Abstract
Coordinated regulation of neuronal progenitor differentiation in the subventricular zone (SVZ) is a fundamental feature of adult neurogenesis. However, the molecular control of this process remains mostly undeciphered. Here, we investigate the role of neuregulins (NRGs) in this process and show that a NRG receptor, ErbB4, is primarily expressed by polysialylated neural cell adhesion molecule immature neuroblasts but is also detected in a subset of GFAP+ astroglial cells, ependymal cells, and Dlx2+ precursors in the SVZ. Of the NRG ligands, both NRG1 and -2 are expressed by immature polysialylated neural cell adhesion molecule neuroblasts in the SVZ. NRG2 is also expressed by some of the GFAP+ putative stem cells lining the ventricles. Infusion of exogenous NRG1 leads to rapid aggregation of Dlx2+ cells in the SVZ and affects the initiation and maintenance of organized neuroblast migration from the SVZ toward the olfactory bulb. In contrast, the infusion of NRG2 increased the number of Sox2 and GFAP+ precursors in the SVZ. An outcome of this NRG2 effect is an increase in the number of newly generated migrating neuroblasts in the rostral migratory stream and GABAergic interneurons in the olfactory bulb. The analysis of conditional null mice that lack NRG receptor, ErbB4, in the nervous system revealed that the observed activities of NRG2 require ErbB4 activation. These results indicate that different NRG ligands affect distinct populations of differentiating neural precursors in the neurogenic regions of the mature forebrain. Furthermore, these studies identify NRG2 as a factor capable of promoting SVZ proliferation, leading to the formation of new neurons in vivo.
Keywords: adult neurogenesis, rostral migratory stream, schizophrenia
The primary sites of neurogenesis in the adult rodent brain are in the hippocampus and the forebrain subventricular zone [SVZ (1–9)]. The multipotential cells in the SVZ are believed to give rise to a collection of migrating neural progenitors that form the rostral migratory stream (RMS). These cells transit from the anterior SVZ to the olfactory bulb and appear to migrate tangentially along each other through tubes composed of astrocytic-like glial cells (3, 4). Upon their arrival at the posterior core of the olfactory bulb, the cells exit the RMS, switch from a tangential to radial mode of migration, and differentiate into functional granule interneurons or periglomerular interneurons (10, 11).
The observation that the receptor tyrosine kinase ErbB4 is selectively expressed at high levels in the SVZ and RMS (12) suggests a possible role for the ErbB4 ligands, the neuregulins (NRGs), as modulators of the proliferation and migration of neural stem and progenitor cells. The NRGs are a collection of related polypeptides characterized by the presence of a single EGF-like domain, capable of binding to and activating ErbB receptors, of which there are four members, ErbB1–4. NRGs serve as ligands for ErbB4 (13) and ErbB3 (14–16) but can be potent activators of ErbB2 and weak activators of the EGF receptor (EGFR) as a result of receptor heterodimerization (17). Four genes encoding NRGs have been identified [NRG1–4 (18, 19)]. Of these, NRG1 is the best-characterized and is known for its biological activities described as glial growth factor (GGF) and the acetylcholine receptor-inducing activity [ARIA (20–24)]. Although >15 alternatively spliced variants of NRG1 have been identified, these can be grouped into three main classes (I, II, and III) based upon the structure of their extracellular regions (18). The type III isoform of NRG1, which contains a cysteine-rich extracellular domain, appears to correspond to the functional roles defined by GGF. Genetic studies imply that the roles of ARIA are served by types I and/or II, which contain an Ig-like domain in their extracellular regions. Types I/II have also been implicated in the formation of muscle spindles, revealing a role for NRG1 in proprioception (25). NRG has also been shown to affect neuronal migration on radial glial guides in cerebral cortex and cerebellum via ErbB2 and ErbB4 receptors, respectively (26, 27). Recently, NRG1 has been identified as a permissive modulator of tangential migration of neurons in the embryonic cortex and the adult RMS (12, 28).
Here, we investigated how NRG1 and -2 influence the proliferation and differentiation of adult neural progenitor cells, and how signaling via the NRG receptor, ErbB4, affects the organization and generation of distinct cell types in the SVZ. We show that NRG1 regulates aggregation of rapidly proliferating Dlx2+ precursor cells, whereas NRG2 promotes GFAP+ cell proliferation and polysialylated neural cell adhesion molecule (PSA-NCAM+) neuroblast generation. The effects of NRG2 depend on ErbB4 receptor activation. In the absence of ErbB4 signaling, the organization and generation of distinct SVZ cell types are disrupted.
Results
Localization of ErbB Receptors and NRG1 and -2 in the SVZ, RMS, and Olfactory Bulb.
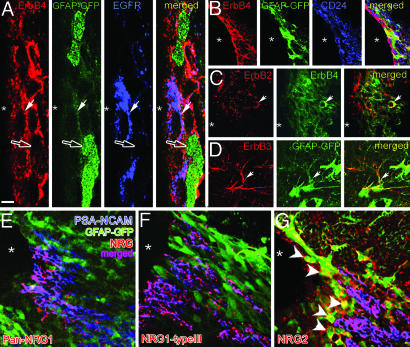
To evaluate the significance of NRG-ErbB signaling in adult neurogenesis, we analyzed the expression pattern and cellular localization of NRGs and ErbBs within distinct cell types in the SVZ, RMS, and the olfactory bulb (Fig. 1; Figs. 8 and 9 and Table 1, which are published as supporting information on the PNAS web site). In the SVZ, ErbB4 and EGFR (ErbB1) had the most extensive labeling compared with other ErbB subtypes. Antibodies against ErbB4 and EGFR-labeled distinct cell types in the SVZ (Fig. 1A). In the lining of the ventricles adjacent to the SVZ, ErbB4 was expressed by the CD24+ ependymal cells (Fig. 1B), and, as noticed (12), by GFAP+ astroglial progenitors (Fig. 1 A and B). Some of the ErbB4+ cells coexpressed EGFR in the SVZ (Fig. 1A). Of the other ErbB4 heterodimer partners, ErbB2 was expressed in the SVZ and occasionally colocalized with ErbB4 (arrow, Fig. 1C). ErbB3 was expressed mainly in the processes of GFAP+ astrocytes (arrow, Fig. 1D).
