Abstract
Differences in T cell receptor (TCR) signaling initiated by interactions among TCRs, coreceptors, and self-peptide–MHC complexes determine the outcome of CD4 versus CD8 lineage of T cell differentiation. The H-2Ld and Kbm3 alloreactive 2C TCR is positively selected by MHC class I Kb and a yet-to-be identified nonclassical class I molecule to differentiate into CD8+ T cells. Here we describe two mechanisms by which CD4+ 2C T cells can be generated in 2C TCR-transgenic mice. In the RAG−/− background, development of CD4+ 2C T cells requires the expression of both I-Ab and the TAP genes, indicating that both MHC class I and II molecules are required for positive selection of these T cells. Notably, only some of the 2C+RAG−/− mice (≈30%) develop CD4+ 2C T cells, with frequencies in individual mice varying from 0.5% to as high as ≈50%. In the RAG+ background, where endogenous TCRα genes are rearranged and expressed, CD4+ 2C T cells are generated because these cells express the 2C TCR as well as additional TCRs, consisting of the 2C TCRβ and endogenous TCRα chains. Similarly, T cells expressing the OT-1 TCR, which is nominally MHC class I-restricted, can also develop into CD4+ T cells through the same two mechanisms. Thus, expression of two TCRs by a single thymocyte, TCR recognition of multiple MHC molecules, and heterogeneity of TCR, coreceptors, and peptide–MHC interactions in the thymus all contribute to the outcome of CD4 versus CD8 lineage development.
Keywords: coreceptors, lineage differentiation, antigen recognition, degeneracy
The antigens recognized by αβ T cell receptors (TCR) are MHC class I and class II molecules in association with peptides (1–3). Most αβ T cells belong to one of the two lineages defined by the mutually exclusive expression of CD8 or CD4 coreceptors, which bind MHC class I and class II molecules, respectively. Because the binding of the coreceptors to MHC molecules stabilizes weak interactions between TCRs and peptide–MHC (pMHC) complexes, CD8+ T cells are MHC class I-restricted whereas CD4+ T cells are MHC class II-restricted. The concordance of CD4 or CD8 lineage development with specificity for class I or II MHC is established during differentiation of CD4+CD8+ double-positive (DP) thymocytes in the thymus by a process referred to as positive selection (4). Whether a DP thymocyte differentiates into a CD4+ or CD8+ T cell depends on differences in TCR signaling as a result of interactions among TCR, coreceptors, and self-peptide–MHC (self-pMHC) complexes (5–8).
Many TCRs are known to interact not only with self- but also with foreign MHC molecules (alloreactivity). Among the best-documented examples is the recognition of different pMHC complexes by a TCR called 2C (9). The 2C TCR was derived from a CD8+ cytotoxic T lymphocyte (CTL) clone from an H-2b mouse (BALB.B) that was injected with H-2d cells (10, 11). The 2C TCR reacts with Ld in association with two overlapping peptides QLSPFPFDL (QL9) and p2Ca derived from α-ketoglutarate dehydrogenase (10, 12, 13). The 2C TCR is also alloreactive to Kbm3 in association with the dEV8 peptide derived from NADH-ubiquinone oxoreductase (14, 15). By constructing transgenic mice expressing the 2C TCR and crossing the TCR transgene onto different H-2 backgrounds, it was shown that the positive selecting MHC for the 2C T cells is Kb (16, 17). Among the naturally derived peptides recognized by the 2C TCR in association with Ld and Kbm3, p2Ca and dEV8 bind weakly to Kb and behave as weak agonists (18). However, several strong agonist peptides have been identified in combinatorial libraries to associate with Kb and activate the 2C T cells (19). Among them, SIYRYYGL (SIY) has been extensively characterized. In addition to recognizing various peptides in association with MHC class I Ld, Kbm3, and Kb molecules, the 2C TCR was also shown recently to recognize a nonclassical class I molecule, leading to differentiation of CD8+ T cells that home to the intestine during fetal and neonatal development (20).
The ability of a given TCR to interact with multiple MHC molecules, especially both class I and class II, can have a significant influence on the development of CD4 versus CD8 T cells. For example, the HY TCR nominally recognizes class I Db for CD8 T cell development but also interacts with class II I-Ab for CD4 T cell development (21). Similarly, the HA and AND TCRs nominally interact with class II MHC for CD4 T cell development but also promote the development of CD8 T cells on either the RAG−/− or the CD4−/− plus TCRα−/− background (22, 23). The development of CD8 HA or AND transgenic T cells was shown to require the interaction of the TCRs with both class I and class II MHC molecules.
Further complicating CD4 and CD8 lineage differentiation is the possibility that a given T cell expresses two different TCRs. Although allelic exclusion at the TCRβ locus is stringently enforced at the DNA rearrangement level, there is no apparent allelic exclusion during the TCRα rearrangement (24–26). Some T cell lines have been shown to possess two functional TCRα rearrangements but to express only one TCRα polypeptide, indicating TCRα allelic exclusion at the transcription and/or posttranscription levels (27–29). However, ≈20% of T cells in humans and 10% of T cells in mice are estimated to express two TCRα chains, resulting in a significant fraction of T cells that express two TCRs (30). When two TCR transgenes are introduced into the same mouse, individual T cells from the resulting transgenic mice can express two different TCRs, as in mice expressing the OT-1 and P14 TCRs (31) or AND and 3A9 TCRs (32). Because DP thymocytes that express two TCRs are likely to recognize additional self-pMHC complexes, the presence of two TCRs could significantly affect CD4 versus CD8 lineage development. However, how the interactions of a single TCR with multiple MHC molecules and how multiple TCRs expressed by the same developing thymocyte affect CD4 versus CD8 lineage differentiation in the thymus have been examined to only a limited extent.
