Abstract
Adhesions of cells to extracellular matrix and adjacent cells are mediated by integrins and VE-cadherin, respectively. Although these adhesion processes play crucial roles in vascular cell migration and angiogenesis, it remains unclear as to how they are coordinated to regulate cellular functions. We report here that integrin engagement by treating bovine endothelial aortic cell monolayers with beads coated with fibronectin (Fn) led to disruption of the VE-cadherin-containing adherens junctions. This disruption was accompanied by increases of tyrosine phosphorylation of β-catenin, γ-catenin, and p120ctn, as well as the dissociation of α-catenin and γ-catenin from VE-cadherin. We applied a membrane-targeted Src reporter based on the fluorescence resonance energy transfer technique to visualize the dynamic Src activation at subcellular levels in live cells. The integrin engagement induced by Fn-coated beads caused the activation of Src around the beads and at adherens junctions, which are subsequently disrupted. The inhibition of Src with PP1 blocked the effects of integrin engagement on adherens junctions. Although Ras can also modulate adherens junctions, the resulting patterns of phosphorylation and association of junction proteins were distinct from those induced by integrin engagement. The inhibition of Ras by RasN17 did not rescue the disruption of adherens junctions induced by integrin engagement or by Src activation. Integrin engagement by Fn-coated beads also induced a significant alteration of cortical actin filaments at adherens junctions. The results indicate that integrin engagement disrupts VE-cadherin-containing adherens junctions via the activation of Src, but not Ras, possibly as a result of modulation of the actin network.
Keywords: adherens junctions, cell–matrix interaction, endothelial cells
Cell–matrix and cell–cell interactions are fundamental processes that regulate cellular functions in relation to the environment. The adhesion of cells to extracellular matrix (ECM) is mainly mediated by integrins, which undergo a conformational change upon activation to recruit structural and signaling molecules (1, 2). Thus, integrins not only mechanically couple the cytoskeleton to the ECM but also transmit molecular signaling cascades to regulate cellular functions in response to extracellular cues.
The adherens junctions between endothelial cells are mainly comprised of VE-cadherin, a single-chain transmembrane protein forming homophilic intercellular adhesion. The extracellular domain of VE-cadherin consists of five homologous repeats, and its intracellular domain is functionally separated into two parts: the juxtamembrane domain (JMD), which is proximal to the membrane, and the catenin-binding domain (CBD), which is located at the distal end. The JMD domain provides putative docking sites for p120ctn, a substrate for Src, and a regulatory protein for adherens junctions (3). The CBD domain binds directly to β-catenin and γ-catenin (also called plakoglobin), that, in turn, associate with α-catenin. α-catenin, by binding to vinculin and α-actinin, bridges the VE-cadherin complex to actin-based cytoskeleton (4, 5).
Because both integrins and cadherins associate with the cytoskeleton and many common signaling molecules (6), it is likely that the cell–ECM and cell–cell adhesion processes mediated by these two types of receptors act in a coordinated manner in regulating cellular functions. Indeed, activation of integrins by plating preaggregated cells on ECM results in a scattering of cells and the loss of adherens junctions in several carcinoma cell lines (7, 8). These reports suggest that integrin expression and its activation may negatively regulate the cadherin-mediated cell–cell adhesion. However, the molecular mechanism by which integrin engagement modulates VE-cadherin-containing adherens junctions remains to be elucidated.
Src plays a critical role in a variety of cellular processes (9) and can be activated by integrins directly or indirectly through FAK or RPTPα (10–12). There is evidence that activated Src perturbs the cadherin-mediated cell–cell adhesion. For example, constitutively active Src protein causes the tyrosine phosphorylation of E-cadherin and a concurrent loss of cell–cell contact (13).
The small GTPase Ras, specifically H-Ras in this context, has a well characterized role in regulating the Raf/ERK signaling pathway (14). Ras can be activated by integrin engagement (15) and is known to modulate adherens junctions (16, 17). Recent studies indicate that Ras is also involved in the regulation of adherens junctions by growth factors (18).
In the current study, we investigated the interaction of integrins and VE-cadherin in bovine endothelial aortic cells (BAECs). The engagement of integrin by beads coated with the integrin-ligand fibronectin (Fn) induced a significant disassembly of VE-cadherin-containing adherens junctions, mainly through the perturbation on γ-catenin-mediated linkage of VE-cadherin complex to actin-based cytoskeleton. This effect of integrin engagement on adherens junctions depends on Src, but not Ras.
Results
The Engagement of Integrins by Ligand-Coated Beads Induces the Disruption of VE-Cadherin-Mediated Adherens Junctions.
