Abstract
A pre-existing malignancy has disqualified patients from solid organ transplantation because of concerns regarding recurrence. We reviewed pre-transplant characteristics and long-term results in patients who underwent heart transplantation with a pre-existing malignancy, because there has been no prior study of these patients in the long term.
All 214 patients who underwent heart transplantation from July 1985 through June 2004 were studied retrospectively. Thirteen of these patients had been treated for a malignancy before transplantation. Pre-transplant characteristics (age, sex, diabetes, and weight) and post-transplant outcomes (rejection, infection, and survival) were compared for the 2 groups.
The patients with pre-existing malignancies were younger (47 vs 54 years, P =0.014), less heavy (73 vs 79 kg, P =0.017), and more likely to be female (54% vs 22%, P =0.010), compared with recipients without a pre-malignancy. Pulmonary vascular resistances, histories of tobacco use, and incidence of pre-transplant diabetes were not different between the 2 groups. The mean duration of follow-up for the 2 groups was similar (2,760 days for the pre-malignancy group vs 2,215 days for the non-pre-malignancy group, P =NS). Episodes of treated rejection and infection for the pre-malignancy group vs the non–pre-malignancy group were similar (1.8 episodes of rejection vs 1.6 episodes, P =NS); and (1.7 episodes of infection vs 0.8 episodes, P =0.098). None of the pre-malignancy patients had recurrence of their original cancer, and long-term survival for the 2 groups was essentially identical (63% vs 62% at 10 years, P =NS). The dissemination of reports such as these may enable more patients with cured malignancies to benefit from transplantation.
Key words: Cardiomyopathies/surgery; heart failure, congestive/surgery; heart transplantation/contraindications; neoplasms/ complications; patient selection; postoperative complications; recurrence; retrospective studies; risk factors; survival rate
Cardiac transplantation has proved to be an excellent treatment for individuals with end-stage heart failure who have no significant concurrent disorders that might preclude cardiac replacement and its attendant need for long-term immunosuppression.
As with many precepts in heart transplantation, early guidelines regarding recipient selection were extrapolated from renal transplantation experiences. Consequently, most heart transplant centers have used the data from the Cincinnati Transplant Tumor Registry, which indicate that the recurrence rate of cancer in kidney transplant recipients who were treated for a pre-transplant malignancy (PTM) is 21%.1 Furthermore, 88% of these recurrences developed within 5 years of transplantation, thus threatening long-term outcomes.
Prompted by a compelling initial case of a 40-year-old woman who suffered radiation-induced cardiomyopathy as a result of her treatment for breast cancer, we undertook heart transplantation in that patient with a “cured” PTM in January of 1986. In 1990, the Stanford Group reported its experiences with 7 patients who underwent heart transplantation in the presence of a PTM. The 1-year survival rate was 71%, with 1 recurrence in a 2-year follow-up period.2 Additional reports,3–5 including our own,6 extended this frontier of heart transplantation to follow-up periods of 39 months7 and 35 months.8 These reports concluded that medium-term survival in “carefully selected” heart transplant recipients with PTM was acceptable.
We performed this investigation to provide long-term follow-up for heart transplant recipients with PTM. Secondarily, we evaluated the pre-transplant characteristics of these PTM patients, compared with those of non-PTM patients, to determine which pre-transplant factors might contribute to “careful selection.”
Patients and Methods
All 214 patients who underwent heart transplantation at the Lutheran Heart Center from July 1985 through June 2004 were studied. Patient charts were retrospectively analyzed in compliance with the Health Information Portability and Privacy Act (HIPPA).
The following pre-transplant data were compared for the PTM and non-PTM groups, in order to determine which patient characteristics in the PTM group were more carefully scrutinized: age, sex, weight, history of tobacco use, pulmonary vascular resistance (PVR) at presentation, and lowest PVR achieved with pharmacologic manipulation. In addition, the presence of insulin-dependent diabetes (IDDM) was compared for the 2 groups.
Follow-up was 100% complete. Mean follow-up was 2,760 days for the PTM group and 2,215 days for the non-PTM group (P =NS), which enabled comparison of infection episodes, rejection episodes, and long-term survival between the 2 groups.
Statistical analysis was performed with the use of the Statistical Package for the Social Sciences 12 for Windows (SPSS, Inc.; Chicago, Ill). Means were compared by independent sample t-tests and Levine's test for equality of variances. Differences in categorical variables were tested by χ2 analysis, with correction for continuity. Survival curves were examined by Kaplan-Meier analysis. A P value of less than 0.05 was considered significant.
