection reduced plant size and fecundity markedly more than clipping. In both bunchgrass species, above-ground biomass production, basal area, height and seed mass were all significantly reduced by virus infection, whereas clipping significantly reduced the growth only of uninfected Nassella, and this by only about one-half as much as that caused by virus infection (Table 1, Fig. 3).
Fig. 3.
Influence of virus infection and clipping on Elymus multisetus (EM) and Nassella pulchra (NP) grown in the absence of competition (0B). Mean ± SEM for (a) above-ground biomass (minus seeds), (b) basal area, (c) height of tallest leaf, and (d) total seed mass. Black bars indicate mock-inoculated bunchgrasses, grey bars indicate virus-inoculated ones. Cl indicates clipping treatment. Statistical comparisons presented in Table 1.
We predicted that virus infection would limit bunchgrass regrowth after clipping, and virus × clipping-species interactions were evident in above-ground biomass (F1,128 = 4.30, P = 0.0402) (Fig. 3a) and basal area (F1,128 = 3.36, P = 0.0691) (Fig. 3b). However, the most negative influence of clipping was seen in uninfected Nassella, rather than in virus-treated individuals of either species. Clipped virus-infected bunchgrasses regrew sufficiently well that their biomass, basal area and heights were statistically indistinguishable from those of unclipped individuals. Overall, therefore, the relative size difference between Nassella and Elymus was reduced with clipping, so that the mean biomass of the two species became almost identical (Fig. 3a) and the influence of virus infection on the size of the two bunchgrasses became more similar when both species were clipped (Table 1).
Among surviving bunchgrasses in competition subplots, those in low-density stands produced c. 40% more tillers than those in high-density ones (P = 0.0057), and Nassella produced c. 50% more tillers than Elymus (P = 0.0030). While significant effects of clipping on bunchgrass size in these subplots were not discernible (P = 0.5091), surviving virus-treated Elymus produced c. 20% fewer tillers than untreated ones (P = 0.0533) (Fig. 2).
Influence of bromus on resource availability
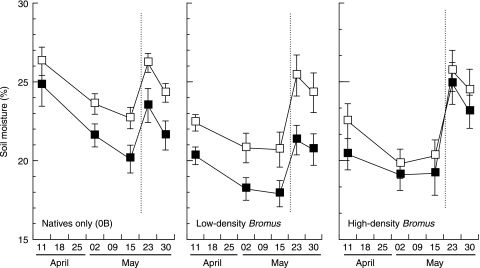
Unclipped Bromus stands were thick and lush in spring, with March LAI values of 6–7 falling to 4–5 in April when senescence and lodging (collapsing of stand structure) began. The LAI of unclipped low- and high-density stands was quite similar, with no difference evident between the stand types in March and only a 10% increase in high-density stands in April (P = 0.0334). Bromus stands reduced light availability substantially, with the fraction of PAR (FPAR) absorbed by the unclipped canopies approaching 90% in April. Bromus stands reduced soil moisture in the top 30-cm of soil by as much as 19% in unclipped plots, with a strong density-effect evident prior to a rain event towards the end of the measurements (P < 0.05 for density effect at first four dates) (Fig. 4).
Fig. 4.
Influence of Bromus and clipping on soil moisture (percentage) in the top 30-cm of soil, from 11 April to 30 May 2002. Closed symbols = without clipping, open symbols = with clipping. Little precipitation occurred after 23 March until a late storm contributed 1.7 cm on 20–21 May (dotted line). Values are mean ± SEM.
Clipping increased light and water availability in Bromus plots. These effects were persistent into April and May after clipping had stopped and Bromus had senesced. In April, clipped stands were c. 50% shorter and displayed 31–40% less leaf area and FPAR was reduced by c. 10%. Clipping increased overall April and May soil moisture values prior to the late storm (P < 0.05), with the effect most evident in low-density Bromus stands (Fig. 4). The additional resources could be utilized by surviving bunchgrasses, which remained green into July, while Bromus was completely senesced by 22 May.