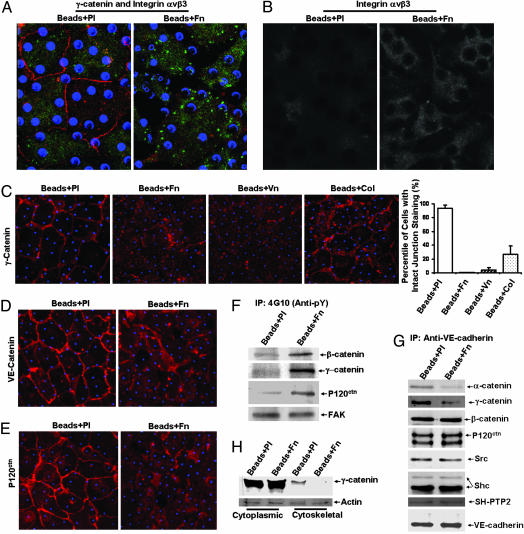
We applied the ECM-coated beads onto monolayers of BAECs to assess the roles of integrin engagement in regulating the adherens junction (19). Fn-coated beads induced a significant focal clustering pattern of integrin αvβ3 on the basal side and a disruption of the γ-catenin staining at the cell–cell junctions (Fig. 1A). Microscopic observation focused on the equator of the beads showed remarkable accumulation of integrin αvβ3 around the beads in cells treated with Fn-coated beads but not in cells treated with control beads coated with polylysine (Pl) (Fig. 1B). Treatments with beads coated with Fn or vitronectin (Vn), but not Pl, induced a significant loss of γ-catenin from cell–cell contact in BAEC monolayers. Beads coated with collagen type I (Col) also caused some discontinuity of γ-catenin staining, but to an extent much less than those seen in the Fn and Vn groups (Fig. 1C). Because Fn (ligands for integrin α5β1 and αvβ3), Vn (ligand for αvβ3), and Col (ligands for α1β1 and α2β1) bind to distinct integrin subtypes, the activations of these integrin subtypes may have different degrees of disruption effect on adherens junctions. Fn-coated beads also induced a significant decrease of the staining of VE-cadherin (Fig. 1D) and P120ctn (Fig. 1E) at cell–cell contacts.
Fig. 1.
The engagement of integrins by ECM-coated beads induced the disruptionof VE-cadherin-containing adherens junctions. Polystyrene beads were coated with polylysine (Beads + Pl; 100 μg/ml), fibronectin (Beads + Fn; 50 μg/ml), collagen (Beads + Col; 50 μg/ml), or vitronectin (Beads + Vn; 50 μg/ml). Beads assays were performed as described in Experimental Procedures. Blue dots represent the beads attached on the BAECs in monolayer. (A) Immunostaining images of γ-cadherin (red) and integrin-αvβ3 (green) collected at the basal side of cells with a confocal microscope. (B) Immunostaining images of integrin-αvβ3 with a view focusing on the equator of the beads. (C) Immunostaining images of γ-catenin (red). (C Right) Results of the percentages of cells with intact γ-catenin staining at cell borders (mean ± SD from three separate experiments). (D and E) Immunostaining images of VE-cadherin (red) (D) and P120ctn (red) (E). (F–H) Cell lysates from monolayers of BAECs treated with Beads + Pl or Beads + Fn were subjected to IP with anti-phosphotyrosine (4G10) (F) or anti-VE-cadherin antibody (G), or were separated into detergent-soluble (Cytoplasmic) and detergent-insoluble (Cytoskeletal) fractions, followed by IB with various antibodies as indicated in H. The results are representative of more than three separate experiments.
We further investigated the role of integrin engagement on adherens junctions with biochemical approaches. Fn-coated beads caused a strong tyrosine phosphorylation of β-catenin, γ-catenin, and p120ctn (Fig. 1F). Cadherins and α-catenin did not show detectable tyrosine phosphorylation in response to integrin engagement (data not shown).
In addition to α-, β-, γ-catenin, and P120ctn, Src, Shc, and a tyrosine phosphatase SH-PTP2 also associate with cadherins and regulate adherens junctions 20–22. Therefore, we studied the presence of α-, β-, γ-catenin, p120ctn, Src, Shc, and SH-PTP2 in the VE-cadherin complex. As shown in Fig. 1G, Fn-coated beads induced marked decreases of α-catenin and γ-catenin in the VE-cadherin complex but no significant change of β-catenin, p120ctn, Src, Shc, and SH-PTP2. These results indicate that integrin engagement selectively induced the disassociation of certain adherens junction proteins from VE-cadherin, particularly γ-catenin and its cytoskeletal-linkage partner, α-catenin.
We then directly assessed the connections of γ-catenin to the cytoskeleton. Proteins in whole cells were separated into cytoplasmic and cytoskeletal fractions (Supporting Text, which is published as supporting information on the PNAS web site). Fn-coated beads induced a marked decrease of γ-catenin in the cytoskeletal portion in comparison to the results with Pl-coated beads (Fig. 1H), suggesting that integrin engagement caused the dissociation of γ-catenin from the cytoskeletal network.
