Abstract
Acute anterior spinal cord ischemia is a rare but disastrous complication of endovascular aortic procedures. Although intravenous thrombolysis with recombinant tissue plasminogen activator is an effective treatment for acute brain ischemia, its use for the treatment of spinal cord ischemia has not previously been reported. We report the case of a patient who developed anterior spinal cord ischemia during diagnostic aortography. He was treated with intravenous recombinant tissue plasminogen activator within 3 hours after the onset of symptoms. The patient had a rapid neurologic improvement and was discharged from the hospital 3 days after thrombolysis, regaining his ability to walk unassisted. We propose that acute spinal cord ischemia can be treated with intravenous recombinant tissue plasminogen activator within 3 hours after the onset of symptoms, as can any other case of acute ischemic stroke.
Keywords: Anterior spinal artery, aortography/ complications, endovascular procedures/complications, spinal cord/blood supply, spinal cord ischemia/diagnosis/complications/therapy, ischemic stroke, thrombolysis, recombinant tissue plasminogen activator/therapeutic use
Acute anterior spinal cord ischemia is a rare condition associated with poor outcomes.1-3 Variable degrees of paraparesis and bladder dysfunction are expected in most patients. Although intravenous thrombolysis is the treatment of choice for acute brain ischemia (if given within 3 hours after the onset of symptoms), it is not known whether spinal cord ischemia may also respond to this therapy. We report a case of acute anterior spinal cord ischemia treated with intravenous recombinant tissue plasminogen activator (rt-PA).
Case Report
In January 2004, a 71-year-old man with a history of severe systemic atherosclerosis (coronary artery disease, bilateral carotid artery stenosis, left renal artery stenosis, and peripheral vascular occlusive disease), underwent diagnostic aortography for the evaluation of a fusiform aneurysm of the abdominal aorta (largest diameter, 5.5 × 5.7 cm on abdominal computed tomography [CT]). At the end of the procedure, the patient began to experience bilateral leg weakness, urinary tenesmus, and low back pain. He had no sensory changes involving the legs, or other focal neurologic symptoms.
On physical examination, the patient's blood pressure was 140/80 mmHg and his heart rate, 60 beats/min. The patient was awake and in mild distress due to low back pain. No unusual results were found on cardiopulmonary examination. He had a solid, pulsatile, non-tender mass in the lower abdomen and substantial bladder distention (about 500 cc of urine was evacuated after Foley catheter placement). The patient had mild acrocyanosis involving the toes of both feet, more on the left side. Pulses (before and after the procedure) were as follows: femoral, 3+ bilaterally; popliteal, 1+ bilaterally; posterior tibialis, 0 bilaterally; and dorsalis pedis, 0 in the left leg and 2+ in the right leg. On neurologic examination, the patient was alert and oriented to time, place, and person. His speech was fluent. He was able to follow complex commands without difficulty. Motor function was normal in the upper extremities. However, muscle power in the lower extremities was as follows: iliopsoas, 3/5 bilaterally; quadriceps, 4/5 bilaterally; hamstrings, 4/5 on the right side and 3/5 on the left side; anterior tibialis, 3/5 on the right side and 2/5 on the left side; and adductors and abductors, 4/5 bilaterally. There was a lack of deep-tendon reflexes in both legs, whereas reflexes were 1+ in the arms. The plantar responses were mute bilaterally. The muscle tone was flaccid in the lower extremities but normal in the upper extremities. Results of the sensory examination were normal. His NIH stroke scale score was 6.
Computed tomographic scanning of the head showed symmetric cortical atrophy, with evidence of bilateral leukomalacia involving both frontal lobes within the watershed distribution, more-so on the left side. The CT of the lumbar spine showed mild osteoarthritis, particularly at L4–L5, without canal stenosis or bony destructive lesions. Results of complete blood cell count and other laboratory tests were within normal limits. The prothrombin and thromboplastin times were normal. The aortogram (Fig. 1) showed an aneurysm that involved a substantial segment of the supra- and infrarenal aorta and both iliac arteries. There was angiographic evidence of mural thrombus in the aorta, but no dissection was noted.
