Abstract
QTc interval prolongation is associated with increased mortality rates in patients with advanced heart failure. We investigated the predictive value of prolonged QTc interval in 567 patients with heart failure who were undergoing coronary artery bypass graft surgery. The patients were in New York Heart Association class III or IV, with left ventricular ejection fractions of 0.40 or less. Before surgery, the QT interval duration was measured in leads II and V4 of the standard electrocardiogram and corrected by use of the Bazett formula. The QTc interval was prolonged (>440 msec) in 243 patients (43%) and normal in 324 (57%). The 2 study groups—prolonged QTc versus normal QTc—did not differ in terms of age (62 ± 11 years vs 64 ± 10 years, P =0.65), sex (80% male vs 76% male, P =0.31), ejection fraction (0.29 ± 0.08 vs 0.29 ± 0.09, P =0.72), hypertension (82% vs 78%, P =0.34), or diabetes (11% vs 7%, P =0.10).
Within 1 month after coronary artery bypass grafting, 22 of 243 patients (9.1%) in the prolonged QTc group died, compared with 5 of 324 in the normal QTc group (1.5%) (P =0.0001). QTc interval prolongation was the only independent predictor of postoperative mortality on multivariate analysis (P =0.002). We conclude that patients with heart failure and preoperative QTc interval prolongation have increased mortality rates after coronary artery bypass grafting.
Key words: Coronary disease/surgery, electrocardiography, heart failure, long QT syndrome, mortality, myocardial revascularization, prognosis, risk factors, treatment outcome
The prevalence of patients with advanced heart failure due to ischemic heart disease is steadily increasing.1 Recently, a number of medical and invasive strategies have been proposed to improve survival in this group of patients. The use of β-blockers, defibrillators, and biventricular pacing has been shown to be of considerable benefit in such patients.2 Yet, in many, the disease still progresses to advanced stages that are associated with profound hemodynamic distress and high mortality rates. For these patients, the only widely available definitive treatment is cardiac transplantation. However, the survival benefit of transplantation is evident only in patients who are in the most severe stages of heart failure, and the shortage of available donor organs further limits the use of this treatment.3 Although the use of left ventricular assist devices is an alternative for selected patients with advanced ischemic heart failure, coronary artery bypass grafting (CABG) remains the most frequently performed surgical procedure in this group of patients.4 Surgical revascularization in patients with severe left ventricular dysfunction has historically carried high perioperative mortality and morbidity rates; however, advances in surgical technique and myocardial protection have improved the safety of CABG in selected patients with ischemic cardiomyopathy.5 Because many patients with advanced ischemic heart failure could potentially benefit from CABG and because a number of alternative treatments are available, it is important to carefully select the patients in whom CABG would be most beneficial. Despite the risk-stratification parameters proposed so far,6 the selection criteria for CABG in patients with advanced ischemic heart failure remain poorly defined.
QTc interval prolongation has been shown to predict mortality in medically treated patients with advanced heart failur7 and in patients with coronary artery disease.8 We therefore sought to evaluate the predictive value of preoperative QTc interval duration in patients with ischemic heart failure who were undergoing CABG.
Patients and Methods
Patients.
We retrospectively reviewed data on all patients referred to our institution for CABG from 1 January 2001 to 1 June 2002. From this group, we selected patients who had a reduced left ventricular ejection fraction (LVEF) on preoperative heart catheterization and who had been in New York Heart Association (NYHA) class III or IV for at least 2 months before referral and evaluation at our institution. Patients with pacemakers or implantable cardioverter-defibrillators and patients taking type-III antiarrhyth-mic medications were excluded.
QTc Interval Measurement.