The Engagement of Integrins by Fn-Coated Beads Induces the Activation of Src.
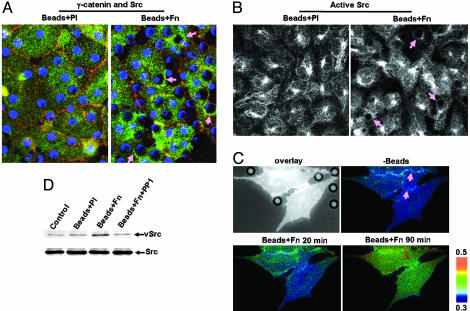
We conducted studies to elucidate the role of Src in the regulation of adherens junctions by integrin engagement. Src formed ring structures around the Fn-coated beads, in contrast to the uniform distribution in control cells treated by Pl-coated beads (Fig. 2A). Active Src was also concentrated in these ring structures (Fig. 2B). These results demonstrate the local recruitment and activation of Src by integrin engagement. A newly developed membrane-targeted fluorescence resonance energy transfer reporter for Src enabled us to visualize the dynamic Src activation upon integrin engagement at subcellular levels. As shown in Fig. 2C (see also Movie 1), a strong Src activation was initiated around the Fn-coated beads and extended to cell–cell contact areas that are subsequently disengaged. Western blots revealed that Fn-coated beads induced a significant phosphorylation at tyrosine-416, indicating elevated Src activities. This effect of the Fn-coated beads was blocked by PP1, a selective inhibitor of Src (Fig. 2D).
Fig. 2.
The engagement of integrins by Fn-coated beads induced the activationof Src. (A) Immunostaining images of γ-cadherin (red) and Src (green). Blue dots indicate the presence of beads. (B) Immunostaining images of active Src. The pink arrows point to the ring structures around beads in A and B. (C) BAECs were transfected with the membrane-targeted Src reporter and subjected to the addition of Beads + Fn for various time periods. The overlay of images of CFP-only fluorescence and beads phase contrast is shown in black and white and the CFP/YFP emission ratio images are shown in color. The pink arrows point to the cell–cell junction areas disrupted by integrin engagement at later time points (Movie 1, which is published as supporting information on the PNAS web site). (D) BAEC monolayers were incubated for 20 min with control medium (control), Beads + Pl, Beads + Fn, or Beads + Fn after 1 h of preincubation with 10 μM PP1 (Beads + Fn + PP1). (D Upper) The Src activities (phospho-416). (D Lower) The Src protein levels. The results are representative of three separate experiments.
Src Mediates the Integrin-Induced Disassembly of Adherens Junctions.
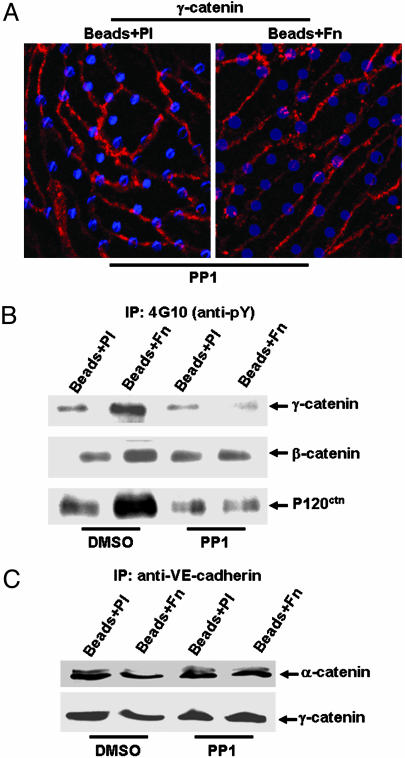
We addressed the question whether Src mediates the effects of integrin engagement on adherens junctions by using its inhibitor PP1. PP1 rescued the Fn-induced disappearance of γ-catenin (Fig. 3A), VE-cadherin, and p120ctn (data not shown) at the cell–cell border. SU6656, another inhibitor of Src, also rescued the Fn-induced disappearance of γ-catenin at junction areas (Fig. 6, which is published as supporting information on the PNAS web site), suggesting the specificity of Src in mediating this process. PP1 also inhibited the integrin engagement-induced tyrosine phosphorylation of β-catenin, γ-catenin, and p120ctn (Fig. 3B) and the dissociation of α-catenin and γ-catenin from VE-cadherin (Fig. 3C). These results indicate that Src plays a crucial role in mediating the integrin regulation of adherens junctions.
Fig. 3.