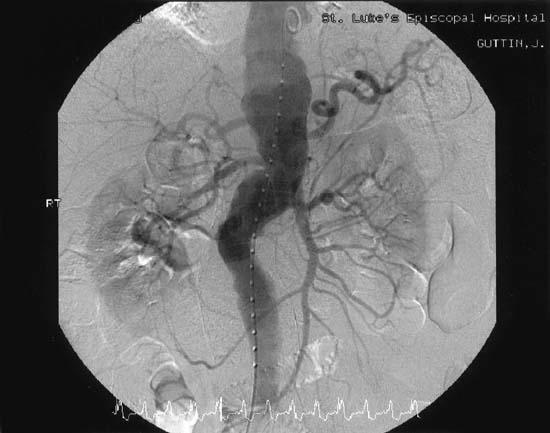
Fig. 1 The aortogram shows an aneurysm involving a substantial segment of the supra- and infrarenal aorta and both iliac arteries.
The femoral artery sheath was removed and manually compressed. An intravenous bolus of rt-PA was injected 110 minutes after the onset of symptoms, and 0.9 mg/kg was infused over the next 60 minutes. At the end of the rt-PA infusion, the patient became hypotensive (blood pressure, 80/40 mmHg) and diaphoretic, with lower extremity cramps and worsening of the low back pain. The neurologic examination revealed worsening of the flaccid paraparesis, accompanied by fine fasciculations involving the quadriceps. No dyspnea, stridor, or rash occurred. The administration of intravenous normal saline and phenylephrine improved the blood pressure within 30 minutes. Analgesia was achieved with intravenous morphine sulfate. The hemoglobin and hematocrit levels remained unchanged. Urgent CT of the abdomen ruled out retroperitoneal bleeding. Plasma creatinine phosphokinase and troponin I levels remained normal. The blood pressure stabilized 1 hour later, with fluctuation of the leg strength and remission of the leg fasciculations. The intravenous phenylephrine and fluids were subsequently discontinued. The patient was given 80 mg of atorvastatin by mouth. He was transferred to the intensive care unit for close monitoring, without new events. Antihypertensive drugs were avoided.
Twenty-four hours after intravenous thrombolysis, the patient was able to stand with assistance and no longer complained of low back pain. He could urinate without difficulty after the Foley catheter was removed. The muscle strength was normal distally and 4/5 proximally, with the weakness more prominent on the left side; the muscle tone appeared normal. Deep tendon reflexes and plantar response were absent. The distal acrocyanosis improved, but the leg pulses remained unchanged. A 300-mg loading dose of clopidogrel and 325 mg of aspirin were administered.
Forty-eight hours after symptom onset, the patient was able to stand and walk without assistance. Muscle power was 5/5 except for the left iliopsoas and hamstrings, which were 4/5. The muscle tone increased slightly in both legs. The deep tendon reflexes were 3+ symmetrically. He had a prominent bilateral Babinski sign. The serum creatinine level remained stable throughout the hospitalization, and there was no hematuria shown on urinalysis. The patient was discharged from the hospital 72 hours after symptom onset, taking clopidogrel, aspirin, and atorvastatin. The National Institutes of Health (NIH) stroke scale score was 1 (mild left leg drift), and the Rankin score was 0 upon discharge. One year after discharge, he remained asymptomatic and able to walk.
Discussion
We have described a case of anterior spinal artery ischemia treated with intravenous thrombolysis. To our knowledge, this is the 1st report of spinal cord ischemia treated with thrombolytic agents. Although we do not have specific imaging or neurophysiologic confirmation (evoked potentials) of acute ischemic myelopathy, the evolving neurologic findings, and the coincidence of the aortography and the onset of symptoms, strongly suggest the occurrence of acute ischemia of the anterior spinal cord circulation during the procedure. Unfortunately, magnetic resonance imaging, which is arguably the method of choice for imaging the spinal cord,4 could not be performed because the patient had a pacemaker. Indeed, the use of magnetic resonance imaging in the setting of acute spinal cord ischemia would have been extremely informative. In particular, diffusion-weighted imaging sequences can disclose evidence of acute spinal cord ischemia 4 hours after the onset of symptoms.5
Nevertheless, the physical examination provided key insights into the pathophysiology of patient's neurologic dysfunction. Initially, the neurologic exam suggested acute spinal shock with flaccid weakness, urinary retention, and absence of reflexes in the legs. Subsequently, the motor recovery was accompanied by signs of upper motor neuron dysfunction, again limited to the lower extremities. Such a sequence of physical signs strongly suggests an acute myelopathy. Furthermore, the striking preservation of lower extremity sensory function is classically associated with anterior spinal cord dysfunction. We postulate that the hypotension and muscle fasciculations coinciding with the end of the rt-PA infusion may have been another manifestation of spinal shock (such as dysautonomia) or perhaps a reperfusion syndrome. Local microbleeding within the spinal cord seems unlikely, given the rapid neurologic improvement.