Fig. 1.
Distribution of ErbB receptors and NRGs in the SVZ. (A–D) In the SVZ of hGFAP promoter–GFP mice, ErbB4 (A) is expressed in a subset of GFP-expressing SVZ astrocytes (green; open arrow), and EGFR+ (blue) cells (filled arrow, A). (B) ErbB4 is also expressed by a subset of CD24+ ependymal cells (blue). (C and D) ErbB2 is coexpressed in occasional ErbB4+ cells in the SVZ, whereas ErbB3 is expressed mainly by hGFAP-GFP+ astrocytes (arrow). (E–G) PSA-NCAM+ cells in the SVZ express NRG1 and -2. NRG2 also labels some hGFAP-GFP+ cells that line the ventricle (G, arrowheads). [Scale bar: 8 μm (A), 25 μm (B), 20 μm (C), 15 μm (D), and 25 μm (E–G)].
Using polyclonal antibodies raised against a peptide sequence from the cysteine-rich domain (“type III”) or EGF-like domain of NRG1, and against recombinant NRG2β, we assessed the expression pattern of the NRG ligands for the ErbBs. A substantial portion of PSA-NCAM+ migrating neuroblasts in the SVZ and RMS expressed NRG1 and -2 (Figs. 1 E–G and 9). NRG2 is also expressed by a subset of GFAP+, putative stem cells, that line the ventricles (Fig. 1G, arrowheads).
In Vivo Effects of ErbB4 Activation by Their NRG Ligands.
The expression patterns of NRGs and their ErbB receptors in the neurogenic regions of the SVZ suggest that they may play a role in distinct aspects of neural progenitor differentiation. To test the effects of NRG ligands in vivo, we infused GST-NRG1 and GST-NRG2 into the lateral ventricles of adult mice (see Supporting Text and Fig. 10, which are published as supporting information on the PNAS web site, for bioactivity of recombinant NRGs). The proliferative effects of the infused factors on specific cell types in the SVZ and RMS were monitored by using BrdUrd incorporation and immunolabeling with cell-type-specific markers (Fig. 11 A–G, which is published as supporting information on the PNAS web site).
Short-Term Infusion of NRGs.
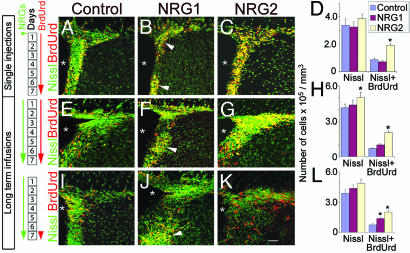
Initially, to determine the short-term effects of ErbB4 ligands on proliferation in the SVZ, short-term infusions (1 μl/h for 24 h, 100 ng/μl; Figs. 2 and 11G) and single injections (5 μl, 500 ng/μl; Fig. 3 A–D) of NRGs were made into the lateral ventricles. In the single-injection experiments, NRG2, but not NRG1, promoted an increase in proliferation in the SVZ (Fig. 3 A–D; Table 2, which is published as supporting information on the PNAS web site). However, the infusion of NRG1 induced the apparent aggregation of BrdUrd+ cells into clusters in the SVZ (Fig. 3B, arrowheads). TUNEL staining of tissue from NRG1, NRG2, and control GST-infused brains indicates no changes in cell death in the SVZ or RMS of the infused brains (data not shown), demonstrating apoptosis is not a notable response to the infusion of recombinant NRGs.
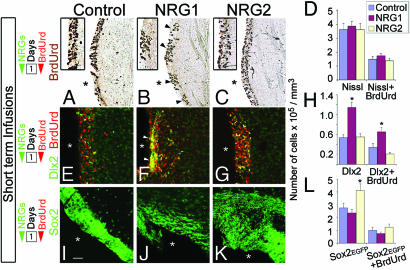
Fig. 2.
Effects of short-term NRG infusion. NRG1, but not NRG2, induced BrdUrd+ cell clustering along the walls of the ventricle (arrowheads in B, Insets in A–C). However, no changes in the density of BrdUrd+ or total number of nuclei (Nissl label) were noticed (D). (E–H) Cells aggregating in response to NRG1 are Dlx2+ progenitors (arrows, F). (I–L) SVZ sections from Sox2-EGFP mice that received NRG infusions indicate that NRG2 induced the expression of the transcription factor Sox2 without a significant increase in BrdUrd incorporation (L). White asterisks denote ventricles. Number of days of NRG and BrdUrd administration is indicated (Left). Data shown are mean ± SEM; ∗, P < 0.01, Student’s t test. [Scale bar: 100 μm (A–C), 65 μm (E–G), and 110 μm (I–K)]. Also see Table 2 and Supporting Text.
Fig. 3.
Long-term effects of NRG infusion on SVZ cell proliferation. Both single (A–D) and continuous (E–G) injections of NRG2 increased the number of BrdUrd+ cells significantly (C and D), whereas NRG1 induced clustering of BrdUrd+ cells (arrowheads, B and F). Similarly, when BrdUrd was administered during the last 24 h of the 7-day NRG infusion period to preferentially label fast-dividing population of SVZ precursors, both NRG1 and -2 increased the number of BrdUrd+ cells. NRG1, as before, induced BrdUrd+ cell clustering (arrowhead, J). White asterisks indicate the position of the ventricles. Number of days of NRG and BrdUrd administration is indicated (Left). Data shown are mean ± SEM; ∗, P < 0.01, Student’s t test. [Scale bar: 50 μm (A, C, E, G, I, K); 65 μm (B, F, J)]. Also see Tables 2, 4, and 5 and Supporting Text.