Within the 24-hour period before surgery, resting, 12-lead electrocardiograms (ECGs) were recorded at a paper speed of 25 mm/sec on a Marquette Resting ECG recorder (Marquette Electronics Inc.; Milwaukee, Wis). Two independent observers who were blinded to the clinical and survival data determined QT interval duration. In accordance with the latest recommendations for clinical QT interval measurement,9 QT interval duration was recorded for 3 consecutive beats through leads II and V4. Using calipers on printed ECGs, each QT interval was measured from the beginning of the QRS complex to the visual return of the T wave to the isoelectric line. When the T wave was interrupted by the U wave, the end of the T wave was defined as the nadir between the T and the U wave. Patients with ECG evidence of arrhythmias or pacemaker rhythms were excluded. Heart rate was corrected using the Bazett formula, and QTc interval duration was defined as the mean duration of all QTc intervals measured. Prolonged QTc was defined as a QTc interval greater than 440 msec.
Cardiac Catheterization and CABG Techniques.
In all patients, cardiac catheterization was performed within the 48 hours before surgery. Coronary angiography was performed using standard techniques. Stenotic lesions were graded subjectively by visual consensus of at least 2 experienced observers on an ordinal scale of 0, 25%, 50%, 75%, 95%, or 100%. The extent of coronary artery disease was characterized by the traditional 1-, 2-, or 3-vessel disease classification. Biplane views were obtained during all ventriculography procedures. Angiographic LVEF and regional wall motion were determined by centerline regional wall motion analysis.10 In the presence of excessive ventricular ectopy or catheter-induced mitral regurgitation, ventriculography was repeated until technically adequate. All ventriculography data were interpreted by at least 2 experienced observers.
All patients underwent isolated CABG surgery in accordance with the standard guidelines for surgical myocardial revascularization.11
Follow-Up and Endpoints.
Patients were followed up for 1 month after CABG. The primary endpoint was death due to these cardiac causes: sudden cardiac death and pump failure. Sudden cardiac death was defined as either a witnessed cardiac arrest or death within 1 hour after the onset of acute symptoms, or an unexpected death in a patient known to have been well within the previous 24 hours.12 Pump-failure death was defined as death resulting from multiorgan failure due to the progression of heart failure.
Statistical Analysis.
Continuous variables are expressed as mean ± SD. Differences between survivors and patients who died during the postoperative period were analyzed using 1-way analysis of variance (ANOVA). Comparisons of categorical variables were made using a χ2 test. Univariate and multivariate stepwise Cox proportional hazard regression analyses were performed to identify independent predictors of postoperative mortality. The P value for entering and staying in the model was set at 0.05. The Kaplan-Meier method was used to analyze and compare survival rates in the prolonged and normal QTc groups. A P value less than 0.05 was considered significant.
Results
Patient Characteristics.
Of 1,112 patients eligible for the study, 201 were excluded because their LVEFs were more than 0.40, and 250 were excluded because they were not in NYHA functional class III or IV. Of the remaining 661 patients, 57 (9%) were excluded because they were being treated with type-III antiarrhythmic drugs, and 37 (6%) were excluded because they exhibited ECG abnormalities such as atrial fibrillation (n=25) and pacemaker rhythm (n=12).
QTc Interval and Outcome.
Of the remaining 567 patients, 27 (5%) died during the 1-month postoperative period. Death was sudden in 3 cases (11%) and due to pump failure in 24 (89%). The preoperative QTc interval duration was significantly longer in patients who died during the early postoperative period than it was in survivors (476 ± 45 msec vs 434 ± 50 msec, respectively; P 0.001).
In our study, the intraobserver relative error of QTc measurements was 2.4%. Using 440 msec as the cutoff value, the QTc interval was prolonged in 243 patients (43%) and normal in 324 (57%). The 2 study groups did not differ in any other clinical or laboratory preoperative characteristics (Table I).