In our studies of CD8+ T cells expressing the 2C TCR, we noticed that a high percentage of 2C TCR-transgenic mice harbor 2C+ T cells that are CD4+ (CD8−), occasionally as many as 25–50% of total 2C T cells in spleen and lymph nodes. Here we show that these CD4+ 2C T cells are generated through two different mechanisms. In the RAG−/− background, the CD4+ 2C T cells are generated because they are positively selected by means of the 2C TCR by class II I-Ab as well as class I Kb molecules. In the RAG+ background, CD4+ 2C T cells are generated because the T cells express other TCRs in addition to the 2C TCR. The findings demonstrate that the 2C TCR recognizes peptides in association with not only the class I Ld, Kbm3, and Kb and a yet-to-be identified nonclassical class I molecule but also the class II I-Ab molecule. Our findings also show that the expression of two TCRs in a cell, TCR recognition of multiple MHC molecules, and the nonuniformity of TCR interactions with coreceptors and pMHC complexes in the thymus all contribute to the outcome of CD4 versus CD8 lineage development.
Results
Development of CD4+ 2C T Cells in 2C+RAG−/− Mice.
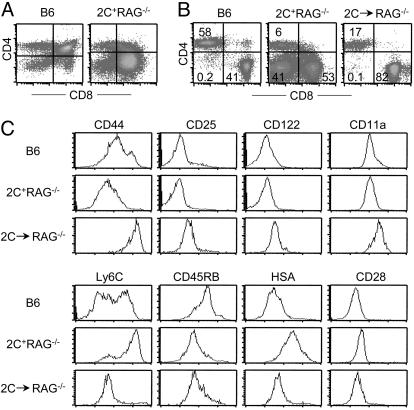
In adult mice, CD8+ T cells that express the 2C TCR are positively selected by the Kb molecule. Consistently, in 2C TCR-transgenic mice on the H-2b (B6) and RAG1−/− backgrounds (referred to as 2C+RAG−/− mice), T cell development in the thymus is biased toward the CD8 lineage and most of the T cells in the periphery are CD8+ (Fig. 1 A and B). Although a substantial fraction of 2C T cells in spleen and lymph nodes were CD4−CD8−, ≈30% of the 2C+RAG−/− mice harbored CD4+ (CD8−) 2C+ T cells, with frequencies in individual mice ranging from 0.5% to as high as ≈50% (Figs. 1B and 2B). The CD4+ 2C T cells were likely generated in the thymus because CD4 single-positive thymocytes were frequently detected in the thymus of 2C+RAG−/− mice (Fig. 1A).
Fig. 1.
Generation of mature CD4+ 2C T cells in 2C+RAG−/− mice. (A) Comparison of CD4 versus CD8 staining profiles of thymocytes from a B6 and a 2C+RAG−/− mouse. (B) Presence of mature CD4+ 2C T cells in the lymph nodes of 2C+RAG−/− mice. Lymph node cells from B6 mice, 2C+RAG−/− mice, and RAG−/− mice that had been adoptively transferred 2 months previously with naïve 2C T cells from 2C+RAG−/− mice were stained with antibodies specific for the 2C TCR (or for TCRβ for B6 mice), CD4, CD8 plus CD44, CD25, CD122, CD11a, Ly6C, CD45RB, HSA, or CD28. The two-dimensional dot plots show CD4 versus CD8 expression by 2C+ cells (or TCRβ+ cells for B6 mice). The numbers indicate percentages of cells in the specific quadrant. (C) Comparison of selected surface markers among CD4+ T cells from B6 mice, 2C+RAG−/− mice, and RAG−/− mice that had been adoptively transferred with naïve 2C T cells. Continued from B, histograms show expression profiles of indicated markers by CD4+ 2C+ T cells or CD4+ TCRβ+ T cells (for B6 mice).
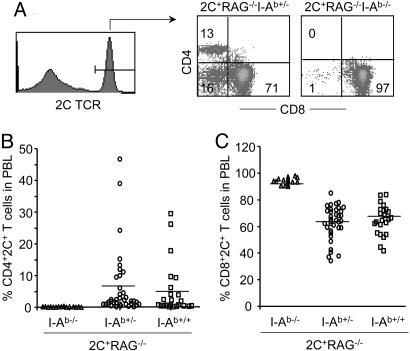
Fig. 2.
Development of CD4+ 2C T cells in 2C+RAG−/− mice requires MHC class II I-Ab. PBMC from 2C+RAG−/−I-Ab+/+, 2C+RAG−/−I-Ab+/−, and 2C+RAG−/−I-Ab−/− mice were analyzed for 2C TCR, CD4, and CD8 expression. (A) A representative histogram showing 2C TCR expression by all PBMC and dot plots showing CD4 versus CD8 expression gating on 2C+ T cells for representative 2C+RAG−/−I-Ab+/− and 2C+RAG−/−I-Ab−/− mice. The numbers indicate percentages of cells in each quadrant. (B and C) Percentages of CD4+ 2C+ T cells and CD8+ 2C+ T cells in PBMC are shown for 2C+RAG−/−I-Ab+/+, 2C+RAG−/−I-Ab+/−, and 2C+RAG−/−I-Ab−/− mice. One symbol represents one mouse. The horizontal line indicates the average within each genotype.
Similar to CD4+ T cells in lymph nodes of B6 mice, the CD4+ 2C T cells in 2C+RAG−/− mice exhibited a naïve phenotype as indicated by the expression patterns of CD44, CD25, CD122, CD11a, and Ly6C (Fig. 1C). However, different from CD4+ T cells in B6 mice, which were CD45RBhi and HSAlo, the CD4+ 2C T cells in 2C+RAG−/− mice were CD45RBlo and HSAhi, resembling the phenotype of CD4 single-positive thymocytes (Fig. 1C and data not shown). Whether these differences are significant or merely the result of comparing a monoclonal population of T cells in 2C+RAG−/− mice with a polyclonal population of T cells in B6 mice is not clear. It is clear, however, that the CD4+ 2C T cells in the lymph nodes of 2C+RAG−/− mice were not short-lived, immature T cells that somehow managed to migrate into the periphery; thus, when adoptively transferred into nonirradiated syngeneic RAG1−/− mice, the CD4+ 2C T cells persisted in the recipients for months and acquired a memory-like CD44hi, CD45RBlo, CD122+, and Ly6Clo phenotype, similar to transferred CD8+ 2C T cells (Fig. 1 B and C and data not shown), indicating that they can undergo lymphopenia-induced development (33, 34). Together, these findings show that mature CD4+ 2C T cells can develop in RAG1−/− mice that express a single 2C TCR known to be restricted by MHC class I.