In contrast to single NRG2 injections, short-term infusions [1 μl/h (100 μg/ml) for 24 h] of NRG2 followed by immediate harvest did not lead to significant changes in cell proliferation (Fig. 2 A–D; Table 3, which is published as supporting information on the PNAS web site). NRG1, however, led to clustering of BrdUrd+ cells in the lateral walls of the ventricles similar to the single NRG1 injection experiments (Fig. 2B, arrowheads). Together, these results suggested that mitotic effects induced by NRG2 required >24 h, whereas NRG1-induced cellular aggregation in the SVZ occurred within 24 h. Analysis of short-term infusion brains with cell-type-specific markers indicated that the cell types most noticeably affected were Dlx2+ and Sox2+ cells in the SVZ (Fig. 2 E–L; Table 3). The clusters of cells aggregated in response to NRG1 are mostly Dlx2+, and their density is significantly higher than in control or NRG2-infused SVZ (Fig. 2 E–H). Moreover, when the SVZ regions from the infused brains were harvested and immunoblotted with anti-Dlx2 antibodies, a noticeable increase in Dlx2 levels was observed in NRG1-infused brains when compared with the control GST-infused brains (Fig. 12, which is published as supporting information on the PNAS web site).
The population of SVZ cells that express the stem cell-specific transcription factor Sox2 increased nearly 2-fold in response to short-term infusions of NRG2 [Fig. 2 I–L (29)]. This increase appears to be a result of induction in the expression of Sox2, without a significant increase in BrdUrd incorporation, suggesting that in response to NRG2 exposure, quiescent SVZ cells may initially express Sox2 without undergoing mitosis (Fig. 2L). In contrast, in NRG1-infused brains, no significant changes in the number of Sox2+ cells in the SVZ were noticed (Fig. 2 J and L). Cells positive for other markers such as Lex/SSEA1 in the SVZ, PSA-NCAM in the RMS, or GABA in the olfactory bulb were not affected after short-term NRG infusions.
Together, our analysis of the short-term exposure of the SVZ cells to distinct NRG isoforms suggests that the response to NRG1 is rapid aggregation of Dlx2+ cells along the lining of the ventricles. On the other hand, NRG2 appears to act rapidly to induce Sox2 expression, and this event precedes the increase in the number of BrdUrd+ cells in the SVZ.
Long-Term Infusion of NRGs.
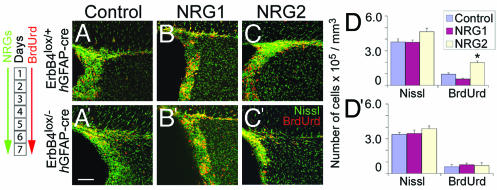
To test the long-term effects of NRGs, mice were infused with NRGs for 7 days [0.5 μl per hour (100 ng/μl)]. We first examined the NRG effects on two sets of slowly dividing putative stem cells. GFAP+ astrocytes that line the ventricles, and Lex/SSEA1+ cells in the SVZ (30). The number of GFAP+ cells significantly increased in response to 7 days of NRG2 infusion, whereas NRG1 had no effect on GFAP+ cells (Fig. 4; Fig. 13, which is published as supporting information on the PNAS web site). Neither NRG1 nor NRG2 induced changes in the number of Lex/SSEA1+ cells.
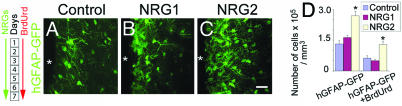
Fig. 4.
Long-term effects of NRG infusion on hGFAP-GFP+ astrocytes in the SVZ. NRG2, but not NRG1 or control GST, infusion for 7 days increased the number of GFAP-GFP+ astrocytes (green) in the SVZ of hGFAP-GFP mice. NRG2 also increased the number of GFAP-GFP+/BrdUrd+ astrocytes in the SVZ (D). Number of days of NRG and BrdUrd administration is indicated (Left). White asterisks indicate the position of the ventricles. Data shown are mean ± SEM; ∗, P < 0.01, Student’s t test. (Scale bar: 30 μm.)
When BrdUrd was administered only during the first day of NRG infusion, to label and be retained undiluted by a majority of the slowly dividing cells, a significant increase in BrdUrd incorporation in putative stem cells lining the lumen of the ventricles was noted in response to NRG2 but not NRG1 infusion (Table 4, which is published as supporting information on the PNAS web site). Together, these experiments suggest that the population of slowly dividing GFAP+ neuronal progenitors is mitogenically responsive to exogenous NRG2.
We next assessed the effects of long-term infusion of NRGs on a resident population of fast-dividing cells by administering BrdUrd during the last 24 h of the 7-day-long NRG infusion period. This schedule of BrdUrd administration preferentially labels fast-dividing progenitors in the SVZ, in addition to labeling many of the slowly dividing cells (6). NRG1 infusion led to aggregated clusters of BrdUrd+ cells in the SVZ (Fig. 3 I and J), and the number of BrdUrd+ cells in the SVZ was significantly higher than controls (Fig. 3L; Table 4). Similarly, NRG2 also significantly increased the number of BrdUrd+ cells (Fig. 3 K and L). The fast-dividing progenitors in the SVZ are known to coexpress Dlx2 and EGFR in the SVZ (6). NRG2, but not NRG1, moderately decreased the EGFR+ cells lining the ventricles (Fig. 13 E–H).