Table I. Preoperative Characteristics of Patients with Prolonged (>440 msec) and Normal QTc Intervals*
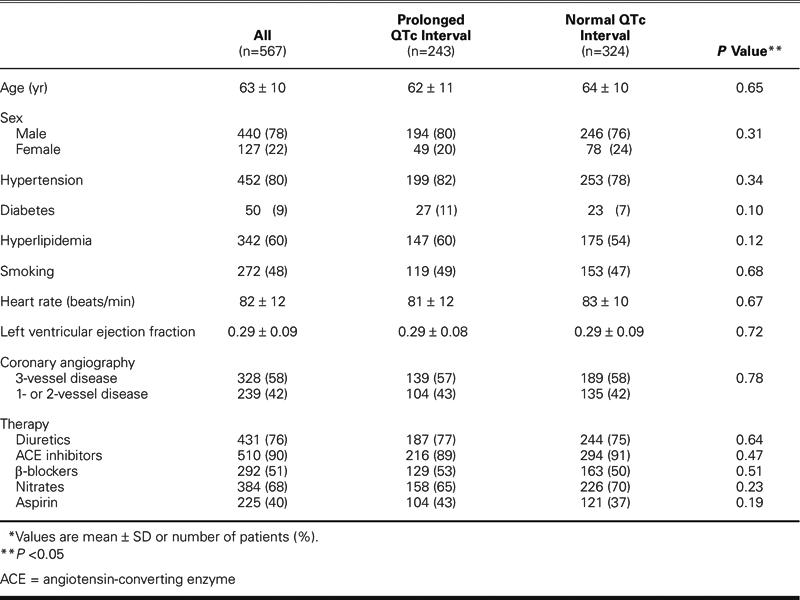
At 1 month after CABG, the cardiac mortality rate was significantly higher in the prolonged QTc group (22/243 [9.1%]) than in the normal QTc group (5/324 [1.5%]) (P =0.0001). The same was true for the pump-failure mortality rate (20/243 [8.2%] vs 4/324 [1.2%], respectively; P =0.0001). The sudden-death mortality rate, however, was not significantly different between the 2 study groups (2/243 [0.8%] vs 1/324 [0.3%], respectively; P =0.40) (Fig. 1).
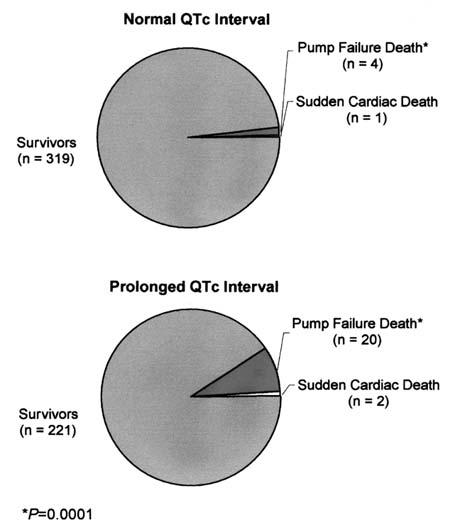
Fig. 1 Early postoperative mortality in heart failure patients with normal versus prolonged QTc intervals.
Univariate and Multivariate Predictors of Outcome.
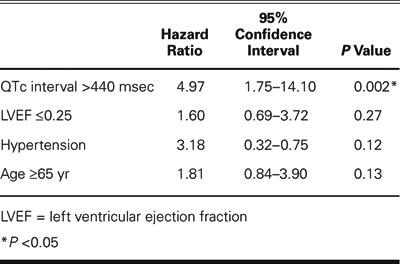
The results of the univariate analysis of potential predictors of outcome are presented in Table II. Parameters reaching or approaching statistical significance in the univariate analysis were included in a multivariate Cox proportional-hazards regression model of cardiac mortality at 1 month (Table III). Both QTc interval prolongation (>440 msec) and severely depressed LVEF (≤0.25) were found to be univariate predictors of outcome after CABG surgery. However, QTc interval prolongation was the only independent predictor of 1-month postoperative mortality on multivariate analysis.