Development of CD4+ 2C T Cells Requires both MHC Class I and II Molecules.
The development of CD4+ 2C T cells in 2C+RAG−/− mice raises the question of whether these T cells are positively selected by MHC class I or class II molecules or both. To answer the question, we generated 2C+RAG−/− mice on an I-Ab+/+, I-Ab+/−, or I-Ab−/− background. Because B6 mice are naturally defective in I-Eα expression, 2C+RAG−/−I-Ab−/− mice are deficient in MHC class II molecules, except for a low level of I-Aα/I-Eβ heterodimer (35). As shown in Fig. 2, CD4+ 2C T cells were undetectable in the peripheral blood of 2C+RAG−/−I-Ab−/− mice, whereas they were readily detected and at the similar frequencies in 2C+RAG−/− mice on either the I-Ab+/+ or the I-Ab+/− background. Thus, the development of CD4+ 2C T cells requires positive selection mediated by the MHC class II I-Ab molecule, indicating that the 2C TCR interacts directly with I-Ab [likely associated with peptide(s)].
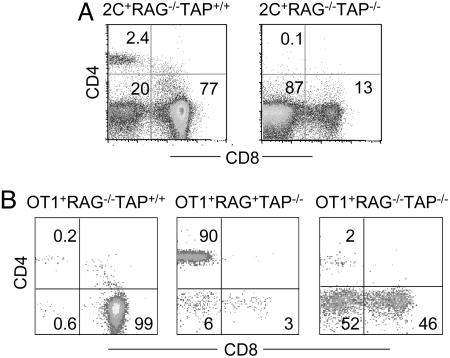
To test the requirement for MHC class I molecules in the development of CD4+ 2C T cells, we generated 2C+RAG−/− mice on a TAP−/− background. As expected, the number and percentage of CD8+ 2C T cells were greatly reduced in 2C+RAG−/−TAP−/− mice (Fig. 3A and data not shown). Despite the normal expression of I-Ab in 2C+RAG−/−TAP−/− mice (data not shown), almost no CD4+ 2C T cells were detected in 20 mice analyzed; most of the 2C T cells in these mice were CD4−CD8−. The absence of CD4+ 2C T cells in 2C+RAG−/−TAP−/− mice suggests that the development of CD4+ 2C T cells requires positive selection by both MHC class I and II molecules.
Fig. 3.
Development of CD4+ T cells in 2C+RAG−/− mice (A) and OT-1+RAG−/− mice (B). (A) PBMC from 2C+RAG−/−TAP+/+ and 2C+RAG−/−TAP−/− mice were analyzed for 2C TCR, CD4, and CD8 expression. CD4 versus CD8 expression profiles are shown for 2C+ T cells. (B) PBMC of OT-1+RAG−/−TAP+/+, OT-1+RAG+TAP−/−, and OT-1+RAG−/−TAP−/− mice were stained with anti-Vα2, anti-CD4, and anti-CD8. CD4 versus CD8 expression profiles are shown for Vα2+ T cells. The numbers indicate the percentages of T cells in the specific quadrants. Data from representative mice are shown.
Development of CD4+ 2C T Cells in 2C+RAG+ Mice.
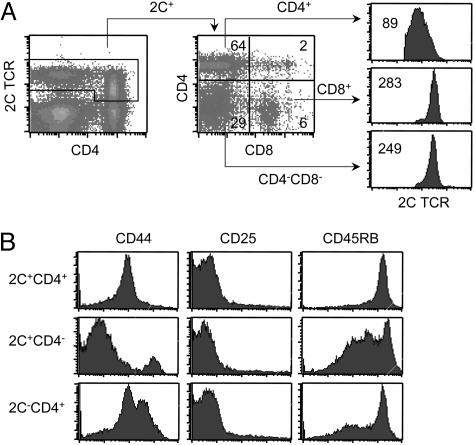
Whereas there were no detectable CD4+ 2C T cells in 2C+RAG−/−TAP−/− mice, in 2C+RAG+TAP−/− mice most of the 2C+ T cells were CD4+ and only a small fraction was either CD8+ or CD4−CD8− (Fig. 4A). Among the three 2C+ fractions, the 2C+CD8+ and the 2C+CD4−CD8− T cells expressed a uniformly high level of the 2C TCR, whereas the 2C+CD4+ T cells expressed a lower level of the 2C TCR, and the expression pattern was broad (Fig. 4A). In addition, 2C+RAG+TAP−/− mice contained a significant fraction of CD4+ cells that did not express the 2C TCR. The 2C−CD4+ cells are likely CD4+ T cells that express endogenous TCRα chains along with the transgenic 2C TCRβ chain so that the resulting TCRs are not reactive with the clonotypic antibody (1B2). Both 2C+CD4+ and 2C−CD4+ T cells exhibited a naïve phenotype as indicated by their CD44lo, CD25−, and CD45RBhi expression profiles (Fig. 4B). 2C+CD4− T cells, which consisted of 2C+CD8+ and 2C+CD4−CD8− cells, were more heterogenous in expression of CD44 and CD45RB. These results show that large numbers of mature CD4+ 2C T cells can be generated in 2C+RAG+TAP−/− mice.
Fig. 4.
Surface phenotype of CD4+ 2C T cells in 2C+RAG+TAP−/− mice. (A) Lymph node cells of 2C+RAG+TAP−/− mice were analyzed for 2C TCR, CD4, and CD8. (Left) 2C TCR versus CD4 staining profile of all live cells. (Center) CD4 versus CD8 expression profile gating on 2C+ T cells. (Right) Comparison of 2C TCR levels among 2C+CD4+, 2C+CD8+, and 2C+CD4−CD8− T cells. Data from one representative mouse are shown. (B) Lymph node cells of 2C+RAG+TAP−/− mice were analyzed for 2C TCR, CD4, plus CD44, CD25, or CD45RB. The expression of CD44, CD25, and CD45RB is shown for 2C+CD4+, 2C+CD4−, and 2C−CD4+ T cells.