To determine whether NRG-induced changes in SVZ cell proliferation are reflected in the progeny of the SVZ cells, we analyzed the effects of long-term infusions on the number of newly generated migrating neuroblasts in the RMS (see Fig. 14 and Table 5, which are published as supporting information on the PNAS web site) and GABAergic interneurons in the olfactory bulb. NRG2 promoted a significant increase in the number of neuroblasts (PSA-NCAM+/BrdUrd+) in the RMS. Similarly, in the olfactory bulb, the number of GABA+ cells that colabeled with BrdUrd in the granule cell layer was increased in response to NRG2 infusions. In contrast, NRG1 infusions induced a reduction in the number of PSA-NCAM+/BrdUrd+ cells in the RMS or GABA+/BrdUrd+ cells in the granule cell layer of the olfactory bulb.
The Role of NRG in the Initiation of Migration from the SVZ.
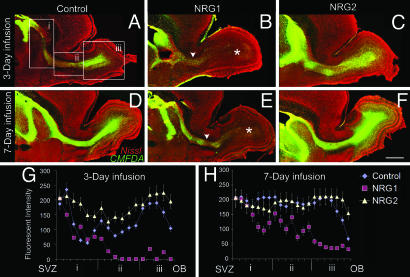
Although NRG1 and -2 differentially affected the proliferation of SVZ cells, it is unclear whether they influenced the initiation of migration of immature neuroblasts from the SVZ. To directly assess the effects of NRG1 and -2 on the initiation of migration from the SVZ, we labeled newly born cells in the SVZ with a fluorescent cell tracker dye [5-chloromethylfluorescein diacetate (CMFDA)] and infused NRGs for 3 or 7 days. CMFDA labeled the streams of migrating neuroblasts from the SVZ to the olfactory bulb (Fig. 5). After 3 days of NRG infusion, there was no significant difference in the extent of migration away from the SVZ into the stream and the olfactory bulbs of control or NRG2-infused brains. In contrast, NRG1 appeared to significantly decrease the initiation of neuroblast migration from the SVZ into the RMS (Fig. 5B). After 7 days of NRG infusion, large numbers of CMFDA-labeled cells had migrated into the RMS and were detected in all layers of the olfactory bulb in both control and NRG2-infused mice. However, only a few of the CMFDA-labeled cells had progressed into the RMS and made their way to the olfactory bulb in NRG1-infused brains (Fig. 5E). Together, these findings suggested that exogenously added NRG1 may prevent the initiation of migration from the SVZ into the RMS by attracting and retaining the cells in the SVZ. In agreement with this hypothesis, when Cos7 cell aggregates, cotransfected with NRG1, were placed adjacent to CMFDA-labeled migrating neuroblasts of the RMS, neuroblasts migrated away from the stream, toward the ectopic source of NRG1 (Fig. 15 and Movies 1 and 2, which are published as supporting information on the PNAS web site).
Fig. 5.
NRG1 inhibits the initiation of migration from the SVZ into the RMS. Single ventricular injections of the cell tracker dye CMFDA (green) were followed by 3 (A–C, G), or 7 days (E–F, H) of continuous infusion of NRGs. Sagittal sections containing the SVZ, RMS, and the olfactory bulbs were Nissl-counterstained (red). SVZ cells and migrating neuroblasts labeled with CMFDA are green. NRG1 infusion for 3 (B and G) or 7 (E and H) days inhibits the emigration of neuroblasts from the SVZ into the RMS. The presence of migrating cells in the RMS (arrowheads, B and E) or the olfactory bulb (asterisks, B and E) is reduced after NRG1 infusion. (G and H) Quantification of the immunofluorescence of CMFDA+ cells from the SVZ to the olfactory bulb (OB) indicates a significant decrease in the number of migrating cells in the RMS or the olfactory bulb after NRG1 infusion. NRG2, however, increased the number of CMFDA+ cells in the RMS and in the olfactory bulb. Boxes in A i–iii depict the regions on the SVZ-RMS-OB axis, where immunofluorescence intensity was measured. Data shown are mean ± SEM; ∗, P < 0.01, Student’s t test. (Scale bar: 500 μm.)
The Effects of NRG Infusions in ErbB4-Deficient Mice.
To determine whether ErbB4, the predominant NRG receptor expressed in the SVZ, plays a critical role in mediating the effects of various NRG isoforms, we used CNS-specific ErbB4 conditional null mice (ErbB4lox/− hGFAP-cre) to test the effects of the NRGs. ErbB4 conditional null mice were infused with NRGs (0.5 μl per hour; 100 ng/μl) for 7 days and administered BrdUrd three times per day during this period. This schedule of BrdUrd administration would label all of the different proliferative populations in the SVZ. The effects on SVZ cell proliferation mediated by NRG2 are not detected in the absence of ErbB4 (Fig. 6; Table 6, which is published as supporting information on the PNAS web site). As with the control mice, no effect on overall proliferation was seen with NRG1 in ErbB4-deficient mice. Therefore, our results with the ErbB4 conditional null mice support the hypothesis that ErbB4 signaling is essential for the mitogenic signaling mediated by NRG2 in the SVZ and RMS.
Fig. 6.
Conditional deletion of ErbB4 retards NRG2-mediated increase in SVZ cell proliferation. In control mice (ErbB4lox/+ hGFAP-cre), 7-day infusion of NRG2 resulted in a significant increase in the number of BrdUrd+ cells in the SVZ (A–D). In ErbB4 mutant mice (ErbB4lox/− hGFAP-cre), infusion of NRG2 did not increase SVZ cell proliferation (A′–D′). NRG1, as in controls, did not change SVZ cell proliferation (B, B′, and D′). Data shown are mean ± SEM; ∗, P < 0.01, Student’s t test. (Scale bar: 130 μm.) Also see Table 5 and Supporting Text.
Changes in the Composition of SVZ in ErbB4-Deficient Mice.