Table II. Univariate Analysis of Potential Predictors of Early Postoperative Mortality
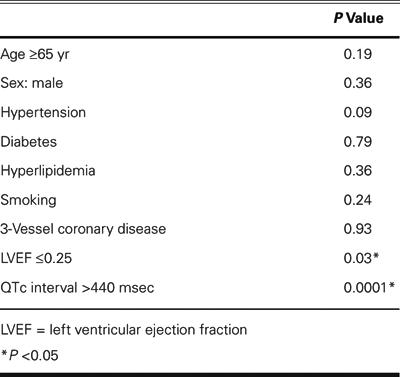
Table III. Multivariate Analysis of Potential Predictors of Early Postoperative Mortality
Kaplan-Meier Survival Estimation.
Survival as evaluated by Kaplan-Meier analysis was 6 times higher in the normal QTc group than in the prolonged QTc group (P =0.0001) (Fig. 2).
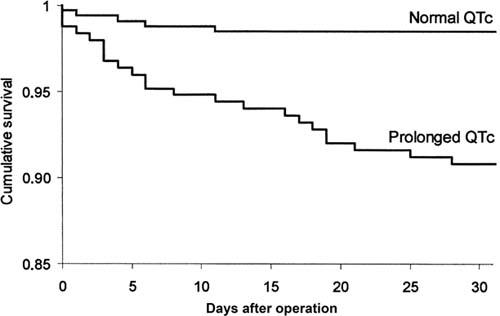
Fig. 2 Kaplan-Meier survival curves in patients classified according to QTc interval duration (prolonged vs normal) (P =0.0001).
Discussion
The results of our study indicate that a prolonged QTc interval (>440 msec) is an adverse prognostic sign in patients with ischemic heart failure undergoing isolated CABG. Preoperative QTc interval prolongation correlates with increased cardiac and pump-failure mortality rates within 1 month after the operation.
QTc Interval Prolongation in Ischemic Heart Failure.
QTc interval on the surface ECG reflects the time between the initial fast depolarization of the left ventricle and its subsequent repolarization. Duration of the QTc interval is highly dependent on T wave morphology, which is determined by the differences in the time course of repolarization of 3 predominant ventricular myocardial cell types (endocardial, epicardial, and M cells).13 In the presence of cardiac disease, ventricular repolarization heterogeneity is increased, leading to QTc interval prolongation.14 It appears that QTc interval duration in heart failure is independent of left ventricular loading conditions;15 however, the duration is affected by various noncardiac stimuli and especially by inflammation and changes in autonomic nervous tone, both of which are present in advanced heart failure.16 Although this may limit the value of QTc interval in the analysis of the electrophysiological properties of ventricular myocardium,17 it offers the possibility of evaluating the combined impact of different disease processes on heart failure. Therefore, QTc prolongation in patients with heart failure appears to be more than merely a manifestation of ventricular repolarization instability; it also appears to be a marker of disease severity.
In our study, QTc interval prolongation was present in 43% of patients, in agreement with the prevalence of prolonged QTc interval in other studies of patients with heart failure.7,18 The preoperative QTc interval prolongation observed in our study should be viewed as a parameter affected by the severity of heart failure.
QTc Interval Duration and Outcome after CABG.
In this study population, a prolonged QTc interval was an independent predictor of 1-month mortality after CABG. The most common cause of death was multiorgan failure caused by progressive worsening of heart failure; sudden cardiac death was rare. The relatively low rate of sudden cardiac death may be due, in part, to the study design, which excluded patients with ECG abnormalities and implantable cardioverter-defibrillators. Previously, in a cohort of heart failure patients awaiting cardiac transplantation, QTc interval prolongation was inversely correlated with peak exercise oxygen consumption and directly associated with increased mortality.15 Furthermore, in patients with heart failure who have brain natriuretic peptide levels greater than 400 pg/mL, QTc interval prolongation appears to be an adverse prognostic sign that predicts both pump failure and sudden cardiac death.7
Worsening of heart failure is one of the most common causes of death and readmission to the hospital after CABG.19 This suggests that CABG may not be an appropriate treatment option for certain heart failure patients, particularly those with advanced disease. Accordingly, we and others20 have found severely depressed preoperative LVEF to be an indicator of adverse outcomes after CABG. No studies have addressed the predictive value of QTc interval in this setting.