Src mediated the integrin-induced disassembly of adherens junctions.(A) BAEC monolayers were incubated with 10 μM PP1 for 1 h before being subjected to Beads + Pl or Beads + Fn. γ-catenin is shown in red, and blue dots represent the beads. (B and C) BAEC monolayers were incubated with DMSO (0.1%) or PP1 (10 μM) for 1 h before being subjected to Beads + Pl or Beads + Fn for 20 min. Cell lysates from various samples were subjected to IP with anti-phosphotyrosine (4G10) antibody in B or anti-VE-cadherin antibody in C, followed by IB with various antibodies as indicated. The results are representative of three separate experiments.
Ras Is Not Essential for the Disassembly of Adherens Junctions Induced by Integrin Engagement.
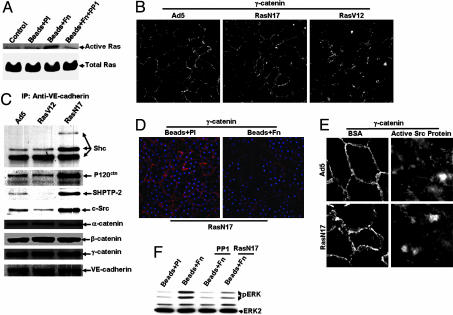
We examined the roles of Ras in mediating the integrin engagement-induced disruption of adherens junctions in BAECs. Affinity precipitation of active Ras (GTP-bound Ras) revealed that Fn-coated beads induced a significant activation of Ras, which was blocked by PP1 (Fig. 4A). These results suggest that, consistent with previous reports (9, 23), Ras is a downstream molecule of Src in response to integrin engagement. We further showed that Ras can modulate adherens junctions in BAECs. An active mutant of Ras (RasV12), but not a negative mutant of Ras (RasN17), disrupted adherens junctions (Fig. 4B). α-, β-, and γ-catenins did not change their associations with VE-cadherin in response to either Ad_RasV12 or Ad_RasN17. But p120ctn, Shc, Src, and SH-PTP2 increased significantly in the VE-cadherin complex in cells overexpressed with Ad_RasN17 and decreased slightly with Ad_RasV12 (Fig. 4C), suggesting that Ras may regulate adherens junctions via the association of these noncatenin signaling molecules with VE-cadherin. RasN17 did not prevent the integrin engagement-induced disappearance of γ-catenin from cell–cell junctions (Fig. 4D), nor did it have any detectable effects on the Src-induced disruptions of adherens junctions (Fig. 4E). Interestingly, inhibition of either Src or Ras blocked or attenuated the integrin engagement-induced ERK activation (Fig. 4F). These results indicate that Ras may act separately from the integrin/Src/γ-catenin pathway in regulating adherens junctions while mediating the integrin engagement-induced ERK activation together with Src.
Fig. 4.
Ras was not essential for the disassembly of adherens junctions inducedby integrin engagement. (A) BAEC monolayers were incubated for 5 min with control medium (control), Beads + Pl, Beads + Fn, or Beads + Fn + PP1. Ras activity was assayed as described in Experimental Procedures. (A Upper) Active Ras shows the Ras activities. (A Lower) Total Ras shows the Ras protein levels. (B and C) BAEC monolayers were infected by control vector Ad5, Ad_RasV12, or Ad_RasN17. After a 4-h incubation with serum-free medium, the cells were stained with anti-γ-catenin antibody (B) or subjected to IP with anti-VE-cadherin antibody and IB with appropriate antibodies to detect the association of VE-cadherin and adherens junction proteins (C). (D) BAEC monolayers were infected by Ad_RasN17 before being subjected to Beads + Pl or Beads + Fn for 20 min. γ-catenin is shown in red and beads in blue. (E) BAEC monolayers were infected by Ad5 or Ad_RasN17 before being subjected to the transfection of BSA or active Src proteins. The images show the γ-catenin staining. (F) BAEC monolayers treated with PP1 (10 μM), Ad_RasN17, or kept as control were treated with Beads + Pl or Beads + Fn. Cell lysates from various samples were probed for phospho-ERK (Upper) and ERK2 (to show comparable protein loading among various samples, Lower). The results are representative of three separate experiments.
Integrin Engagement Causes Alteration in Cortical Actin Structure.
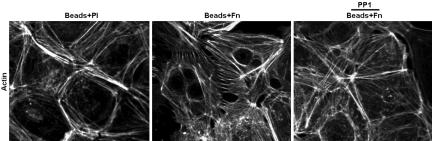
Because Src regulates cortical actin network, which has been shown to be critical for stable junction formation (24–26), we examined whether integrin engagement alters the cortical actin cytoskeleton via Src. As shown in Fig. 5, in contrast to the thick actin belts that run along (parallel to) the cortical cell–cell contact seen with Pl-coated beads, Fn-coated beads induced thin bundles of actin filaments perpendicular to the cell–cell contact line. Pretreating the cells with PP1 prevented this action of integrin engagement. These results suggest that integrin engagement and its subsequent Src activation modulate actin organization at cell–cell contacts.