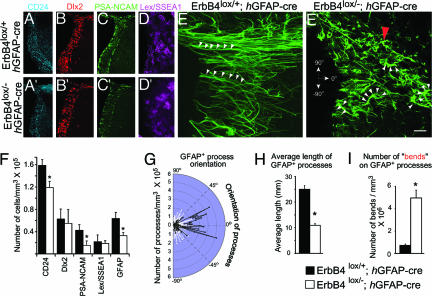
Because ErbB4 receptors expressed in the SVZ are critical for NRG, we examined changes in the organization and numbers of distinct cell types in the SVZ as a result of ErbB4 inactivation. We immunohistochemically labeled distinct cell types in the SVZ using antibodies to the following markers: CD24 for ependymal cells, GFAP for astrocytes and stem cells, Lex/SSEA1 for a subpopulation of stem cells, Dlx2 for rapidly proliferating precursors, and PSA-NCAM for migrating neuroblasts. Overall, the number of CD24+, GFAP+, and PSA-NCAM+ cells was significantly lower in the ErbB4 conditional null mice (Fig. 7; Table 6). In contrast, the density of Dlx2+ and Lex/SSEA1+ cells was not changed.
Fig. 7.
Conditional deletion of ErbB4 disrupts the cellular organization of the SVZ. Distribution of CD24+ ependymal cells, Dlx2+ progenitors, PSA-NCAM+ neuroblasts, Lex/SSEA1+ stem cells, and GFAP+ astrocytic cells in the SVZ of control (A–E) and conditional mutant mice lacking the ErbB4 receptor (ErbB4lox/− hGFAP-cre; A′–E′). The characteristic elongated GFAP+ processes oriented radially away from the ventricular surface (arrowheads, E) are shorter and disrupted in their normal orientation in ErbB4 mutants (arrowheads, E′). (F) Quantification of changes in density (number of cells per mm3) of distinct cell types in control and ErbB4 mutant SVZ indicates reduced density of CD24+, PSA-NCAM+, and GFAP+ cells in the SVZ. (G–I) Quantification of GFAP+ process orientation, relative to the ventricular surface indicates that that orientation of GFAP+ processes is widely dispersed in ErbB4 conditional mutants compared with controls (G). In addition, the length and number of “bends” (red arrowhead, E′) of GFAP+ processes are also altered in ErbB4 mutants (H and I). Data shown are mean ± SEM; ∗, P < 0.01, Student’s t test. [Scale bar: 240 μm (A,A′–C,C′); 140 μm (D,D′); 20 μm (E,E′).]
To determine whether changes in the density of distinct cell populations in the absence of NRG-ErbB4 signaling leads to changes in the organization of distinct cell types in the SVZ, we examined the interrelationship of cell types present in the SVZ. First, we noticed that the radial processes of GFAP+ cells within the anterior lining of the ventricles appeared disrupted compared with control littermates (Fig. 7 E and E′). Next, to assess the effects of ErbB4 deletion and the abnormalities in the GFAP+ cells on positioning and anatomical distribution of other cell types in the SVZ, we mapped their position in the SVZ (Fig. 16, which is published as supporting information on the PNAS web site). Although there were no changes in the overall positional distribution pattern of these cells, the number of GFAP+, Dlx2+, and PSA-NCAM+ cell clusters was significantly lower in the SVZ of ErbB4 conditional null mice.
Discussion
The characterization of factors and guidance molecules that regulate proliferation and direct or organize the migration of neuroblasts within the SVZ and the RMS is essential to decipher the functional significance of adult neurogenesis. Here, we show that ErbB4 receptors and their ligands, the NRGs, are expressed by cells in the SVZ, and that distinct NRG ligands can trigger specific mitogenic and motogenic activities in distinct cell types of the adult SVZ in vivo.
NRGs and Their ErbB Receptors Are Expressed by Distinct Cell Types in the SVZ and RMS.
ErbB receptors and their activating NRG ligands are expressed by distinct cell types in the SVZ and RMS. Of the receptors, ErbB4 and EGFR exhibit the highest level of expression and are expressed by distinct cell types in the SVZ. ErbB4 is primarily expressed by PSA-NCAM+ neuroblasts that migrate toward the olfactory bulb and by a subset of GFAP+ and CD24+ ependymal cells lining the ventricles. EGFR, as previously demonstrated, is expressed by distinct Dlx2+ progenitors and by a few GFAP+ cells that contact the ventricles (6). ErbB3 is restricted to a subset of GFAP+ astrocytes lining the ventricles and within the RMS.
Of the ligands for the ErbB receptors, NRG1 had the most extensive expression in the SVZ and RMS, exhibiting near-perfect colocalization with PSA-NCAM+ neuroblasts. NRG2 was expressed by the PSA-NCAM+ neuroblasts in the SVZ and by a subset of GFAP+ cells lining the ventricles in the SVZ. The NRG1-expressing PSA-NCAM+ cells and the subset of GFAP+ cells that express NRG2 together account for most of the observed NRG expression in the SVZ. The expression of the ErbBs is more complex, with subpopulations of each of the different SVZ cell types expressing distinct combinations of these receptors (Table 1). Therefore the endogenous NRG-mediated effects on distinct cell types within the SVZ are likely combinatorial effects determined by these specific patterns of NRG ligand and ErbB receptor expression. Whether these effects are mediated through autocrine mechanisms as detected in epithelial cells (31), through juxtacrine mechanisms as is believed to occur in axon–Schwann cell interactions, or through paracrine mechanisms as seen in cardiac endothelial cell–myocardiocyte interactions (18) remains to be elucidated. Additionally, NRGs can induce their effects on distinct SVZ cell types indirectly and the relative in vivo contribution of other ErbB4 ligands such as betacellulin, heparin-binding EGF, and epiregulin in SVZ cell differentiation remains unclear at this juncture.
NRG2 Increases Mitotic Events in the SVZ, Number of Migrating Neuroblasts in the RMS, and Young Interneurons in the Olfactory Bulb.