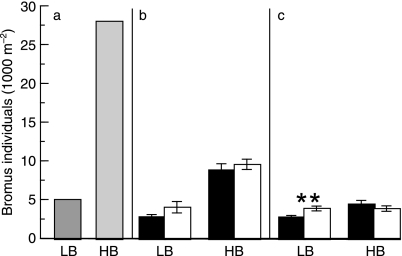
Clipping exerted its greatest influence on survivorship of Bromus in low-density stands, the same stands in which this factor had the greatest influence on soil moisture and bunchgrass survivorship. Bromus densities fell during the experiment because the stands self-thinned, as seen in natural grasslands (Biswell & Graham 1956), with the greatest mortality occurring in the high-density subplots (Fig. 5). Clipping increased Bromus survivorship in low-density stands by 41% (P = 0.0092), but it had no effect in high-density stands (P = 0.2122).
Fig. 5.
Influence of clipping on Bromus density within subplots seeded at low (LB) and high (HB) densities. Mean ± SEM (a) Initial seeding rate in December (no SEM calculated) Clipping and no-clipping subplots were sown at equal rates (b) Measured densities on 4 March. Black bars: no clipping White bars: with clipping. (c) Measured densities on 25 May Asterisks indicate significance of clipping effect: **P < 0.01.
Discussion
Survivorship of native bunchgrass seedlings in a competitive environment
Although often overlooked, pathogen-induced seedling mortality can have broad consequences for community structure, as indicated by work with pathogenic fungi (Augspurger 1983; Packer & Clay 2000). Like fungal pathogens, plant viruses are common in natural communities (MacClement & Richards 1956; Nienhaus & Castello 1989; Fraile et al. 1997; Raybould et al. 1999) and can induce mortality in young wild plants (Maskell et al. 1999; Yahara & Oyama 1993). The significance of virus-mediated seedling mortality in the context of that driven by other stressors is unknown but, in a given community, will depend on both the extent of infection and its consequences.
To quantify the significance of B/CYDV-mediated mortality and the potential for apparent competition in natural grasslands requires conservative, step-wise experimentation, in part because regulatory constraints limit the release and manipulation of viruses in natural settings. In this study, we extended the realism of past investigations by examining the consequences of virus infection for bunchgrass seedlings experiencing the additional stresses of interspecific competition and grazing, both of which are common in natural grasslands. We tested the hypothesis that survivorship would be lowest when bunchgrasses had simultaneously to contend with infection, competition and grazing, a prediction based on the idea that multiple stresses generally increase plant mortality. We were also implicitly testing the contrasting prediction that, in competitive settings, virus infection would exert little influence on survivorship. However unlikely the latter prediction may sound, it reflects a surprisingly persistent assumption that plant disease has minimal consequences in nature.
Our results demonstrate that, within an environment in which competition strongly reduces seedling survivorship (as in natural grasslands), virus infection further increases seedling mortality and alters patterns of seedling establishment. However, regular community-wide defoliation increased the survivorship of infected seedlings in a competitive environment, demonstrating that stressors can exert counteracting influences and suggesting that grazing may sometimes enhance the persistence of infected bunchgrasses.
Virus infection intensifies effects of competition
BYDV infection halved the first-year survivorship of bunchgrass seedlings competing with exotic annuals, but not that of seedlings growing alone. This result indicates both that infection exacerbates the effects of interspecific competition and that the consequences of virus infection become more severe in a competitive environment. Competition made BYDV infection a fatal disease for N. pulchra, a species not previously found to suffer virus-induced mortality (Malmstrom et al. 2005a), and it accelerated mortality of infected E. multisetus. With competition, both bunchgrasses experienced greater first-year mortality when infected (76–84%) than either experienced in 3 years without it (EMT: 59%; NPY: 5%), as reported previously (Malmstrom et al. 2005a). These findings are consistent with agricultural research indicating that most B/CYDV-related mortality in cereals occurs when infected plants experience a second stress (Haber 1995), but this has not previously been documented in wild hosts.