Fig. 5.
Integrin engagement caused significant alteration in cortical actin structure. BAEC monolayers were subjected to Beads + Pl, Beads + Fn, or Beads + Fn + PP1 for 20 min. Actin filaments were stained with FITC-phalloidin. The results are representative of three separate experiments.
Discussion
Cell adhesion is critical for cell migration, formation of tissue architecture, and angiogenesis (4, 27, 28). These physiological processes require an intimate cooperation between cell–ECM and cell–cell adhesions. Little is known about the regulatory mechanisms by which cells coordinate signaling events spatially and temporally to achieve such cooperation. In our study using the fluorescence resonance energy transfer-based Src reporter, the activation of integrins by treating cells with ECM-coated beads induced an activation of Src around the beads and at cell–cell contact. This activation of Src is accompanied by the disassembly of adherens junctions. Although both constitutively active Src and Ras can cause disruption of the adherens junctions, only Src and its downstream cytoskeletal reorganization, but not Ras, are involved in this integrin-induced disassembly of adherens junctions.
ECM-coated beads cause the aggregation and ligand-occupancy of integrins, which lead to activation of the signaling events involving integrins and cytoskeleton (19). In our study, upon placement of Fn-coated beads on the apical side of the cells, integrin αvβ3 accumulated around the beads and formed a strong focal contact pattern on the basal side, demonstrating both local and global activations of integrins.
Integrin activations are accompanied by the disassembly of adherens junctions, as well as the tyrosine phosphorylation of β-catenin, γ-catenin, and p120ctn, which were previously shown to correlate with the decrease of adherens junctions. Interestingly, among them, only γ-catenin is found to have a decreased association with VE-cadherin upon integrin activation, and the tyrosine phosphorylation of β-catenin and p120ctn does not alter their association with VE-cadherin. Our findings suggest a hierarchy by which modulations of catenins cause junction disassembly upon integrin activation, i.e., the phosphorylation of γ-catenin is followed by the dissociation of γ- and α- catenins from the VE-cadherin complex and, finally, the possible dissociation of β-catenin and p120ctn from the complex at a later time, thus disrupting the cell–cell junctions.
With the use of fluorescence resonance energy transfer, we observed Src activation locally around the beads and distally at cell–cell adhesion sites. It is possible that Src directly binds to integrins upon integrin engagement (10) and becomes activated locally. The integrin-mediated adhesions may also induce an increase in mechanical tension across the BAEC monolayer. This tension may then accumulate at the anchoring cell–cell adhesion sites and activate Src, which, in turn, induces the cytoskeleton remodeling at the cell–cell contact. This scenario may explain our observations of the actin-based filopodia-like formation at the cell–cell contact and the concomitant disruption of adherens junctions (Fig. 5 and Movie 1). Interestingly, inactive Src is abundant at junction areas in cells before integrin engagement (Fig. 7, which is published as supporting information on the PNAS web site), suggesting that inactive Src may function as an adaptor protein through its SH2 and SH3 domains to provide structural support for cell–cell adhesions, which are disrupted when Src is activated. This hypothesis is supported by the finding that the catalytic activity of Src is essential for the disruption of E-cadherin-containing adherens junctions (29).
The size and density of focal contact spots stained by the anti-phospho-FAK397 antibody did not change upon integrin engagement (Fig. 8, which is published as supporting information on the PNAS web site), suggesting that the phosphorylation of FAK Y397 may not be involved in recruiting and activating Src upon integrin engagement.
Recent reports suggest that Src regulates the adherens junctions in epithelial cancer cells via the Ras-ERK pathway and its downstream molecule MLCK, which leads to the myosin-based increase in contractility at cell–cell contacts and the breakage of adherens junctions (9, 15, 30–33). However, blockage of Ras, the main upstream regulator of ERK, by Ad_RasN17 did not inhibit the disassembly of adherens junctions induced by either Fn-coated beads or constitutively active Src protein. Therefore, Ras may not be involved in the integrin-regulated disassembly of adherens junctions. This view is supported by the findings that integrin engagement and Ras-transformation caused different patterns of association of adherens junction proteins with VE-cadherin. As shown in Table 1, which is published as supporting information on the PNAS web site, integrin engagement only regulated the association of γ-catenin and α-catenin with VE-cadherin, whereas Ras transformation altered the association of p120ctn, Src, Shc, and SHPTP-2 with VE-cadherin. Therefore, Ras, unlike integrin engagement, may not target the VE-cadherin/catenin linkage to regulate adherens junctions. Rather, Ras may control adherens junctions through the regulation of noncatenin junction proteins, including SHPTP2 and p120ctn, which are known to maintain the dephosphorylation status of junction proteins and the stability of VE-cadherin at plasma membrane (34, 35). The association between β-catenin and VE-cadherin was not perturbed either by integrin engagement or by Ras, consistent with previous reports that β-catenin may be dispensable for a strong-to-weak shift of adherens junctions (36).