Infusion of NRG1 and -2 into the ventricles led to distinct outcomes. NRG2 was notable for its ability to promote cell proliferation, whereas NRG1 was not mitogenic but instead fostered changes in the cellular organization of the SVZ. Infusion of NRG2 increased the number of GFAP+ cells near the lining of the ventricles, which are thought to include the slowly dividing putative stem cells (6). The increase in GFAP+ cells is preceded by an increase in the expression of the transcription factor Sox2. This transcription factor is thought to regulate DNA bending (32) and has been implicated in the maintenance of progenitor cells in a proliferative state (33, 34). The increase in Sox2 expression in response to NRG2 may enable normally quiescent SVZ cells to become mitotic, resulting ultimately in an increase in GABA+ interneurons in the granule cell layer of the olfactory bulb in response to NRG2 infusion.
The effects of infused NRG2 differ from that observed for FGF2, TGF-α, and EGF. The infusion of FGF2 promoted proliferation but had a modest inhibitory effect on migration to the bulb (35), whereas NRG2 increased both proliferation and migration. The infusion of EGF and TGF-α, two ligands for ErbB1/EGFR, led to a preferential increase in the number of astrocytes but to a marked decrease in cell migration and reduced interneuronal production in the olfactory bulb, even after the termination of infusions (6, 35, 36). Disruption of eph/ephrin signaling has also been shown to increase cell proliferation and alter migration in the SVZ (37). Therefore, although NRG2, FGF2, EGF, TGF-α, and ephrin all promote mitotic events in the SVZ, NRG2 is unique in that it enhances both the proliferation of SVZ cells as well as their differentiation into interneurons. The conditional nervous system-specific loss of the NRG2 receptor ErbB4 resulted in the abrogation of the effects of the infusion of this factor. However, it remains to be determined whether SVZ cell proliferation or neuroblast migration is disrupted in NRG2-deficient mice (38).
The Effects of NRG1 in the SVZ.
In contrast to the effects mediated by NRG2, NRG1 induced aggregation of neuronal progenitors into clusters in regions near the lining of the ventricles. Intriguingly, NRG1 increased the density of Dlx2+ progenitors in the SVZ (Fig. 2F) without an overall increase in SVZ proliferation as indicated by BrdUrd incorporation (Fig. 3F). Many PSA-NCAM+ cells in the SVZ, before their migration into the RMS, coexpress Dlx2 (6). One possible explanation for the increased density of Dlx2+ cells in the absence of increased proliferation is that ventricle-infused NRG1 may attract the PSA-NCAM+/Dlx2+ cells to aggregate into clusters in the SVZ. Thus, it would be possible to have an increase in the density of Dlx2+ cells without an overall increase in their proliferation in the SVZ. An alternative but not mutually exclusive hypothesis is that NRG1 may be capable of inducing the expression of Dlx2 in the absence of proliferation.
NRG1 in the ventricle may attract and retain immature neuroblasts in the SVZ region, thus preventing their normal emigration into the RMS and promoting their apparent aggregation in the SVZ. Ectopic sources of NRG1 presented along the route of the RMS can function to attract RMS neuroblasts. Previously, NRG1 has been shown to function in vitro as a chemoattractant for the tangentially migrating neurons of the embryonic cortex and as a promoter of SVZ-derived neuroblast migration in the postnatal forebrain as well as of glial-guided neuronal migration in the developing cortex (12, 26, 28). It should be noted, however, that in situ hybridization studies have not provided evidence for graded NRG1 expression in the SVZ or RMS, suggestive of a chemoattractive role. Thus the chemoattractive function of NRG1 observed in vitro in the SVZ/RMS is likely to be context-dependent and may rely on the concomitant activity of other cell surface receptors such as integrins.
An alternative explanation for the NRG1-induced BrdUrd+ cell clustering is the focal proliferation of precursors in response to NRG1. The presence of BrdUrd+ cell-sparse regions surrounding the BrdUrd+ cell clusters and the observation that ErbB4 is expressed in many but not all GFAP+ precursors lining the ventricular zone suggest that BrdUrd+ cell clusters may occur as a result of focal proliferation of NRG1-activated neural precursors in the SVZ. Clustered proliferation of precursor cells in the SVZ may facilitate the coordinated generation of appropriate subsets of neurons, as observed in the embryonic cortex (39).
Role of ErbB4 in the Cellular Organization of the SVZ.
NRG signaling is mediated by ErbB receptors. Analysis of the cellular organization of the SVZ in ErbB4 mutant mice indicates that inactivation of ErbB4 leads to significantly lower cellular density and disrupted intercellular organization of Dlx2+ cells and GFAP+ astroglia lining the ventricles, as well as the PSA-NCAM+ neuroblasts in the SVZ. The generation and differentiation of these cell types may be in part regulated via NRG-ErbB4 signaling. The GFAP+ astrocytes adjacent to the ventricles display significant disruption in the polarized orientation of their processes. This GFAP+ population of cells may be multifunctional, some functioning as neuronal precursors, and others helping to orient and guide initial neuroblast migration away from the SVZ. Thus, the disruption in PSA-NCAM+ neuroblast chain organization observed in ErbB4 mutants and the resultant mal-positioning of newly generated interneurons in the olfactory bulb (12) may occur in part due to disrupted organization of GFAP+ glial cells in the SVZ.
Together, these findings have revealed that two different NRG ligands can exert markedly distinct effects on the cell types present in the mature SVZ (Fig. 17, which is published as supporting information on the PNAS web site). Whether this ability of distinct NRG ligands to directly or indirectly modulate distinct cell types in the SVZ depends on specific NRG–ErbB receptor combinations or the cellular context provided by the engagement of other cell surface receptors, such as integrins, remains to be elucidated. However, the ability to modulate the proliferation, migration, and differentiation of discrete subsets of cells in the SVZ will be of use in using endogenous neurogenesis as a potential avenue of CNS repair. Furthermore, NRG1 was recently identified as a susceptibility gene for schizophrenia (40, 41). Abnormal NRG1 signaling in the SVZ and the resultant changes in neuroblast generation and placement may alter the neural circuitry of the brain and contribute to neurodevelopmental disorders such as schizophrenia.