Bromus stands reduced survivorship of both infected and infected bunchgrasses (Fig. 1), most likely by reducing light levels and soil moisture (Fig. 4), as reported in previous competition studies (Dyer & Rice 1999; Hamilton et al. 1999). Infected individuals most likely experienced greater mortality because they were smaller and thus less able either to compete for light and water or to withstand resource shortages (Haber 1995).
Simulated grazing moderates effects of competition
In competition subplots without virus, simulated grazing increased survivorship of bunchgrasses (Fig. 1), most likely because defoliating and shortening Bromus canopies increased light and soil moisture available to bunchgrasses (Fig. 4), but had little direct influence on above-ground bunchgrass growth (Figs 2 and 3, Table 1). These findings are consistent with reports that grazing can increase soil moisture availability in grasslands (Bremer et al. 2001) and that established bunchgrass populations can tolerate some grazing (White 1967; Stromberg & Griffin 1996), although the extent of this tolerance remains controversial (Edwards 1992; Painter 1995). Our results are consistent with the experience of practising restoration ecologists in California that controlled livestock grazing can be an effective tool for reducing weed pressure and facilitating bunchgrass restoration (Stromberg & Kephart 1996; http://science.calwater.ca.gov/pdf/SIA_grasslands_063005.pdf). However, the finding that clipping more strongly reduced growth of N. pulchra than that of E. multisetus (Fig. 3, Table 1) suggests that grazing may also alter competitive relations between these two bunchgrasses.
Counteracting influences of clipping and virus infection
Based on the expectation that multiple stresses would increase plant mortality, we predicted that bunchgrass survivorship would be lowest when seedlings contended simultaneously with infection, competition and clipping. However, when infected bunchgrasses grew within competition plots that were subjected to clipping, both their absolute and relative survivorship increased (Fig. 1). This result may be explained in part by the finding that clipping had even less direct influence on the growth and size of infected plants than on that of uninfected ones (Fig. 3, Table 1), contradicting our prediction that physiological constraints imposed by virus infection would limit the capacity of infected plants to regrow. Several factors control plant regrowth capacity, including internal and external resource availability (Coughenour et al. 1985; Chapin & McNaughton 1989), and interactions with mycorrhizas and other soil fungi (Callaway et al. 2001; Kula et al. 2005). The influence of virus infection on these factors merits further investigation.
The disproportionate response of infected bunchgrasses to the relaxation of resource constraints brought about by clipping may have occurred in part because infected bunchgrasses were smaller than uninfected ones and closer to the threshold size for mortality. Soil moisture values (Fig. 4) and Bromus survivorship (Fig. 5c) both suggest that clipping increased resource availability most strongly in low-density Bromus stands, in which the disproportionate release of infected bunchgrasses was seen (Fig. 1). Structural differences between stand types may have given rise to this differential response. In low-density stands, individual Bromus were generally bigger, and the soil moisture data suggest that this may have allowed unclipped individuals to plumb the soil profile more effectively (Fig. 4). Following clipping, however, resource use may have been more strongly reduced because the more widely spaced individuals took longer to achieve canopy closure after defoliation (C.M.M., pers. observ.). Alternatively, crowding in high-density stands may have permanently altered the grasses’ growth trajectories (Biswell & Graham 1956) so that individuals were less able to respond to increased resource availability after clipping. An additional possibility is that infected bunchgrasses benefited more strongly from resource changes that improved their water and carbon status because of virus-induced constraints on their root growth and carbohydrate transport (Esau 1957; Kolb et al. 1991; Jensen & D’Arcy 1995), as has been found experimentally in oats (Malmstrom & Field 1997).
The observed effects of clipping suggest that, under some conditions, non-selective livestock grazing or mowing could enhance survivorship of infected bunchgrass seedlings and persistence of virus in grasslands. Such a differential response to general herbivory would thus have effects similar to preferential herbivory on a competitive dominant (Lubchenco 1978), i.e. an increased abundance of previously inferior competitors and a concomitant rise in diversity, in this case including that of infected and uninfected types. This prediction is supported by the observation that clipping increased the dominance of BYDV-infected Lolium perenne in simulated swards in Wales, UK (Catherall 1966). Longer-term feedbacks arising from the influence of grazing animals on nitrogen availability or soil structure might alter outcomes but were not considered here.