Src is closely related to the cytoskeleton and associates with cortactin, a cortical actin-binding protein (21, 37). Because the cadherin–catenin complex connects to an actin-based cytoskeleton (4), the Src activation by integrin engagement may exert its effects on adherens junctions via the cytoskeletal network. In our study, integrin engagement induced a marked modulation of the cortical actin network at cell–cell contacts (Fig. 5). Modulation of the dynamic equilibrium of actin polymerization/depolymerization by either cytochalasin D or Jasplakinolide disrupts the adherens junctions in human keratinocytes (29) and BAECs (data not shown). These results indicate that actin cytoskeleton is critical in mediating the integrin regulation of adherens junctions. Although active Src colocalizes with microtubules (Fig. 9, which is published as supporting information on the PNAS web site), stabilization of microtubules by Taxol (1.0 μM) (38) does not have any detectable effect on adherens junctions in control or beads-treated cells (data not shown). Hence, it appears that microtubules are not involved in the integrin regulation of adherens junctions.
Both integrins and VE-cadherin in endothelial cells play important roles in angiogenesis, cell migration, and wound healing (4, 27, 39). The angiogenesis inhibitors endostatin, tumstatin, and angiostatin mainly interact with integrins as targets (39, 40). The disruption of the VE-cadherin-mediated cell–cell adhesion enhances angiogenesis (41). Hence, the decrease of VE-cadherin-mediated cell–cell adhesion as a result of integrin activation may provide a molecular basis for the coordination of different adhesion processes to regulate angiogenesis.
Experimental Procedures
Immunoprecipitation (IP) and Immunoblotting (IB).
The antibodies used were monoclonal anti-SHPTP-2, polyclonal anti-Src (Santa Cruz Biotechnology), polyclonal anti-VE-cadherin (Research Diagnostics , Flanders, NJ), monoclonal anti-α-catenin, anti-β-catenin, anti-γ-catenin, anti-p120ctn, anti-p-FAK397, polyclonal anti-Shc (Transduction Laboratories), monoclonal anti-αvβ3 LM609 (Chemicon), polyclonal anti-p-Src416 (BioSource International, Carmarillo, CA), monoclonal anti-phosphotyrosine 4G10, and polyclonal anti-Ras (Upstate Biotechnology). IP and IB were conducted as described in ref. 42.
Beads Assays.
Polystyrene microspheres (mean diameter 9.7 μm; Bangs Laboratories, Fishers, IN) were coated with polylysine or various ligands of integrins as described in ref. 19. Coating conditions were chosen to yield comparable binding of these proteins to beads (19). A monolayer of BAECs on a 22 × 22-mm cover slide were incubated for 20 min with 2 × 106 polylysine- or ECM-coated beads before being subjected to IB analysis.
Recombinant Adenovirus Infection of BAEC Monolayers.
The recombinant adenoviruses expressing a control adenovirus type 5 (Ad5), a constitutively active Ras mutant with Gly-12-to-Val substitution linked to Ad5 (Ad_RasV12), or a dominant negative Ras mutant with Ser-17-to-Asn substitution linked to Ad5 (Ad_RasN17) were described in ref. 43. Monolayers of BAECs were infected in DMEM supplemented with 2% FBS for 24 h before being subjected to further experimental procedures as indicated.
Transfection of Src proteins.
The transfection of proteins with the lipofectamine method (Invitrogen) was described in ref. 44. In brief, monolayers of BAECs were transfected with lipofectamine and constitutively active Src enzyme (Upstate Biotechnology), or with BSA as control. After incubation for 3 h in serum-free DMEM, the transfected cells were washed and fixed for IP and IB.
The Membrane-Targeted Src Reporter and Fluorescence Resonance Energy Transfer Image Acquisition.
The membrane-targeted Src reporter was described in ref. 45. BAECs expressing the membrane-targeted Src reporter were starved with 0.5% FBS for 24 h before being subjected to Fn-coated beads. During imaging, the cells were maintained in CO2-independent medium without serum (GIBCO/BRL) at 37°C in a thermostatic chamber. Images were collected by using metafluor 6.2 software (Universal Imaging, Downingtown, PA) with a 440DF20 excitation filter, a 455DRLP dichroic mirror, and two emission filters controlled by a filter changer (480DF30 for CFP and 535DF25 for YFP).