Results
Characteristics for each of the 13 patients with pre-existing malignancies are listed in Table I. The average disease-free interval before transplantation was 74.23 months.
Table I. Patients with Pre-Existing Malignancies
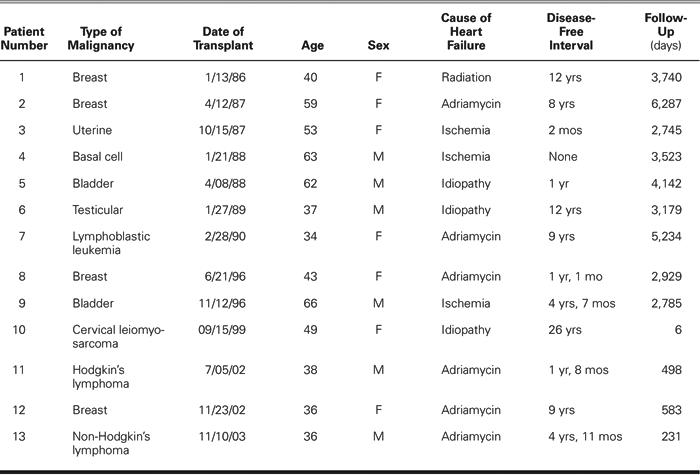
The PTM group differed from the non-PTM group during pre-transplant evaluation, in that patients were younger (47 vs 54 years, P =0.014), weighed less (73 kg vs 79 kg, P =0.017), and were more likely to be female (54% vs 22%, P =0.010). The PTM group and non-PTM group had similar pulmonary vascular resistance elevations (mean highest PVR, 3.05 vs 2.59 Wood units; P =NS; and mean lowest achievable PVR, 2.12 vs 1.63 Wood units; P =NS). Also, the 2 groups had similar low incidences of insulin-dependent diabetes during evaluation (none in PTM vs 10% in non-PTM, P =NS) and similar histories of tobacco use.
On average, the PTM group waited a shorter period for a donor organ after being placed on the waiting list (104 days vs 277 days; P =0.018).
Eleven of the 13 PTM patients (85%) received induction therapy with antithymocyte globulin or monoclonal antibody after transplantation. In the non- PTM group, 156 of 201 (78%) received induction therapy. Long-term immunosuppression was also similar for the 2 groups, consisting of a triple-drug regimen with attempted steroid weaning by 6 months postoperatively. The incidence of treated post-transplant rejection episodes during the entire follow-up period was not different for the PTM and non-PTM groups (1.82 ± 1.85 vs 1.55 ± 1.52, respectively; P = NS). The incidence of treated post-transplant infection episodes for this time period tended toward a higher infection incidence in the PTM group (1.69 ± 1.80 episodes in the PTM group vs 0.79 ± 0.99 episodes in the non-PTM group). This difference, however, was not statistically significant (P =0.098).
Finally, the most important result is shown in the survival graph (Fig. 1). This graph shows that 10-year survival was very similar in the 2 groups: 63% for the non-PTM patients and 62% for the PTM patients. None of the PTM patients experienced recurrence of his or her original malignancy.
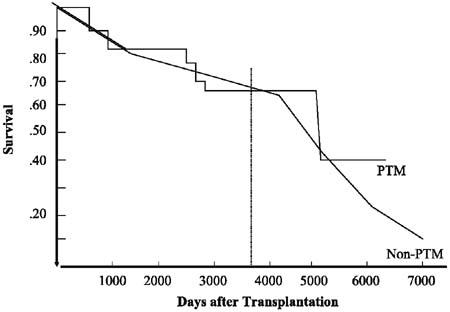
Fig. 1 This graph shows that 10-year survival was very similar in the 2 groups: 63% for the non-PTM patients and 62% for the PTM patients (P =NS).
PTM = pre-transplant malignancy
Discussion
This study presents the clinical long-term results of heart transplantation in 13 patients who had a history of malignancy before transplantation. These results were compared with the outcomes of 201 heart transplant recipients who did not have a pre-existing malignancy. The mean follow-up of the groups was statistically similar: 2,760 days for the PTM group and 2,215 days for the non-PTM group. During this time, the episodes of rejection for the 2 groups were similar, which suggests that the groups had similar levels of immunosuppression after transplantation. Although the incidence of infection was higher for the PTM group, which indicates possible immunodeficiency or over-immunosuppression, this difference did not reach statistical significance. The 10-year Kaplan-Meier survival for PTM and non-PTM patients was essentially identical, and there was no recurrence of the original malignancies in the PTM patients.