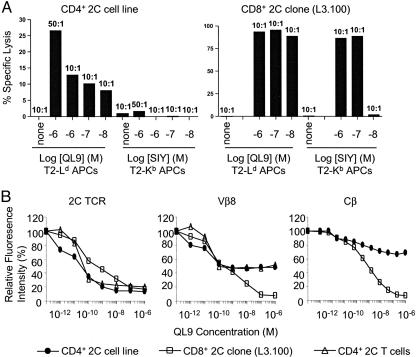
To examine their functional properties, CD4+ 2C T cells were purified from 2C+RAG+TAP−/− mice by cell sorting and then stimulated weekly with irradiated splenocytes from β2-microglobulin−/− mice in the presence of cytokines. In culture, the CD4+ 2C T cells proliferated slowly but established a CD4+ 2C T cell line. CTL activity of the CD4+ 2C T cell line was compared with that of a CD8+ 2C T cell clone, L3.100, by using SIY-labeled T2–Kb target cells in a 6-h 51Cr-release assay. As expected, L3.100 cells lysed SIY-pulsed T2–Kb target cells effectively: At an effector-to-target cell ratio (E:T ratio) of 10:1 and SIY at 0.1 μM, 85% of target cells were lysed (Fig. 5A). In contrast, the CD4+ 2C T cell line did not significantly lyse SIY-labeled T2–Kb targets cells, even at an E:T ratio of 50:1 and a SIY concentration of 1 μM, indicating that either the CD4+ 2C T cell line did not possess much cytolytic activity and/or coreceptor CD8 is required for lysis of the T2–Kb target cells.
To distinguish between the two possibilities, QL9-labeled T2–Ld cells were used as target cells because the QL9–Ld complex can activate the 2C TCR independent of CD8, in contrast to SIY–Kb, which requires CD8 coreceptor to activate 2C T cells (9). As shown in Fig. 5A, L3.100 cells lysed QL9-pulsed T2–Ld target cells extremely effectively; i.e., lysis remained at a near maximal level (≈85%) at an E:T ratio of 10:1 and 0.01 μM QL9 peptide. Although the CD4+ 2C T cell line exhibited significant lysis of QL9-labeled T2–Ld target cells, its cytolytic activity was far lower than that of the L3.100 clone: Even at an E:T ratio of 50:1 and 1 μM QL9 peptide, there was only ≈27% target cell lysis (Fig. 5A). Thus, the CD4+ 2C T cell line exhibited little cytolytic activity and resembled CD4+ T cells in general.
Fig. 5.
CD4+ 2C T cells respond to allogeneic QL9–Ld but not to syngeneic SIY–Kb stimulations. (A) Comparison of cytolytic activities between a CD4+ 2C T cell line and a CD8+ 2C CTL clone (L3.100). See Materials and Methods for details. The ratios indicate E:T ratios. Percentages of specific lysis are shown. (B) Comparison of TCR down-regulation in the CD4+ 2C T cell line, the CD8+ 2C clone L3.100, and the freshly isolated CD4+ 2C T cells in response to QL9–Ld stimulation. CD4+ 2C T cells from 2C+RAG+TAP−/− mice (triangle) or from the cultured CD4+ 2C line (circle) and the L3.100 cells (square) were incubated with T2–Ld cells in the presence of the indicated concentrations of QL9 peptide at 37°C overnight. The cells were then stained for the 2C TCR (1B2), anti-Vβ8, or anti-Cβ antibodies. Fluorescence intensities of 2C TCR, Vβ8, and Cβ on the three types of T cells without QL9 stimulation are arbitrarily defined as 100%, and fluorescence intensities of treated samples are normalized to that of the untreated samples. The relative fluorescence intensities of 2C TCR, Vβ8, and Cβ are shown as a function of QL9 peptide concentrations for the three cell types.
Expression of Two TCRs by CD4+ 2C T Cells in 2C+RAG+ Mice.
Because the development of CD4+ 2C T cells in 2C+RAG−/− mice requires the presence of both MHC class I and II molecules, it was surprising that large numbers of CD4+ 2C T cells were generated in 2C+RAG+TAP−/− mice. Because endogenous TCRα genes can be rearranged in 2C+RAG+TAP−/− but not in 2C+RAG−/−TAP−/− mice, CD4+ 2C T cells generated in 2C+RAG+TAP−/− mice probably express additional TCRs, which likely promote CD4 lineage development. Consistent with this hypothesis, CD4+ 2C T cells expressed much lower levels of 2C TCR than did CD8+ or CD4−CD8− 2C T cells in the same 2C+RAG+TAP−/− mice (Fig. 4A). To further test this hypothesis, we compared the extent of TCR down-regulation in the CD4+ 2C T cell line and the CD8+ 2C clone L3.100 after TCR ligation. To minimize a requirement for CD8, T cells were cultured overnight in the presence of T2–Ld cells plus various amounts of the QL9 peptide, and the levels of 2C TCR, total TCR, and Vβ8 (used by 2C TCRβ) were measured by flow cytometry by using antibodies specific for the 2C TCR, Cβ, and Vβ8, respectively. When measured with the 1B2 antibody, specific for the 2C TCR, both the CD4+ 2C T cell line and L3.100 clone exhibited a QL9 dosage-dependent down-regulation of the 2C TCR (Fig. 5B Left). Importantly, the extent of 2C TCR down-regulation was the same and nearly complete for both cell types.
Similarly, when measured with antibodies specific for the Cβ or the Vβ8, both the CD4+ 2C T cell line and L3.100 clone exhibited a QL9 dosage-dependent TCR down-regulation. However, whereas the TCR down-regulation was nearly complete with L3.100 cells, it was only ≈50% or less with the CD4+ 2C T cell line (Fig. 5B Center and Right). To exclude any possible artifact due to the use of a cell line, we performed the same assay with freshly isolated CD4+ 2C T cells from spleen and lymph nodes of 2C+RAG+TAP−/− mice. Down-regulation of the 2C TCR on primary CD4+ 2C T cells was QL9 dosage-dependent and nearly complete when measured with 1B2 antibody (Fig. 5B Left), whereas TCR down-regulation was ≈50% when measured with anti-Vβ8. Together, these results strongly suggest that CD4+ 2C T cells in 2C+RAG+TAP−/− mice express other TCRs in addition to the 2C TCR. Based on the incomplete down-regulation of Vβ8, the other TCRs likely consist of the transgenic TCRβ and endogenous TCRα chains and do not interact with the QL9–Ld complexes.