Methods
Recombinant NRG Proteins.
Recombinant NRG proteins were constructed by expressing the EGF-like domains of NRG1β and -2β as C-terminal GST fusion proteins. See Supporting Text for details on their production and analysis of bioactivity.
Infusion of NRGs.
We infused 0.1 μg/μl GST-NRG1, GST-NRG2, or GST in artificial cerebrospinal fluid, using Alzet (Palo Alto, CA) pumps (Durect, Cupertino, CA) for either short-term (24-h) or long-term (7-day) survival periods. Pumps were implanted into the lateral ventricles in 8-week-old adult CD-1 ErbB4, conditional null, littermate control, Sox2-EGFP, or hGFAP-GFP mice. The SVZ and RMS ipsilateral to the infusion sites were analyzed. See Supporting Text for details.
Analysis of NRG-Infused Brains.
After the implantation of pumps, the animals were administered BrdUrd (100 mg/kg) three times during the 24-h short-term infusion and on three different schedules during the long-term infusion experiments [Fig. 11G (42, 43)]. See Supporting Text for details on BrdUrd administration, BrdUrd-labeled cell analysis (44), and characterization of cell types in the SVZ, RMS, and olfactory bulb.
Analysis of Neuroblast Migration from the SVZ into the RMS.
To directly assess the effects of infused NRGs on the migration of neuroblasts from the SVZ into the RMS, a single ventricular injection [0.5 μl (10 nM)] of the cell tracker dye 5-chloromethylfluorescein diacetate (CMDFA; Molecular Probes) was made before the continuous infusion of GST, NRG1, or NRG2 for either 3 or 7 days (1 μl/h; 100 ng/μl). After NRG infusion, mice were perfused intracardially with 4% paraformaldehyde, brains were sagittally sectioned (50 μm thick), and the extent of migration of CMFDA-labeled cells away from the SVZ into the RMS was quantified. See Supporting Text for details.
Analysis of Cellular Organization of the SVZ.
Tissue sections from control and ErbB4 conditional null mice were immunostained with markers for distinct cell types in the SVZ. Changes in the cellular organization of the SVZ in these sections were evaluated by using confocal imaging and the stereoinvestigator software (MicroBrightField Inc., Williston, VT), as described in Supporting Text.
Slice Overlay Assay for the Function of NRG1.
CMFDA-labeled neuroblasts in the RMS were presented with Cos cell explants expressing different NRG1 isoforms, and changes in neuroblast migration were analyzed in real time. See Supporting Text for details.
Supplementary Material
Acknowledgments
We thank S. Spangler for technical assistance and Drs. F. Longo and J. Weimer for helpful comments. This work was supported by National Institutes of Health Grant NS39411 (to C.L. and E.S.A.), by a National Research Service Award fellowship (to H.T.G.), and by the confocal imaging core of the University of North Carolina Neuroscience Center (National Institute of Neurological Disorders and Stroke Center Grant P30NS045892).
Abbreviations
- RMS
rostral migratory stream
- NRG
neuregulin
- SVZ
subventricular zone
- EGFR
EGF receptor
- CMFDA
5-chloromethylfluorescein diacetate
- PSA-NCAM+
polysialylated neural cell adhesion molecule.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Altman J., Das G. D. J. Comp. Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 2.Levison S. W., Chuang C., Abramson B. J., Goldman J. E. Development (Cambridge, U.K.) 1993;119:611–622. doi: 10.1242/dev.119.3.611. [DOI] [PubMed] [Google Scholar]
- 3.Lois C., Alvarez-Buylla A. Proc. Natl. Acad. Sci. USA. 1993;90:2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luskin M. B. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- 5.Coskun V., Luskin M. B. J. Neurosci. Res. 2002;69:795–802. doi: 10.1002/jnr.10336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doetsch F., Petreanu L., Caille I., Garcia-Verdugo J. M., Alvarez-Buylla A. Neuron. 2002;36:1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- 7.Seaberg R. M., Van Der Kooy D. J. Neurosci. 2002;22:1784–1793. doi: 10.1523/JNEUROSCI.22-05-01784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seri B., Garcia-Verdugo J. M., McEwen B. S., Alvarez-Buylla A. J. Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Temple S. Nature. 2001;414:112–117. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- 10.Belluzzi O., Benedusi M., Ackman J., LoTurco J. J. J. Neurosci. 2003;23:10411–10418. doi: 10.1523/JNEUROSCI.23-32-10411.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Betarbet R., Zigova T., Bakay R. A., Luskin M. B. Int. J. Dev. Neurosci. 1996;14:921–930. doi: 10.1016/s0736-5748(96)00066-4. [DOI] [PubMed] [Google Scholar]
- 12.Anton E. S., Ghashghaei H. T., Weber J. L., McCann C., Fischer T. M., Cheung I. D., Gassmann M., Messing A., Klein R., Schwab M. H., et al. Nat. Neurosci. 2004;7:1319–1328. doi: 10.1038/nn1345. [DOI] [PubMed] [Google Scholar]
- 13.Plowman G. D., Green J. M., Culouscou J. M., Carlton G. W., Rothwell V. M., Buckely S. Nature. 1993;366:473–475. doi: 10.1038/366473a0. [DOI] [PubMed] [Google Scholar]