Supplementary Material
Acknowledgments
We thank Dr. Hisaaki Kawakatsu at the University of California, San Francisco, for providing the antibody recognizing the active Src (clone 28) and Dr. Kenneth M. Yamada at the National Institutes of Health (NIH) for his helpful suggestions on beads assay. This work was supported, in part, by NIH Research Grants HL43026, HL64382, and HL-80518 (to S.C.).
Abbreviations
- BAEC
bovine aortic endothelial cell
- ECM
extracellular matrix
- Fn
fibronectin
- IB
immunostaining
- IP
immunoprecipitation
- Pl
polylysine.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Li R., Mitra N., Gratkowski H., Vilaire G., Litvinov R., Nagasami C., Weisel J. W., Lear J. D., DeGrado W. F., Bennett J. S. Science. 2003;300:795–798. doi: 10.1126/science.1079441. [DOI] [PubMed] [Google Scholar]
- 2.Carman C. V., Springer T. A. Curr. Opin. Cell Biol. 2003;15:547–556. doi: 10.1016/j.ceb.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds A. B., Herbert L., Cleveland J. L., Berg S. T., Gaut J. R. Oncogene. 1992;7:2439–2445. [PubMed] [Google Scholar]
- 4.Dejana E., Bazzoni G., Lampugnani M. G. Exp. Cell Res. 1999;252:13–19. doi: 10.1006/excr.1999.4601. [DOI] [PubMed] [Google Scholar]
- 5.Ranscht B. Curr. Opin. Cell Biol. 1994;6:740–746. doi: 10.1016/0955-0674(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 6.Brunton V. G., MacPherson I. R., Frame M. C. Biochim. Biophys. Acta. 2004;1692:121–144. doi: 10.1016/j.bbamcr.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Genda T., Sakamoto M., Ichida T., Asakura H., Hirohashi S. Lab. Invest. 2000;80:387–394. doi: 10.1038/labinvest.3780043. [DOI] [PubMed] [Google Scholar]
- 8.Kawano K., Kantak S. S., Murai M., Yao C. C., Kramer R. H. Exp. Cell Res. 2001;262:180–196. doi: 10.1006/excr.2000.5083. [DOI] [PubMed] [Google Scholar]
- 9.Thomas S. M., Brugge J. S. Annu. Rev. Cell Dev. Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 10.Arias-Salgado E. G., Lizano S., Sarkar S., Brugge J. S., Ginsberg M. H., Shattil S. J. Proc. Natl. Acad. Sci. USA. 2003;100:13298–13302. doi: 10.1073/pnas.2336149100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eide B. L., Turck C. W., Escobedo J. A. Mol. Cell Biol. 1995;15:2819–2827. doi: 10.1128/mcb.15.5.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Wichert G., Jiang G., Kostic A., De Vos K., Sap J., Sheetz M. P. J. Cell Biol. 2003;161:143–153. doi: 10.1083/jcb.200211061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Behrens J., Vakaet L., Friis R., Winterhager E., Van Roy F., Mareel M. M., Birchmeier W. J. Cell Biol. 1993;120:757–766. doi: 10.1083/jcb.120.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marshall C. J. Curr. Opin. Cell Biol. 1996;8:197–204. doi: 10.1016/s0955-0674(96)80066-4. [DOI] [PubMed] [Google Scholar]
- 15.Schlaepfer D. D., Hanks S. K., Hunter T., van der Geer P. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- 16.Espada J., Perez-Moreno M., Braga V. M., Rodriguez-Viciana P., Cano A. J. Cell Biol. 1999;146:967–980. doi: 10.1083/jcb.146.5.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mareel M. M., Behrens J., Birchmeier W., De Bruyne G. K., Vleminckx K., Hoogewijs A., Fiers W. C., Van Roy F. M. Int. J. Cancer. 1991;47:922–928. doi: 10.1002/ijc.2910470623. [DOI] [PubMed] [Google Scholar]
- 18.Terauchi R., Kitamura N. Exp. Cell Res. 2000;256:411–422. doi: 10.1006/excr.2000.4850. [DOI] [PubMed] [Google Scholar]
- 19.Miyamoto S., Akiyama S. K., Yamada K. M. Science. 1995;267:883–885. doi: 10.1126/science.7846531. [DOI] [PubMed] [Google Scholar]
- 20.Xu Y., Guo D. F., Davidson M., Inagami T., Carpenter G. J. Biol. Chem. 1997;272:13463–13466. doi: 10.1074/jbc.272.21.13463. [DOI] [PubMed] [Google Scholar]
- 21.Calautti E., Cabodi S., Stein P. L., Hatzfeld M., Kedersha N., Paolo Dotto G. J. Cell Biol. 1998;141:1449–1465. doi: 10.1083/jcb.141.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ukropec J. A., Hollinger M. K., Salva S. M., Woolkalis M. J. J. Biol. Chem. 2000;275:5983–5986. doi: 10.1074/jbc.275.8.5983. [DOI] [PubMed] [Google Scholar]