Development of CD4+ T Cells in OT-1 TCR-Transgenic Mice.
To extend the generality of the observations with the 2C TCR, we analyzed T cell development in transgenic mice expressing the OT-1 TCR in the absence of RAG1 and/or TAP (on the H-2b background). As with the 2C TCR, the OT-1 TCR is positively selected by MHC class I Kb. Because there is no antibody specific for the OT-1 TCR, the OT-1 T cells were identified by anti-Vα2 antibody specific for the OT-1 TCRα chain. In >50 OT-1+RAG−/−TAP+/+ mice analyzed, almost all Vα2+ T cells were CD8+ and only very few were CD4+ or CD4−CD8− (Fig. 3B). However, in two OT-1+RAG−/−TAP−/− mice analyzed, of the Vα2+ T cell population a large fraction were still CD8+, but the majority were CD4−CD8−, and a significantly higher percentage were CD4+ than in OT-1+RAG−/−TAP+/+ mice (2% versus 0.2%). Therefore, in the absence of TAP and positive selection mediated by Kb, the OT-1 TCR probably has an increased probability of interacting with MHC class II, thereby leading to thymocyte differentiation into CD4+ T cells. Most dramatically, ≈90% of Vα2+ T cells in OT-1+RAG+TAP−/− mice were CD4+ (Fig. 3B). The OT-1 TCRβ chain has been shown to pair with TCRα chains that differ only in the CDR3 region to generate both CD4+ and CD8+ T cells in mice (36, 37). Thus, rearrangement of endogenous TCRα genes in OT-1+RAG+TAP−/− mice and expression of TCRs consisting of OT-1 TCRβ and endogenous TCRα chains, in addition to the OT-1 TCR, likely have promoted the observed development of CD4 T cells in the absence of TAP.
Discussion
The 2C TCR Recognizes both Class I and Class II MHC Molecules.
The 2C TCR has been shown to recognize syngeneic MHC class I Kb (16, 17, 19), allogeneic Ld and Kbm3 (10, 12–15), and a yet-to-be identified nonclassical class I molecule (20). In this report, we show that the 2C TCR also recognizes the MHC class II I-Ab for the development of CD4+ 2C T cells (Figs. 1 and 2). Similarly, the development of CD4+ OT-1 T cells in OT-1+RAG−/−TAP−/− mice suggests that the OT-1 TCR also interacts with I-Ab (Fig. 3B). Thus, like AND, HA, and HY TCRs (21–23), the 2C and OT-1 TCRs recognize peptides presented by both class I and class II MHC molecules. To determine whether these TCRs are unusual, we reviewed the literature for T cell development in TCR-transgenic mice that have been crossed onto the RAG−/− background. Among 24 TCR/RAG transgenic lines that we found, both CD4+ and CD8+ single-positive T cells were reported in the thymus and/or peripheral lymph organs in 22 lines (Table 1, which is published as supporting information on the PNAS web site). In most of these transgenic lines development of T cells was usually biased to one lineage; however, in many lines the other lineage T cells were as abundant or only 2- to 3-fold fewer. Although the minor selecting MHC molecules have not been identified for most of these TCRs, these observations strengthen the view that the individual TCR can recognize diverse MHC molecules.
Recent studies suggest that, when only a limited set of self-pMHC complexes is available in the thymus for positive selection, the TCRs of the selected mature T cells are especially degenerate and tend to recognize both MHC class I and MHC class II as well as multiple foreign (allogeneic) MHC (38, 39). The 2C TCR arose in a BALB.B (H2b) mouse (10, 11). It is possible that in the BALB.B environment the set of self-peptides that binds to Kb is not entirely congruent with the set that binds to Kb in the B6 environment, but it is not obvious that the peptide–Kb set in the BALB.B thymus would be unusually constricted. Moreover, the well known prevalence of allogeneic reactivity seen in polyclonal antivirus and other T cell responses (40, 41) suggests that the extensive reactivity of 2C TCR with diverse MHC molecules is not unusual. This feature is likely quite common based on the extremely high percentage of TCRs that are capable of promoting both CD4+ and CD8+ T cell development in RAG−/− background (Table 1). There is little evidence to suggest that a TCR should be restricted by any particular MHC molecule (42). The observed restriction by predominantly one MHC molecule for a given TCR likely arises from thymic selection with small but critical contributions of peptide(s) bound to that MHC. Taken together with the ability of a single TCR to recognize a large number of different peptides in association with the same MHC molecule (43–46), the ability of the same TCR to interact with multiple MHC molecules, including both class I and class II, reinforces the view that TCR recognition of peptide–MHC complexes can be highly degenerate.
Development of CD4+ 2C T Cells Requires Positive Selection by both MHC Class I and MHC Class II Molecules.
One of the functional consequences of the 2C TCR recognition of I-Ab is the development of CD4+ 2C T cells in the thymus. A significant fraction of 2C+RAG−/− mice (≈30%) develop CD4+ 2C T cells, comprising 0.5–50% of total 2C T cells (Figs. 1 and 2). These CD4+ 2C T cells are evidently positively selected by I-Ab, because they were absent in 2C+RAG−/−I-Ab−/− mice. Interestingly, development of these CD4+ 2C T cells also requires the TAP gene because they were absent in 2C+RAG−/−TAP−/− mice (Fig. 3A). In the absence of TAP, the levels of class I expression on the cell surface are dramatically reduced, impairing both TCR–class I and CD8–class I interactions. Although it is formally possible that CD8 and class I MHC molecules have to interact in order for CD4 T cells to develop, the absence of CD4+ 2C T cells in 2C+RAG−/−TAP−/− mice most likely results from the reduced interaction between the 2C TCR and the class I MHC (Kb) molecule.