In the present study, heart failure patients who exhibited QTc interval prolongation had a 6-fold higher mortality rate after CABG than did patients who exhibited a normal QTc interval. Because QTc interval has been shown to be prolonged after CABG,21 this procedure may not be of benefit to patients with ischemic heart failure and preoperative QTc interval prolongation. On the other hand, QTc duration has been shown to decrease significantly after implantation of a left ventricular assist device, which suggests a beneficial effect of left ventricular mechanical support.22 Therefore, in selected patients with advanced heart failure and QTc interval prolongation, earlier left ventricular assist device implantation may be warranted instead of a high-risk CABG procedure.
Study Limitations.
Our study and its results have several limitations. First, the study excluded 2 large subgroups of heart failure patients: patients in whom heart failure progression was associated with atrial arrhythmias or with the need for permanent pacing, and those who were taking type-III antiarrhythmic medications or had implantable cardioverter-defibrillators. Therefore, our results cannot be directly applied to all patients with advanced heart failure. Second, we did not attempt to analyze QT interval dispersion in our study. Even though QT dispersion has been proposed as a risk marker in patients with advanced heart failure, the reproducibility of QT dispersion measurements has been poor.23 We did, however, minimize intraobserver variability in our study by using only leads II and V4 to determine QT interval length. Finally, the postoperative follow-up of our patients was relatively short, which could account for the low mortality rates observed in our study.
Conclusions
QTc intervals greater than 440 msec are associated with adverse outcomes in ischemic heart failure patients undergoing CABG. Although the mechanism underlying QTc prolongation is not fully understood, it appears that QTc prolongation may be a good adjunct in risk stratification of patients with advanced heart failure who are being considered for surgical revascularization. Further studies are needed to determine its pathophysiologic significance and to determine whether it can be used with other markers as part of a multivariate risk stratification protocol for therapeutic decision-making in patients with advanced heart failure.
Footnotes
Address for reprints: Branislav Radovancevic, MD, Department of Cardiopulmonary Transplantation, Texas Heart Institute, MC 2-114A, P.O. Box 20345, Houston, TX 77225-0345
E-mail: bradovancevic@heart.thi.tmc.edu
References
- 1.Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med 2002;347:1397–402. [DOI] [PubMed]
- 2.Jessup M, Brozena S. Heart failure. N Engl J Med 2003; 348:2007–18. [DOI] [PubMed]
- 3.Deng MC, De Meester JM, Smits JM, Heinecke J, Scheld HH. Effect of receiving a heart transplant: analysis of a national cohort entered on to a waiting list, stratified by heart failure severity. Comparative Outcome and Clinical Profiles in Transplantation (COCPIT) Study Group. BMJ 2000; 321:540–5. [DOI] [PMC free article] [PubMed]
- 4.Ascione R, Narayan P, Rogers CA, Lim KH, Capoun R, Angelini GD. Early and midterm clinical outcome in patients with severe left ventricular dysfunction undergoing coronary artery surgery. Ann Thorac Surg 2003;76:793–9. [DOI] [PubMed]
- 5.Trachiotis GD, Weintraub WS, Johnston TS, Jones EL, Guyton RA, Craver JM. Coronary artery bypass grafting in patients with advanced left ventricular dysfunction. Ann Thorac Surg 1998;66:1632–9. [DOI] [PubMed]
- 6.Gardner SC, Grunwald GK, Rumsfeld JS, Mackenzie T, Gao D, Perlin JB, et al. Risk factors for intermediate-term survival after coronary artery bypass grafting. Ann Thorac Surg 2001;72:2033–7. [DOI] [PubMed]