Thrombolysis is considered the only specific therapy for acute brain ischemia. However, the use of rt-PA is limited by its narrow therapeutic window of 3 hours, as well as several contraindications.6 One of these contraindications is aortic dissection, although this particular entity was not one of the explicit exclusion criteria of the National Institutes of Neurological Diseases and Stroke rt-PA trial.6,7 Before the administration of thrombolysis to our patient, the angiogram was carefully reviewed to rule out dissection. In addition, at the end of the rt-PA infusion, CT of the abdomen was performed to ascertain that no abdominal bleeding had occurred.
Other important aspects of patient care include the aggressive treatment of hypotension in an intensive-care setting and subsequent use of dual antiplatelet therapy and a high-dose statin, which probably contributed to the documented good outcome in our patient. To date, there are no specific therapeutic regimens for spinal cord infarction.8 Our patient did not receive steroids, which is a therapy that has been proposed for spinal cord ischemia on the grounds of a randomized controlled trial of methylprednisolone for spinal cord trauma.9 We decided against steroid therapy because of a lack of evidence showing the benefit of steroids in the specific context of spinal cord ischemia. Controlled trials have shown no clear benefit of steroids in cases of acute brain ischemia.10
In the absence of direct pathologic confirmation, the nature of the embolic débris that caused anterior spinal cord ischemia in our patient is a matter of speculation, although thrombotic and atherosclerotic material were probable culprits. We are aware of well-documented cases of atherothrombotic embolization during endovascular aortic procedures, including intra-aortic balloon pump placement11 and aortography.12 Cerebral ischemia is a rare but well-recognized complication of endovascular procedures, including cardiac catheterization.13 Contrast-dye toxicity is another conceivable mechanism of neurologic dysfunction during angiography. The overall incidence of neurologic complications is similar with use of both ionic and nonionic contrast dyes.14
Surgical repair of thoracoabdominal aneurysms is associated with perioperative spinal cord ischemia in about 1% to 21% of cases.15-17 With the advent of nonsurgical endovascular procedures for the repair of thoracoabdominal aneurysms, an increased number of patients are likely to receive this type of treatment. However, spinal cord ischemia can also complicate endovascular repair of aortic aneurysms.15,16 Consequently, the number of periprocedural neurologic complications may be on the rise. Patients who are particularly likely to benefit from these novel endovascular treatments for aortic aneurysms include the elderly; those who have compromised cardiac, pulmonary, or renal status; individuals with previous aortic operations; and those with comorbidities.16 Concomitant or previous abdominal aortic aneurysm repair and long-segment thoracic aortic exclusion appear to be important risk factors.15 A randomized, controlled trial comparing conventional surgery and endovascular repair of abdominal aortic aneurysms showed that endovascular procedures are associated with a significant reduction in patient morbidity.17 However, spinal cord ischemia occurred with similar frequency in both treatment groups (surgical group, 2/174; endovascular group, 1/171).17
The causes of anterior spinal cord ischemia are diverse and include surgical repair of aortic aneurysms, aortic dissection, aortic rupture and thrombosis, systemic hypotension, repair or thrombosis of spinal arteriovenous malformations, cardiac surgery, hematomyelia, epidural hematoma, cervical osteophytosis, vertebral artery dissection, celiac plexus block, systemic lupus erythematosus, polyarteritis nodosa, coagulopathy, and decompression sickness.1,2 A substantial proportion of patients may have no apparent cause for spinal cord infarction, which is referred to as cryptogenic stroke.
Another interesting aspect of the present case was the rapid neurologic improvement over a 72-hour period. Spontaneous and substantial improvement of spinal cord ischemia is rare but well-documented. Rapid improvement during the first 24 hours (as with a spinal transient ischemic attack) may occur in about 7% of patients.1 Although one third of patients with spinal cord ischemia eventually improve, only 10% of these experience substantial motor recovery within the first 2 to 4 weeks.1 Retrospective studies of spinal cord infarction have shown a mortality rate of 9% to 20%, and most survivors become moderately or severely disabled (20% to 57% of patients become wheelchair-bound).1-3 In addition to motor disabilities, patients with spinal cord infarction often experience autonomic dysfunction, chronic pain, paresthesia, and depression, which may interfere with recovery.1-3
Considering the outcome in our patient and the lack of specific therapy provided in the literature, the use of thrombolysis may be a sensible therapeutic option for this devastating condition. We conclude that intravenous thrombolysis could be considered for those patients with signs and symptoms of spinal cord ischemia who are evaluated within 3 hours after the onset of symptoms. Further studies are needed to measure the potential benefits of intravenous thrombolytic agents in spinal cord ischemia against the potential complications—which could be fatal—including hemorrhage (involving the brain, spinal cord, epidural space, or abdominal cavity), dislodging of thromboembolic débris with subsequent ischemia of downstream circulation (involving the legs, kidneys, and bowel), and allergic reactions (such as anaphylaxis and angioedema).