The requirement of both class I and class II MHC for the development of CD4+ 2C T cells resembles the development of CD8+ T cells in transgenic mice expressing the nominally MHC class II-restricted HA and AND TCRs (22, 23). The difference is that, in case of the 2C TCR, most 2C T cells generated in the RAG−/− background are CD8+, and the development of CD4+ 2C T cells requires positive selection by not only class II (I-Ab) but also class I. In the case of HA and AND TCRs, most T cells generated in the RAG−/− or CD4−/− and TCRα−/− background are CD4+, and the development of CD8+ T cells requires not only MHC class I but also MHC class II.
How is CD4 versus CD8 lineage commitment accomplished if both class I and class II MHC molecules are involved in positive selection? Although the precise mechanisms underlying the development of CD4 versus CD8 lineage T cells are still controversial, interactions among TCRs, coreceptors, and self-pMHC are critical for the outcome (4, 5, 7). Recent studies suggest that the duration of TCR signaling may control CD4 versus CD8 lineage differentiation (47). According to the kinetic signaling model (48), upon the initial interaction among TCR, coreceptors, and pMHC, DP thymocytes down-regulate CD8 to become CD4+CD8lo cells (49). Transient TCR signaling in CD4+CD8lo cells results in CD8 lineage differentiation, and persistent TCR signaling in CD4+CD8lo cells results in CD4 lineage differentiation (47). The requirement of both class I and class II MHC for the development of CD4+ 2C T cells from a nominally class I-restricted TCR is consistent with this model. Perhaps 2C TCR interaction with some pMHC class II complexes, in addition to its interaction with pMHC class I complexes, might provide the sustained TCR signaling in CD4+CD8lo cells required for CD4+ 2C T cell development. However, this model does not explain the requirement of both class I and class II MHC for the development of CD8+ HA and AND T cells from these nominally class II-restricted TCRs.
Our findings in 2C+RAG−/− mice appear to provide a clue to this conundrum. In 2C+RAG−/− mice, only a fraction of T cells develop into CD4+ 2C T cells (0.5–50%), and only a fraction of the mice harbor the CD4+ 2C T cells (≈30%). Similarly, in the HA and AND TCR-transgenic (RAG−/− or TCRα−/−) mice, only a fraction of T cells are CD8+, and the percentages of mice developing CD8+ T cells were not reported (22, 23). The variation among mice could result from background differences, although the 2C+RAG−/− mice have been backcrossed onto the B6 background for 13 generations. That only a fraction of 2C T cells are CD4+ in 2C+RAG−/− mice suggests that not all developing DP thymocytes have equal access to I-Ab. Variability in the pMHC complexes available at a given time or location in the thymus likely affects a thymocyte’s development into a CD4 or CD8 T cell (50). Thus, when, where, and how long TCR, coreceptors, and self-pMHC interact probably has a significant effect on CD4 versus CD8 lineage differentiation.
Development of CD4+ 2C T Cells as a Consequence of Expression of Additional TCR.
In the 2C TCR-transgenic mice on the RAG+ background, large numbers of CD4+ 2C+ T cells are generated (Fig. 4), consistent with a previous report (51). Two lines of evidence suggest that, because of the lack of TCRα allelic exclusion (25, 28), these T cells express two different TCRs. First, although the level of total TCR among CD4+, CD8+, and CD4−CD8− 2C T cells was similar, the CD4+ 2C T cells had a lower level of the 2C TCR than did the other 2C T cells (Figs. 4 and 5 and data not shown), similar to other transgenic T cells that express two TCRs (52). Second, after QL9–Ld stimulation the 2C TCR was almost as completely down-regulated from CD4+ 2C T cells (as from CD8+ 2C T cells), but 50% of the TCR persisted when assayed with anti-Cβ or anti-Vβ8 antibodies (Fig. 5). The 2C TCRβ utilizes the Vβ8 gene segment (16), and the fact that the persistent TCRs still react with anti-Vβ8 (Fig. 5) suggests that the second TCR is composed of the transgenic TCRβ and an endogenous TCRα chain. Some of these nontransgenic TCRs expressed on 2C T cells are likely positively selected by class II I-Ab molecules and therefore promote the development of CD4+ T cells. However, the precise nature of the 2C TCR–class I MHC interaction that contributes to the development of CD4+ T cells in 2C+RAG+ mice is unclear.
The same mechanisms likely underlie the development of large numbers of CD4+ OT-1 T cells in OT-1+RAG+TAP−/− mice (Fig. 3B), especially because the OT-1 TCRβ chain is known to pair with different TCRα chains to generate both CD4+ and CD8+ T cells (36, 37). Unlike these previous observations, however, the CD4+ OT-1 T cells in OT-1+RAG+TAP−/− mice likely express two TCRs: one consisting of the transgenic TCRβ and transgenic TCRα chains and the other consisting of the transgenic TCRβ and endogenous TCRα chains. In humans, ≈20% of T cells are estimated to express two TCRs, and in mice ≈10% of T cells express two TCRs (30). Thus, the effect of expressing two TCRs in the same developing thymocyte could significantly affect its ultimate development into a CD4 or CD8 T cell.
Materials and Methods
Mice and Cell Lines.
2C TCR-transgenic mice on either the RAG1−/− or RAG1+ background were backcrossed onto C57BL/6 background for 13 generations (33). Similarly, OT-1 TCR-transgenic mice on either the RAG1−/− or RAG1+ background were backcrossed onto the B6 background for 10 generations. B6 mice and B6 mice deficient in I-Ab, TAP, or β2-microglobulin were purchased from The Jackson Laboratory. RAG1−/− mice, backcrossed with B6 mice for 13 generations, were used as adoptive transfer recipients. All mice were kept under specific pathogen-free facilities and used between 6 and 12 weeks of age. L3.100 is a CD8+ CTL clone from a 2C TCR-transgenic mouse. T2–Ld and T2–Kb cell lines are human B cell lymphoma T2 cells stably expressing the mouse Ld or Kb gene, respectively.
Antibodies and Flow Cytometry Analysis.