Patients with a PTM were younger and weighed less. This may reflect a subconscious institutional effort to select leaner and more youthful patients for transplantation when relaxing another selection criterion (that is, the presence of a PTM). These patients were also more likely to be female, perhaps reflecting the fact that 6 of the 13 PTM patients had a breast or gynecologic PTM.
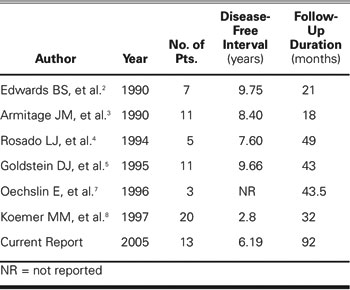
Before this report, there have been reports of similar series involving heart transplantation in patients with treated malignancies. These are summarized in Table II. The current report adds to the existing literature by extending the post-transplant follow-up of these patients into the “long-term” range. With the dissemination of reports such as these, it is to be hoped that more patients with “cured” PTMs will be able to benefit from transplantation. However, it goes without saying that the PTM must be cured as well as possible, because the immunosuppressed patient remains more vulnerable to the development of malignancies than does the population at large. All of our PTM patients had been treated aggressively for their original malignancies; these treatments included bone marrow transplantation to treat non-Hodgkin's lymphoma, and adriamycin or high-dose radiation for all cases of breast cancer.
Table II. Summary of Literature Regarding Heart Transplantation in Patients with Pre-Existing Malignancies
Finally, a prolonged disease-free interval does reassure all concerned that the malignancy is as well controlled as possible. For more aggressive cancers (such as breast cancer), we would recommend at least 1 year of malignancy-free survival before transplantation. In some cases, this has required a several-month course of continuous intravenous administration of inotropic agents to control heart failure, while we establish that the patient is indeed free of overt recurrence. The PTM patient who is referred for heart transplantation must undergo additional testing (tumor markers or radiographic studies) to rule out covert recurrence.
In conclusion, carefully selected patients (defined as younger and leaner, with a disease-free interval of at least 1 year for more aggressive types of cancer) with PTM that is cured can undergo heart transplantation with long-term survival rates similar to those of recipients without PTM.
Acknowledgment
With great thanks to Roger C. Hoversland, PhD, for help with statistical analysis.
Footnotes
Address for reprints: J.S. Ladowski, MD, 7910 W. Jefferson Blvd., Suite 102, Fort Wayne, IN 46804
E-mail: jsl@ioheart.com
This material was presented in oral form to the World Society of Cardio-Thoracic Surgeons' 15th Congress, 20 June 2005, Vilnius, Lithuania.
This study was funded by the August Tomusk Foundation.
References
- 1.Penn I. Evaluation of transplant candidates with pre-existing malignancies. Ann Transplant 1997;2:14–7. [PubMed]
- 2.Edwards BS, Hunt SA, Fowler MB, Valantine HA, Stinson EB, Schroeder JS. Cardiac transplantation in patients with preexisting neoplastic diseases. Am J Cardiol 1990;65:501–4. [DOI] [PubMed]
- 3.Armitage JM, Kormos RL, Griffith BP, Fricker FJ, Hardesty RL. Heart transplantation in patients with malignant disease. J Heart Transplant 1990;9:627–30. [PubMed]
- 4.Rosado LJ, Wild JC, Huston CL, Sethi GK, Copeland JG. Heart transplantation in patients with treated breast carcinoma. J Heart Lung Transplant 1994;13:246–9. [PubMed]
- 5.Goldstein DJ, Seldomridge A, Addonizio L, Rose EA, Oz MC, Michler RE. Orthotopic heart transplantation in patients with treated malignancies. Am J Cardiol 1995;75:968–71. [DOI] [PubMed]
- 6.Dillon TA, Sullivan M, Schatzlein MH, Peterson AC, Scheeringa RH, Clark WR Jr, Ladowski JS. Cardiac transplantation in patients with preexisting malignancies. Transplantation 1991;52:82–5. [DOI] [PubMed]
- 7.Oechslin E, Kiowski W, Schneider J, Follath F, Turina M, Gallino A. Pre-transplant malignancy in candidates and posttransplant malignancy in recipients of cardiac transplantation. Ann Oncol 1996;7:1059–63. [DOI] [PubMed]
- 8.Koerner MM, Tenderich G, Minami K, Mannebach H, Koertke H, zu Knyphausen E, et al. Results of heart transplantation in patients with preexisting malignancies. Am J Cardiol 1997;79:988–91. [DOI] [PubMed]