Implications for understanding natural grassland dynamics
Increasing evidence indicates that B/CYDVs are common in grasslands in many world regions (Lindsten & Gerhardson 1969; Guy et al. 1987; Garrett et al. 2004), but their role in shaping grassland dynamics is poorly understood. The effects of BYDV infection on seedling survivorship seen in this study demonstrate that infection can substantially augment mortality arising from resource competition or other interspecific interactions, and that it can do so almost invisibly because infection rarely can be detected by eye. The degree to which virus-mediated mortality or apparent competition has been erroneously ascribed to resource competition in past studies is thus unknown and merits significant attention. We recommend further investigation of patterns of infection in natural grasslands, including interactions among different species and life stages.
In invaded California grasslands, bunchgrass populations have often been found to be recruitment limited (Dyer & Rice 1997; Hamilton et al. 1999; Seabloom et al. 2003). Young bunchgrasses are particularly likely to die in their first few seasons, i.e. before they have developed sufficient stature and root system to compete effectively with the fast-growing exotic annuals for resources (Jackson & Roy 1986; Dyer & Rice 1997; Hamilton et al. 1999). Any factor that places additional constraints on seedling establishment in the first year or two is thus likely to have significant consequences for bunchgrass population dynamics. BYDV-PAV infection markedly reduced first-year survivorship of bunchgrass seedlings competing with exotic annuals, suggesting that in natural grasslands, where such competition is the norm, virus infection could exert a notable influence on bunchgrass establishment and population dynamics. Perennial bunchgrass populations may be more likely to decline under virus pressure than those of the invasive annual grasses because of differences in the population-level buffering provided by their stand structures and seed banks, as suggested previously (Malmstrom et al. 2005a).
Although limited to an experimental field, this study captured many of the critical elements of nearby grassland environments − including the same host populations, virus type and weather – and closely replicated the nature of local restoration projects. BYDV-PAV-PH2 exerted strong effects on hosts in experiments conducted in different years (Malmstrom et al. 2005a) and in different fields (cf. Malmstrom et al. 2005a, and this study), increasing our confidence that similar results would likely occur elsewhere. The two bunchgrass species represent opposite types in terms of the long-term mortality they experienced under the influence of inoculation alone, as well as in their current abundance in California; that both showed similar patterns of survivorship further suggests that our findings are likely to be broadly applicable. Because little is known about the molecular diversity of B/CYDVs in natural grasslands, we selected the experimental virus at random from among several BYDV-PAV isolates collected from wild hosts. To enhance understanding of the landscape-level influences of B/CYDVs, we thus recommend further investigation of the viruses’ molecular diversity and their geographical distribution among wild grasses.
Conclusions
Together with previous work, this study suggests that virus-mediated apparent competition has the power to significantly influence patterns of seedling establishment in California grasslands and thus influence interactions among native species and invasive exotics. More generally, our findings demonstrate the potential significance of multitrophic interactions in virus ecogy. Although sometimes treated collectively as plant ‘predators’, viruses and herbivores may exert influences that are distinctly different, even counteracting.
Acknowledgments
We are indebted to B. Falk, T. Kominek, the UC Davis Department of Plant Pathology, Hedgerow Farms, and Rana Creek Restoration for field facility use and technical assistance. We are grateful to Syngenta Crop Protection Inc. for its donation of pesticides and to B. Anderson, H. S. Butterfield, C. Distefano, C. Hoe, C. Hughes, P. Malmstrom, E. Malmstrom-Partridge, N. Milan, B. Morriss, N. Rashid, R. Shu and the rest of the team for their invaluable contributions in the lab and field. We thank A. Dyer, A. Jarosz, P. Hujik, K. Rice, M. Stromberg and J. Wirka for helpful discussions, and Z. Cardon, V. Eviner, T. Persson, A. Schrotenboer and three anonymous referees for valuable comments on the manuscript. This work was supported by the National Science Foundation (DEB 9983373) and the Michigan Agriculture Experiment Station. Experiments complied with the laws of the United States of America.