Antibodies to CD8, CD25, CD44, CD11a, CD45RB, HSA, CD28, Ly-6C, CD122, Vβ8, and TCRβ (Cβ) were purchased as conjugates from BD Pharmingen. Clonotypic antibody 1B2, specific for the 2C TCR, was conjugated to biotin. Single-cell suspensions were prepared from thymus, lymph nodes, spleen, and peripheral blood mononuclear cells (PBMC). Red blood cells were lysed by treating splenocytes and PBMC with lysis buffer. Cells were stained in the presence of 2.5 μg/ml anti-FcR antibody in PBS containing 0.1% BSA and 0.1% NaN3 and analyzed on a FACSCalibur. Analyses were carried out with cellquest software.
Establishment of a CD4+ 2C T Cell Line, T Cell Activation Assay, and CTL Assay.
CD4+ 2C T cells were isolated from lymph nodes and spleen of 2C+RAG+TAP−/− mice by cell sorting. The cells were then stimulated weekly with irradiated splenocytes from β2-microglobulin−/− mice in the presence of supernatants of Con A-stimulated rat splenocytes (a source of cytokines). To assay T cell activation, the resulting CD4+ 2C T cell line and a CD8+ 2C CTL clone were cultured at ≈5 × 105 cells per ml at 37°C in the presence of QL9 peptide-loaded T2–Ld cells. Twenty-four hours later, the levels of TCR on 2C T cells were measured by staining with antibodies specific for the 2C TCR, Vβ8, or TCRβ. For CTL assays, the CD4+ 2C T cell line or L3.100 clone were incubated for 6 h with 51Cr-labeled T2–Ld target cells in the presence of various concentrations of the QL9 peptide or with 51Cr-labeled T2–Kb target cells in the presence of various concentrations of the SIY peptide. Specific lysis was calculated as [(experimental counts − spontaneous counts)/(total counts − spontaneous counts)] × 100.
Supplementary Material
Acknowledgments
We thank Carol McKinley for technical support and members of the J.C. laboratory for advice, comments, and review of the manuscript. This work was supported in part by National Institutes of Health Grants AI50631 and AI40146 (to J.C.) and CA60686 (to H.N.E.) and Core Grant CA140451 to the Massachusetts Institute of Technology Center for Cancer Research (to Tyler Jacks).
Abbreviations
- TCR
T cell receptor
- DP
double-positive
- CTL
cytotoxic T lymphocyte
- SIY
SIYRYYGL
- QL9
QLSPFPFDL
- E:T ratio
effector-to-target cell ratio
- PBMC
peripheral blood mononuclear cell.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Davis M. M., Boniface J. J., Reich Z., Lyons D., Hampl J., Arden B., Chien Y. Annu. Rev. Immunol. 1998;16:523–544. doi: 10.1146/annurev.immunol.16.1.523. [DOI] [PubMed] [Google Scholar]
- 2.Goldrath A. W., Bevan M. J. Nature. 1999;402:255–261. doi: 10.1038/46218. [DOI] [PubMed] [Google Scholar]
- 3.Rudolph M. G., Luz J. G., Wilson I. A. Annu. Rev. Biophys. Biomol. Struct. 2002;31:121–149. doi: 10.1146/annurev.biophys.31.082901.134423. [DOI] [PubMed] [Google Scholar]
- 4.von Boehmer H. Cell. 1994;76:219–228. doi: 10.1016/0092-8674(94)90330-1. [DOI] [PubMed] [Google Scholar]
- 5.Starr T. K., Jameson S. C., Hogquist K. A. Annu. Rev. Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 6.von Boehmer H. Adv. Immunol. 2004;84:201–238. doi: 10.1016/S0065-2776(04)84006-9. [DOI] [PubMed] [Google Scholar]
- 7.Singer A., Bosselut R. Adv. Immunol. 2004;83:91–131. doi: 10.1016/S0065-2776(04)83003-7. [DOI] [PubMed] [Google Scholar]
- 8.Robey E., Fowlkes B. J. Annu. Rev. Immunol. 1994;12:675–705. doi: 10.1146/annurev.iy.12.040194.003331. [DOI] [PubMed] [Google Scholar]
- 9.Chen J., Eisen H. N., Kranz D. M. Microbes Infect. 2003;5:233–240. doi: 10.1016/s1286-4579(03)00016-9. [DOI] [PubMed] [Google Scholar]
- 10.Kranz D. M., Sherman D. H., Sitkovsky M. V., Pasternack M. S., Eisen H. N. Proc. Natl. Acad. Sci. USA. 1984;81:573–577. doi: 10.1073/pnas.81.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saito H., Kranz D. M., Takagaki Y., Hayday A. C., Eisen H. N., Tonegawa S. Nature. 1984;309:757–762. doi: 10.1038/309757a0. [DOI] [PubMed] [Google Scholar]
- 12.Udaka K., Tsomides T. J., Eisen H. N. Cell. 1992;69:989–998. doi: 10.1016/0092-8674(92)90617-l. [DOI] [PubMed] [Google Scholar]
- 13.Udaka K., Tsomides T. J., Walden P., Fukusen N., Eisen H. N. Proc. Natl. Acad. Sci. USA. 1993;90:11271–11276. doi: 10.1073/pnas.90.23.11272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tallquist M. D., Pease L. R. J. Immunol. 1995;155:2419–2426. [PubMed] [Google Scholar]
- 15.Sha W. C., Nelson C. A., Newberry R. D., Pullen J. K., Pease L. R., Russell J. H., Loh D. Y. Proc. Natl. Acad. Sci. USA. 1990;87:6186–6190. doi: 10.1073/pnas.87.16.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sha W. C., Nelson C. A., Newberry R. D., Kranz D. M., Russell J. H., Loh D. Y. Nature. 1988;335:271–274. doi: 10.1038/335271a0. [DOI] [PubMed] [Google Scholar]
- 17.Sha W. C., Nelson C. A., Newberry R. D., Kranz D. M., Russell J. H., Loh D. Y. Nature. 1988;336:73–76. doi: 10.1038/336073a0. [DOI] [PubMed] [Google Scholar]