- 14.Carraway K. L., III, Sliwkowski M. X., Akita R, Platko J. V., Guy P. M., Nuijens A., Diamonti A. J., Vandlen R. L., Cantley L. C., Cerione R. A. J. Biol. Chem. 1994;269:14303–14306. [PubMed] [Google Scholar]
- 15.Tzahar E., Levkowitz G., Karunagaran D., Yi L., Peles E., Lavi S., Chang D., Liu N., Yayon A., Wen D., et al. J. Biol. Chem. 1994;269:25226–25233. [PubMed] [Google Scholar]
- 16.Sliwkowski M. X., Schaefer G., Akita R. W., Lofgren J. A., Fitzpatrick V. D., Nuijens A., Fendly B. M., Cerione R. A., Vandlen R. L., Carraway K. L., III J. Biol. Chem. 1994;269:14661–14665. [PubMed] [Google Scholar]
- 17.Yarden Y., Sliwkowski M. X. Nat. Rev. Mol. Cell. Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 18.Falls D. L. Exp. Cell. Res. 2003. 2003;284:14–30. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- 19.Buonanno A., Fischbach G. D. Curr. Opin. Neurobiol. 2001;11:287–296. doi: 10.1016/s0959-4388(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 20.Michailov G. V., Sereda M. W., Brinkmann B. G., Fischer T. M., Haug B., Birchmeier C., Role L., Lai C., Schwab M. H., Nave K. A. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- 21.Garratt A. N., Britsch S., Birchmeier C. BioEssays. 2000;22:987–996. doi: 10.1002/1521-1878(200011)22:11<987::AID-BIES5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 22.Fischbach G. D., Rosen K. M. Annu. Rev. Neurosci. 1997;20:429–458. doi: 10.1146/annurev.neuro.20.1.429. [DOI] [PubMed] [Google Scholar]
- 23.Rimer M. J. Neurocytol. 2003;32:665–675. doi: 10.1023/B:NEUR.0000020615.79831.51. [DOI] [PubMed] [Google Scholar]
- 24.Mejat A., Ravel-Chapuis A., Vandromme M., Schaeffer L. Ann. N.Y. Acad. Sci. 2003;998:53–65. doi: 10.1196/annals.1254.008. [DOI] [PubMed] [Google Scholar]
- 25.Hippenmeyer S., Shneider N. A., Birchmeier C., Burden S. J., Jessell T. M., Arber S. Neuron. 2002;36:1035–1049. doi: 10.1016/s0896-6273(02)01101-7. [DOI] [PubMed] [Google Scholar]
- 26.Anton E. S., Marchionni M. A., Lee K. F., Rakic P. Development (Cambridge, U.K.) 1997;124:3501–3510. doi: 10.1242/dev.124.18.3501. [DOI] [PubMed] [Google Scholar]
- 27.Rio C., Rieff H. I., Qi P., Khurana T. S., Corfas G. Neuron. 1997;19:39–50. doi: 10.1016/s0896-6273(00)80346-3. [DOI] [PubMed] [Google Scholar]
- 28.Flames N., Long J. E., Garratt A. N., Fischer T. M., Gassmann M., Birchmeier C., Lai C., Rubenstein J. L., Marin O. Neuron. 2004;44:251–261. doi: 10.1016/j.neuron.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 29.Ellis P., Fagan B. M., Magness S. T., Hutton S., Taranova O., Hyashi S., McMahon A., Rao M., Pevny L. Dev. Neurosci. 2004;26:148–165. doi: 10.1159/000082134. [DOI] [PubMed] [Google Scholar]
- 30.Capela A., Temple S. Neuron. 2002;35:865–875. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 31.Vermeer P. D., Einwalter L. A., Moninger T. O., Rokhlina T., Kern J. A., Zabner J., Welsh M. J. Nature. 2003;422:322–326. doi: 10.1038/nature01440. [DOI] [PubMed] [Google Scholar]
- 32.Scaffidi P., Bianchi M. E. J. Biol. Chem. 2001;276:47296–47302. doi: 10.1074/jbc.M107619200. [DOI] [PubMed] [Google Scholar]
- 33.Graham V., Khudyakov J., Ellis P., Pevny L. Neuron. 2003;39:749–765. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- 34.Bylund M., Andersson E., Novitch B. G., Muhr J. Nat. Neurosci. 2003;6:1162–1168. doi: 10.1038/nn1131. [DOI] [PubMed] [Google Scholar]
- 35.Kuhn H. G., Winkler J., Kempermann G., Thal L. J., Gage F. H. J. Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Craig C. G., Tropepe V., Morshead C. M., Reynolds B. A., Weiss S., van der Kooy D. J. Neurosci. 1996;16:2649–2658. doi: 10.1523/JNEUROSCI.16-08-02649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conover J. C., Doetsch F., Garcia-Verdugo J. M., Gale N. W., Yancopoulos G. D., Alvarez-Buylla A. Nat. Neurosci. 2000;3:1091–1097. doi: 10.1038/80606. [DOI] [PubMed] [Google Scholar]
- 38.Britto J. M., Lukehurst S., Weller R., Fraser C., Qiu Y., Hertzog P., Busfield S. J. Mol. Cell. Biol. 2004;24:8221–8226. doi: 10.1128/MCB.24.18.8221-8226.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weissman T. A., Riquelme P. A., Ivic L., Flint A. C., Kriegstein A. R. Neuron. 2004;43:647–661. doi: 10.1016/j.neuron.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 40.Stefansson H., Sigurdsson E., Steinthorsdottir V., Bjornsdottir S., Ghosh S., Brynjolfsson J., Gunnarsdottir S., Ivarsson O., Chou T. T., et al. Am. J. Hum. Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corfas G., Roy K., Buxbaum J. D. Nat. Neurosci. 2004;7:575–580. doi: 10.1038/nn1258. [DOI] [PubMed] [Google Scholar]
- 42.Doetsch F., Garcia-Verdugo J. M., Alvarez-Buylla A. Proc. Natl. Acad. Sci. USA. 1999;96:11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doetsch F., Garcia-Verdugo J. M., Alvarez-Buylla A. J. Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palmer T. D., Willhoite A. R., Gage F. H. J. Comp. Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.