References
- Augspurger CK. Seed dispersal of the tropical tree, Platypodium elegans, and the escape of its seedlings from fungal pathogens. Journal of Ecology. 1983;71:759–771. [Google Scholar]
- Bartolome JW, Klukkert SE, Barry WJ. Opal phytoliths as evidence for displacement of native Californian grassland. Madroño. 1986;33:217–222. [Google Scholar]
- Bisnieks M, Kvarnheden A, Sigvald R, Valkonen JPT. Molecular diversity of the coat protein-encoding region of Barley yellow dwarf virus-PAV and Barley yellow dwarf virus-MAV from Latvia and Sweden. Archives of Virology. 2004;149:843–853. doi: 10.1007/s00705-003-0242-2. [DOI] [PubMed] [Google Scholar]
- Biswell HH, Graham CA. Plant counts and seed production on California annual-type ranges. Journal of Range Management. 1956;9:116–118. [Google Scholar]
- Bremer DJ, Auen LM, Ham JM, Owensby CE. Evapotranspiration in a prairie ecosystem: effects of grazing by cattle. Agronomy Journal. 2001;93:338–348. [Google Scholar]
- Callaway RM, Newingham B, Zabinski CA, Mahall BE. Compensatory growth and competitive ability of an invasive weed are enhanced by soil fungi and native neighbours. Ecology Letters. 2001;4:429–433. [Google Scholar]
- Catherall PL. Effects of barley yellow dwarf virus on the growth and yield of single plants and simulated swards of perennial rye-grass. Annals of Applied Biology. 1966;57:155–162. [Google Scholar]
- Chapin FS, III, McNaughton SJ. Lack of compensatory growth under phosphorus deficiency in grazing-adapted grasses from the Serengeti Plains. Oecologia. 1989;79:551–557. doi: 10.1007/BF00378674. [DOI] [PubMed] [Google Scholar]
- Clements FE. The relict method in dynamic ecology. Journal of Ecology. 1934;22:39–68. [Google Scholar]
- Corbin JD, D’Antonio CM. Competition between native perennial and exotic annual grasses: implications for an historical invasion. Ecology. 2004;85:1273–1283. [Google Scholar]
- Coughenour MB, McNaughton SJ, Wallace LL. Responses of an African graminoid (Themeda traindra Forsk.) to frequent defoliation, nitrogen, and water: a limit of adaptation to herbivory. Oikos. 1985;68:80–86. doi: 10.1007/BF00379481. [DOI] [PubMed] [Google Scholar]
- Davis FW, Stoms DM, Hollander AD, Thomas KA, Stine PA, Odion D, Borchert MI, Thorne JH, Grey MV, Walker RE, Warner K, Graae J. The California Gap Analysis Project – Final Report. Santa Barbara, CA: University of California; 1998. [ http://www.biogeog.ucsb.edu/projects/gap/gap_rep.html] [Google Scholar]
- Dyer AR, Rice KJ. Intraspecific and diffuse competition: the response of Nassella pulchra in a California grassland. Ecological Applications. 1997;7:484–492. [Google Scholar]
- Dyer AR, Rice KJ. Effects of competition on resource availability and growth of a California bunchgrass. Ecology. 1999;80:2697–2710. [Google Scholar]
- Edwards SW. Observations on the prehistory and ecology of grazing in California. Fremontia. 1992;20:3–11. [Google Scholar]
- Esau K. Phloem degeneration in Gramineae affected by the barley yellow-dwarf virus. American Journal of Botany. 1957;44:245–251. [Google Scholar]
- Fraile A, Escriu F, Aranda MA, Malpica JM, Gibbs AJ, García-Arenal F. A century of tobamovirus evolution in an Australian population of Nicotiana glauca. Journal of Virology. 1997;71:8316–8320. doi: 10.1128/jvi.71.11.8316-8320.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett KA, Dendy SP, Power AG, Blaisdell GK, Alexander HM, McCarron JK. Barley yellow dwarf disease in natural populations of dominant tallgrass prairie species in Kansas. Plant Disease. 2004;88:574. doi: 10.1094/PDIS.2004.88.5.574B. [DOI] [PubMed] [Google Scholar]