- 18.Dutz J. P., Tsomides T. J., Kageyama S., Rasmussen M. H., Eisen H. N. Mol. Immunol. 1996;31:967–975. doi: 10.1016/0161-5890(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 19.Udaka K., Wiesmuller K. H., Kienle S., Jung G., Walden P. J. Immunol. 1996;157:670–678. [PubMed] [Google Scholar]
- 20.Maurice M. M., Gould D. S., Carroll J., Vugmeister Y., Ploegh H. L. Proc. Natl. Acad. Sci. USA. 2001;98:7437–7442. doi: 10.1073/pnas.141143298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arsov I., Vukmanovic S. J. Immunol. 1999;162:2008–2015. [PubMed] [Google Scholar]
- 22.Kirberg J., Baron A., Jakob S., Rolink A., Karjalainen K., von Boehmer H. J. Exp. Med. 1994;180:25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matechak E. O., Killeen N., Hedrick S. M., Fowlkes B. J. Immunity. 1996;4:337–347. doi: 10.1016/s1074-7613(00)80247-2. [DOI] [PubMed] [Google Scholar]
- 24.Sleckman B. P., Gorman J. R., Alt F. W. Annu. Rev. Immunol. 1996;14:459–481. doi: 10.1146/annurev.immunol.14.1.459. [DOI] [PubMed] [Google Scholar]
- 25.von Boehmer H. Annu. Rev. Immunol. 1990;8:531–556. doi: 10.1146/annurev.iy.08.040190.002531. [DOI] [PubMed] [Google Scholar]
- 26.Casanova J.-L., Romero P., Widmann C., Kourilsky P., Maryanski J. L. J. Exp. Med. 1991;174:1371–1373. doi: 10.1084/jem.174.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lacorazza H. D., Nikolich-Zugich J. J. Immunol. 2004;173:5591–5600. doi: 10.4049/jimmunol.173.9.5591. [DOI] [PubMed] [Google Scholar]
- 28.Gascoigne N. R., Alam S. M. Semin. Immunol. 1999;11:337–347. doi: 10.1006/smim.1999.0190. [DOI] [PubMed] [Google Scholar]
- 29.Borgulya P., Kishi H., Uematsu Y., von Boehmer H. Cell. 1992;69:529–537. doi: 10.1016/0092-8674(92)90453-j. [DOI] [PubMed] [Google Scholar]
- 30.Padovan E. G., Casorati P., Dellabona P., Meyer S., Brockhaus M., Lanzavecchia A. Science. 1993;262:422–424. doi: 10.1126/science.8211163. [DOI] [PubMed] [Google Scholar]
- 31.Gladow M., Uckert W., Blankenstein T. Eur. J. Immunol. 2004;34:1882–1891. doi: 10.1002/eji.200425041. [DOI] [PubMed] [Google Scholar]
- 32.Robertson J. M., Evavold B. D. J. Immunol. 1999;163:1750–1754. [PubMed] [Google Scholar]
- 33.Cho B., Varada R., Ge Q., Eisen H. E., Chen J. J. Exp. Med. 2000;192:549–556. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jameson S. C. Nat. Rev. Immunol. 2002;2:547–556. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- 35.Martin B., Bourgeois C., Dautigny N., Lucas B. Proc. Natl. Acad. Sci. USA. 2003;100:6021–6026. doi: 10.1073/pnas.1037754100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Correia-Neves M., Waltzinger C., Mathis D., Benoist C. Immunity. 2001;14:21–32. doi: 10.1016/s1074-7613(01)00086-3. [DOI] [PubMed] [Google Scholar]
- 37.Correia-Neves M., Mathis D., Benoist C. Eur. J. Immunol. 2001;31:2583–2592. doi: 10.1002/1521-4141(200109)31:9<2583::aid-immu2583>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 38.Logunova N. N., Viret C., Pobezinsky L. A., Miller S. A., Kazansky D. B., Sundberg J. P., Chervonsky A. V. J. Exp. Med. 2005;202:73–84. doi: 10.1084/jem.20050198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huseby E. S., White J., Crawford F., Vass T., Becker D., Pinilla C., Marrack P., Kappler J. W. Cell. 2005;122:247–260. doi: 10.1016/j.cell.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 40.Nahill S. R., Welsh R. M. J. Exp. Med. 1993;177:317–327. doi: 10.1084/jem.177.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindahl K. F., Wilson D. B. J. Exp. Med. 1977;145:507–522. doi: 10.1084/jem.145.3.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zerrahn J., Held W., Raulet D. H. Cell. 1997;88:627–636. doi: 10.1016/s0092-8674(00)81905-4. [DOI] [PubMed] [Google Scholar]
- 43.Malissen B. Immunity. 2003;19:463–464. doi: 10.1016/s1074-7613(03)00271-1. [DOI] [PubMed] [Google Scholar]
- 44.Wucherpfennig K. W. Mol. Immunol. 2004;40:1009–1017. doi: 10.1016/j.molimm.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Cohn M. Mol. Immunol. 2005;42:1419–1443. doi: 10.1016/j.molimm.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 46.Eisen H. N., Sykulev Y., Tsomides T. J. Adv. Protein Chem. 1996;49:1–56. doi: 10.1016/s0065-3233(08)60487-8. [DOI] [PubMed] [Google Scholar]
- 47.Liu X., Bosselut R. Nat. Immunol. 2004;5:280–288. doi: 10.1038/ni1040. [DOI] [PubMed] [Google Scholar]
- 48.Bosselut R. Nat. Rev. Immunol. 2004;4:529–540. doi: 10.1038/nri1392. [DOI] [PubMed] [Google Scholar]
- 49.Brugnera E., Bhandoola A., Cibotti R., Yu Q., Guinter T. I., Yamashita Y., Sharrow S. O., Singer A. Immunity. 2000;13:59–71. doi: 10.1016/s1074-7613(00)00008-x. [DOI] [PubMed] [Google Scholar]
- 50.Canelles M., Park M. L., Schwartz O. M., Fowlkes B. J. Nat. Immunol. 2003;4:756–764. doi: 10.1038/ni953. [DOI] [PubMed] [Google Scholar]
- 51.Chen F.-L., Kung J. T. J. Immunol. 1996;156:2036–2044. [PubMed] [Google Scholar]
- 52.Heath W. R., Miller J. F. J. Exp. Med. 1993;178:1807–1811. doi: 10.1084/jem.178.5.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.