Footnotes
Address for reprints: Lucas Restrepo, MD, Department of Physiological Science, Interdepartmental Program in Molecular, Cellular, & Integrative Physiology, 2317 Life Science Building; Box 951606, University of California–Los Angeles, Los Angeles, CA 90095-1606
E-mail: lrestre1@jhmi.edu
References
- 1.Cheshire WP, Santos CC, Massey EW, Howard JF Jr. Spinal cord infarction: etiology and outcome. Neurology 1996;47: 321–30. [DOI] [PubMed]
- 2.Salvador de la Barrera S, Barca-Buyo A, Montoto-Marques A, Ferreiro-Velasco ME, Cidoncha-Dans M, Rodriguez-Sotillo A. Spinal cord infarction: prognosis and recovery in a series of 36 patients. Spinal Cord 2001;39:520–5. [DOI] [PubMed]
- 3.Nedeltchev K, Loher TJ, Stepper F, Arnold M, Schroth G, Mattle HP, Sturzenegger M. Long-term outcome of acute spinal cord ischemia syndrome. Stroke 2004;35:560–5. [DOI] [PubMed]
- 4.Rovira A, Pedraza S, Comabella M, Alvarez J, Salgado A. Magnetic resonance imaging of acute infarction of the anterior spinal cord. J Neurol Neurosurg Psychiatry 1998;64: 279–81. [DOI] [PMC free article] [PubMed]
- 5.Weidauer S, Dettmann E, Krakow K, Lanfermann H. Diffusion-weighted MRI of spinal cord infarction. Description of two cases and review of the literature [in German]. Nervenarzt 2002;73:999–1003. [DOI] [PubMed]
- 6.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med 1995;333:1581–7. [DOI] [PubMed]
- 7.Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on the Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction). Circulation 2004;110:588–636. [DOI] [PubMed]
- 8.Benavente O, Barnett HJ. Spinal cord ischemia. In: Barnett HJ, Mohr JP, Stein BM, Yatsu FM, editors. Stroke: pathophysiology, diagnosis, and management. 3rd ed. New York: Churchill Livingstone; 1998. p. 751–65.
- 9.Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA 1997;277:1597–604. [PubMed]
- 10.Norris JW, Hachinski VC. High dose steroid treatment in cerebral infarction. Br Med J (Clin Res Ed) 1986;292:21–3. [DOI] [PMC free article] [PubMed]
- 11.Harris RE, Reimer KA, Crain BJ, Becsey DD, Oldham HN Jr. Spinal cord infarction following intraaortic balloon support. Ann Thorac Surg 1986;42:206–7. [DOI] [PubMed]
- 12.Mullier JP, Vanderhaeghen JJ, Capon A. Spinal cord infarction caused by cholesterol embolisms and embolic complications of retrograde aortography [in French]. Acta Clin Belg 1979;34:272–7. [DOI] [PubMed]
- 13.Lazar JM, Uretsky BF, Denys BG, Reddy PS, Counihan PJ, Ragosta M. Predisposing risk factors and natural history of acute neurologic complications of left-sided cardiac catheterization. Am J Cardiol 1995;75:1056–60. [DOI] [PubMed]
- 14.Davidson CJ, Mark DB, Pieper KS, Kisslo KB, Hlatky MA, Gabriel DA, Bashore TM. Thrombotic and cardiovascular complications related to nonionic contrast media during cardiac catheterization: analysis of 8,517 patients. Am J Cardiol 1990;65:1481–4. [DOI] [PubMed]
- 15.Gravereaux EC, Faries PL, Burks JA, Latessa V, Spielvogel D, Hollier LH, Marin ML. Risk of spinal cord ischemia after endograft repair of thoracic aortic aneurysms. J Vasc Surg 2001;34:997–1003. [DOI] [PubMed]
- 16.Fann JI, Miller DC. Endovascular treatment of descending thoracic aortic aneurysms and dissections. Surg Clin North Am 1999;79:551–74. [DOI] [PubMed]
- 17.Prinssen M, Verhoeven EL, Buth J, Cuypers PW, van Sambeek MR, Balm R, et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Eng J Med 2004;351:1607–18. [DOI] [PubMed]