- Griesbach JA, Falk BW, Valverde RA. Incidence of barley yellow dwarf viruses in California cereals. Plant Disease. 1990a;74:111–114. [Google Scholar]
- Griesbach JA, Steffenson BJ, Brown MP, Falk BW, Webster RK. Infection of grasses by barley yellow dwarf viruses in California. Crop Science. 1990b;30:1173–1177. [Google Scholar]
- Guy PL, Johnstone GR, Morris DI. Barley yellow dwarf virus in, and aphids on, grasses (including cereals) in Tasmania. Australian Journal of Agricultural Research. 1987;38:139–152. [Google Scholar]
- Haber S. Interactions of barley yellow dwarf viruses: cross-protection with other pathogens and interactions with other pathogens and abiotic factors. In: D’ Arcy CJ, Burnett PA, editors. Barley Yellow Dwarf: 40 Years of Progress. St. Paul, Minnesota: The American Phytopathological Society; 1995. pp. 145–164. [Google Scholar]
- Hamilton JG. Changing perceptions of pre-European grasslands in California. Madroño. 1997;44:311–333. [Google Scholar]
- Hamilton JG, Holzapfel C, Mahall BE. Coexistence and interference between a native perennial grass and non-native annual grasses in California. Oecologia. 1999;121:518–526. doi: 10.1007/s004420050958. [DOI] [PubMed] [Google Scholar]
- Heady HF. Valley grassland. In: Barbour MG, Major J, editors. Terrestrial Vegetation of California. New York: John WileySons; 1977. pp. 491–514. [Google Scholar]
- Holmes TH, Rice KJ. Patterns of growth and soil-water utilization in some exotic annuals and native perennial bunchgrasses of California. Annals of Botany. 1996;78:233–243. [Google Scholar]
- Holt RD. Predation, apparent competition, and the structure of prey communities. Theoretical Population Biology. 1977;12:197–229. doi: 10.1016/0040-5809(77)90042-9. [DOI] [PubMed] [Google Scholar]
- Irwin ME, Thresh JM. Epidemiology of barley yellow dwarf: a study in ecological complexity. Annual Review of Phytopathology. 1990;28:393–424. [Google Scholar]
- Jackson LE, Roy J. Growth patterns of Mediterranean annual and perennial grasses under simulated rainfall regimes of southern France and California. Acta Oecologia. 1986;7:191–212. [Google Scholar]
- Jensen SG, D’Arcy CJ. Effects of barley yellow dwarf on host plants. In: D’Arcy CJ, Burnett PA, editors. Barley Yellow Dwarf: 40 Years of Progress. St. Paul, Minnesota: The American Phytopathological Society; 1995. pp. 55–74. [Google Scholar]
- Kolb FL, Cooper NK, Hewings AD, Bauske FM, Teyker RH. Effects of barley yellow dwarf virus on root growth in spring oat. Plant Disease. 1991;75:143–145. [Google Scholar]
- Kula AAR, Hartnett DC, Wilson GWT. Effects of mycorrhizal symbiosis on tallgrass prairie plant–herbivore interactions. Ecology Letters. 2005;8:61–69. [Google Scholar]
- Lindsten K, Gerhardson B. Investigations on barley yellow dwarf virus (BYDV) in leys in Sweden. Meddelanden. 1969;14:261–280. [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS System for Mixed Models. Cary, North Carolina: SAS Institute Inc; 1996. [Google Scholar]
- Lubchenco J. Plant species diversity in a marine intertidal community: importance of herbivore food preference and algal competitive abilities. American Naturalist. 1978;112:23–39. [Google Scholar]
- MacClement WD, Richards MG. Virus in wild plants. Canadian Journal of Botany. 1956;34:793–799. [Google Scholar]