- 23.Jalali S., Li Y. S., Sotoudeh M., Yuan S., Li S., Chien S., Shyy J. Y. Arterioscler. Thromb. Vasc. Biol. 1998;18:227–234. doi: 10.1161/01.atv.18.2.227. [DOI] [PubMed] [Google Scholar]
- 24.Adams C. L., Chen Y. T., Smith S. J., Nelson W. J. J. Cell Biol. 1998;142:1105–1119. doi: 10.1083/jcb.142.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vasioukhin V., Bauer C., Yin M., Fuchs E. Cell. 2000;100:209–219. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- 26.Vasioukhin V., Fuchs E. Curr. Opin. Cell Biol. 2001;13:76–84. doi: 10.1016/s0955-0674(00)00177-0. [DOI] [PubMed] [Google Scholar]
- 27.Lauffenburger D. A., Horwitz A. F. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 28.Gumbiner B. M. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 29.Owens D. W., McLean G. W., Wyke A. W., Paraskeva C., Parkinson E. K., Frame M. C., Brunton V. G. Mol. Biol. Cell. 2000;11:51–64. doi: 10.1091/mbc.11.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu Q., Paredes M., Zhang J., Kosik K. S. Mol. Cell. Biol. 1998;18:3257–3265. doi: 10.1128/mcb.18.6.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Avizienyte E., Fincham V. J., Brunton V. G., Frame M. C. Mol. Biol. Cell. 2004;15:2794–2803. doi: 10.1091/mbc.E03-12-0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maru Y., Yamaguchi S., Takahashi T., Ueno H., Shibuya M. J. Cell Physiol. 1998;176:223–234. doi: 10.1002/(SICI)1097-4652(199808)176:2<223::AID-JCP1>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 33.Potempa S., Ridley A. J. Mol. Biol. Cell. 1998;9:2185–2200. doi: 10.1091/mbc.9.8.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zanetti A., Lampugnani M. G., Balconi G., Breviario F., Corada M., Lanfrancone L., Dejana E. Arterioscler. Thromb. Vasc. Biol. 2002;22:617–622. doi: 10.1161/01.atv.0000012268.84961.ad. [DOI] [PubMed] [Google Scholar]
- 35.Kowalczyk A. P., Reynolds A. B. Curr. Opin. Cell Biol. 2004;16:522–527. doi: 10.1016/j.ceb.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Takeda H., Nagafuchi A., Yonemura S., Tsukita S., Behrens J., Birchmeier W., Tsukita S. J. Cell Biol. 1995;131:1839–1847. doi: 10.1083/jcb.131.6.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burr J. G., Dreyfuss G., Penman S., Buchanan J. M. Proc. Natl. Acad. Sci. USA. 1980;77:3484–3488. doi: 10.1073/pnas.77.6.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Enomoto T. Cell Struct. Funct. 1996;21:317–326. doi: 10.1247/csf.21.317. [DOI] [PubMed] [Google Scholar]
- 39.Eliceiri B. P., Cheresh D. A. Curr. Opin. Cell Biol. 2001;13:563–568. doi: 10.1016/s0955-0674(00)00252-0. [DOI] [PubMed] [Google Scholar]
- 40.Rehn M., Veikkola T., Kukk-Valdre E., Nakamura H., Ilmonen M., Lombardo C. R., Pihlajaniemi T., Alitalo K., Vuori K. Proc. Natl. Acad. Sci. USA. 2001;98:1024–1029. doi: 10.1073/pnas.031564998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagashima H., Okada M., Hidai C., Hosoda S., Kasanuki H., Kawana M. Heart Vessels Suppl. 1997;12:110–112. [PubMed] [Google Scholar]
- 42.Wang Y., Miao H., Li S., Chen K. D., Li Y. S., Yuan S., Shyy J. Y., Chien S. Am. J. Physiol. 2002;283:C1540–C1547. doi: 10.1152/ajpcell.00222.2002. [DOI] [PubMed] [Google Scholar]
- 43.Jin G., Chieh-Hsi Wu J., Li Y. S., Hu Y. L., Shyy J. Y., Chien S. J. Surg. Res. 2000;94:124–132. doi: 10.1006/jsre.2000.6014. [DOI] [PubMed] [Google Scholar]
- 44.Kreisberg J. I., Ghosh-Choudhury N., Radnik R. A., Schwartz M. A. Am. J. Physiol. 1997;273:F283–F288. doi: 10.1152/ajprenal.1997.273.2.F283. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y., Botvinick E. L., Zhao Y., Berns M. W., Usami S., Tsien R. Y., Chien S. Nature. 2005;434:1040–1045. doi: 10.1038/nature03469. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.