- 7.Vrtovec B, Delgado R, Zewail A, Thomas CD, Richartz BM, Radovancevic B. Prolonged QTc interval and high B-type natriuretic peptide levels together predict mortality in patients with advanced heart failure. Circulation 2003;107: 1764–9. [DOI] [PubMed]
- 8.Elming H, Brendorp B, Kober L, Sahebzadah N, Torp-Petersen C. QTc interval in the assessment of cardiac risk. Card Electrophysiol Rev 2002;6:289–94. [DOI] [PubMed]
- 9.Toivonen L. More light on QT interval measurement. Heart 2002;87:193–4. [DOI] [PMC free article] [PubMed]
- 10.Sheehan FH, Bolson EL, Dodge HT, Mathey DG, Schofer J, Woo HW. Advantages and applications of the centerline method for characterizing regional ventricular function. Circulation 1986;74:293–305. [DOI] [PubMed]
- 11.Eagle KA, Guyton RA, Davidoff R, Ewy GA, Fonger J, Gardner TJ, et al. ACC/AHA guidelines for coronary artery bypass graft surgery: executive summary and recommendations: A report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines (Committee to revise the 1991 guidelines for coronary artery bypass graft surgery). Circulation 1999;100:1464–80. [DOI] [PubMed]
- 12.Stevenson WG, Stevenson LW, Middlekauff HR, Saxon LA. Sudden death prevention in patients with advanced ventricular dysfunction. Circulation 1993;88:2953–61. [DOI] [PubMed]
- 13.Yan GX, Antzelevitch C. Cellular basis for the normal T wave and the electrocardiographic manifestations of the long-QT syndrome. Circulation 1998;98:1928–36. [DOI] [PubMed]
- 14.Antzelevitch C, Shimizu W. Cellular mechanisms underlying the long QT syndrome. Curr Opin Cardiol 2002;17:43–51. [DOI] [PubMed]
- 15.Boccalandro F, Velasco A, Thomas C, Richards B, Radovancevic B. Relations among heart failure severity, left ventricular loading conditions, and repolarization length in advanced heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol 2003;92:544–7. [DOI] [PubMed]
- 16.Kjaer A, Hesse B. Heart failure and neuroendocrine activation: diagnostic, prognostic and therapeutic perspectives. Clin Physiol 2001;21:661–72. [DOI] [PubMed]
- 17.Magnano AR, Holleran S, Ramakrishnan R, Reiffel JA, Bloomfield DM. Autonomic nervous system influences on QT interval in normal subjects. J Am Coll Cardiol 2002; 39:1820–6. [DOI] [PubMed]
- 18.Brendorp B, Elming H, Jun L, Kober L, Malik M, Jensen GB, et al. Qtc interval as a guide to select those patients with congestive heart failure and reduced left ventricular systolic function who will benefit from antiarrhythmic treatment with dofetilide. Circulation 2001;103:1422–7. [DOI] [PubMed]
- 19.Hannan EL, Racz MJ, Walford G, Ryan TJ, Isom OW, Bennett E, Jones RH. Predictors of readmission for complications of coronary artery bypass graft surgery. JAMA 2003; 290:773–80. [DOI] [PubMed]
- 20.De Carlo M, Milano A, Borzoni G, Pratali S, Barzaghi C, Tartarini G, et al. Predicting outcome after myocardial revascularization in patients with left ventricular dysfunction. Cardiovasc Surg 1998;6:58–66. [DOI] [PubMed]
- 21.Wranicz JK, Ruta J, Strzondala M, Kosmider M, Bolinska H, Zaslonka J. QT interval duration in 24-hour Holter monitoring after different interventional treatment of coronary artery disease in patients after the myocardial infarction. Med Sci Monit 2000;6:100–2. [PubMed]
- 22.Harding JD, Piacentino V 3rd, Gaughan JP, Houser SR, Margulies KB. Electrophysiological alterations after mechanical circulatory support in patients with advanced cardiac failure. Circulation 2001;104:1241–7. [DOI] [PubMed]
- 23.Malik M, Batchvarov VN. Measurement, interpretation and clinical potential of QT dispersion. J Am Coll Cardiol 2000;36:1749–66. [DOI] [PubMed]