- Malmstrom CM. Barley yellow dwarf virus in native California grasses. Grasslands. 1998;8(1):6–10. [Google Scholar]
- Malmstrom CM, Field CB. Virus-induced differences in the response of oat plants to elevated carbon dioxide. Plant, Cell, and Environment. 1997;20:178–188. [Google Scholar]
- Malmstrom CM, Hughes CC, Newton LA, Stoner CJ. Virus infection in remnant native bunchgrasses from invaded California grasslands. New Phytologist. 2005a;168:217–230. doi: 10.1111/j.1469-8137.2005.01479.x. [DOI] [PubMed] [Google Scholar]
- Malmstrom CM, McCullough AJ, Newton LA, Johnson HA, Borer ET. Invasive annual grasses indirectly increase virus incidence in California native perennial bunchgrasses. Oecologia. 2005b;145:153–164. doi: 10.1007/s00442-005-0099-z. [DOI] [PubMed] [Google Scholar]
- Malmstrom CM, Shu R. Multiplexed RT-PCR for streamlined detection and separation of barley and cereal yellow dwarf viruses. Journal of Virological Methods. 2004;120:69–78. doi: 10.1016/j.jviromet.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Maskell LC, Raybould AF, Cooper JI, Edwards M-L, Gray AJ. Effects of turnip mosaic virus and turnip yellow mosaic virus on the survival, growth and reproduction of wild cabbage (Brassica oleracea) Annals of Applied Biology. 1999;135:401–407. [Google Scholar]
- Nienhaus F, Castello JD. Viruses in forest trees. Annual Review of Phytopathology. 1989;27:165–186. [Google Scholar]
- Oswald JW, Houston BR. A new virus disease of cereals, transmissible by aphids. Plant Disease Reporter. 1951;35:471–475. [Google Scholar]
- Packer A, Clay K. Soil pathogens and spatial patterns of seedling mortality in a temperate tree. Nature. 2000;404:278–281. doi: 10.1038/35005072. [DOI] [PubMed] [Google Scholar]
- Painter EL. Threats to the California flora: ungulate grazers and browsers. Madroño. 1995;42:180–188. [Google Scholar]
- Pike KS, Allison D, Boydston L, Qualset CO, Vogt HE, Summers CG. Suction trap reveals 60 wheat aphid species, including Russian wheat aphid. California Agriculture. 1989;43:22–24. [Google Scholar]
- Raybould AF, Maskell LC, Edwards M-L, Cooper JI, Gray AJ. The prevalence and spatial distribution of viruses in natural populations of Brassica oleracea. New Phytologist. 1999;141:265–275. doi: 10.1046/j.1469-8137.1999.00339.x. [DOI] [PubMed] [Google Scholar]
- Seabloom EW, Harpole WS, Reichman OJ, Tilman D. Invasion, competitive dominance, and resource use by exotic and native California grassland species. Proceedings of the National Academy of Science USA. 2003;100:13384–13389. doi: 10.1073/pnas.1835728100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes ME, Davis CS, Koch GG. Categorical Data Analysis Using the SAS System. Cary, North Carolina: SAS Institute Inc; 2000. [Google Scholar]
- Stromberg MR, Griffin JR. Long-term patterns in coastal California grasslands in relation to cultivation, gophers, and grazing. Ecological Applications. 1996;6:1189–1211. [Google Scholar]
- Stromberg MR, Kephart P. Restoring native grasses in California old fields. Restoration and Management Notes. 1996;14:102–111. [Google Scholar]
- White KL. Native bunchgrass (Stipa pulchra) on Hastings Reservation, California. Ecology. 1967;48:949–955. [Google Scholar]
- Yahara T, Oyama K. Effects of virus infection on demographic traits of an agamospermous population of Eupatorium chinense (Asteraceae) Oecologia. 1993;96:310–315. doi: 10.1007/BF00317499. [DOI] [PubMed] [Google Scholar]