Abstract
IFN-α is used to suppress the replication of hepatitis C virus (HCV) in chronically infected patients with partial success. Here we present evidence showing that a ligand of Toll-like receptor 7 (TLR7) can induce anti-HCV immunity not only by IFN induction, but also through an IFN-independent mechanism. Human hepatocyte line Huh-7 carrying an HCV replicon expressed TLR7, and activation of the receptor induced several antiviral genes including IFN regulatory factor-7. Inhibitors of the enzyme inosine monophosphate dehydrogenase augmented both IFN-dependent and -independent antiviral effect. Prolonged exposure of Huh-7 cells to a TLR7 ligand [SM360320 (9-benzyl-8-hydroxy-2-(2-methoxyethoxy)adenine)], alone or in combination with an inosine monophosphate dehydrogenase inhibitor, reduced HCV levels dose dependently. Immunohistochemical analysis of livers shows that TLR7 is expressed in hepatocytes of normal or HCV-infected people. Because TLR7 agonists can impede HCV infection both via type I IFN and independently of IFN, they may be considered as an alternative treatment of chronic HCV infection, especially in IFN-α-resistant patients.
Keywords: inosine monophosphate dehydrogenase, IFN regulatory factor
More than 170 million people, ≈3% of the world’s population, are infected with hepatitis C virus (HCV) and 55–85% of patients become chronically infected (1, 2). Many develop chronic liver disease, leading to cirrhosis and hepatocellular carcinoma. Combination therapy with polyethylene glycol modified IFN-α and ribavirin suppresses HCV replication in 40–80% of patients (3–5). However, severe side effects are associated with this treatment, leading to poor patient compliance. For these reasons, it is crucial to develop alternative therapies (6). These might include agents that selectively stimulate the production of IFN-α, and drugs that potentiate its anti-HCV effects.
The type I IFNs (IFN-α and IFN-β) are required for the efficient induction of a T helper 1 (Th1) immune response in humans (7). The activation of certain Toll-like receptors (TLRs), particularly TLR7 and TLR9, induces the production of type I IFNs, and thus primes the host for a Th1 adaptive immune response. Various synthetic nucleoside analogs related to guanosine protect mice in several models of RNA viral infection by induction of type I IFN via TLR7 stimulation (8, 9).
Recent reports have uncovered the key molecules in the TLR-induced signaling pathways that lead to type I IFN induction (11). Induction of type I IFN via TLR7 or TLR9 depends on the adaptor molecule MyD88 (10). TANK Binding Kinase 1 and IκB kinase i/ε (IKKi/ε) are activators of IFN regulatory factor-3 (IRF-3) and IRF-7 during TLR-mediated type I IFN production (11–13). These IRF molecules are phosphorylated in the cytosol and are translocated to the nucleus upon activation (13). DNA-dependent protein kinase (DNA-PK) has also been shown recently to phosphorylate and activate IRF-3 (14, 15). Activity of IRF-3 (16) and IRF-7 expression (17) are inhibited in HCV replicon cells. The IRFs can both foster type I IFN production (18) and enhance the antiviral activity of these cytokines (19). Thus, pharmacologic agents that activate IRFs in hepatocytes may exert direct anti-HCV effects, as well as potentiate IFN action.
Pharmacologic inhibitors of the enzyme inosine monophosphate dehydrogenase (IMPDH), including ribavirin and mizoribine, have been reported to have activity against RNA viruses (18–20). A recent report suggested that ribavirin could induce IRF-7 in cells infected with respiratory syncitial virus (21). This finding implied that IMPDH inhibitors might augment the antiviral activity of drugs that similarly induce IRF signaling.
Here we show that a TLR7 ligand 9-benzyl-8-hydroxy-2-(2-methoxyethoxy)adenine (SM360320), can inhibit HCV replication in hepatocytes via a type I IFN-independent mechanism in addition to its IFN-mediated activity. The antiviral activity is enhanced by IMPDH inhibition in both cases. TLR7 is detectable in cultured Huh-7 hepatocytes and in the livers of most HCV-infected patients. These results raise the possibility that high-affinity TLR7 stimulants may be able to control chronic HCV infection in vivo, both by induction of IFN and by direct activation of antiviral mechanisms in infected hepatocytes.
Results
TLR7 Is Expressed in HCV-Infected Hepatocytes.
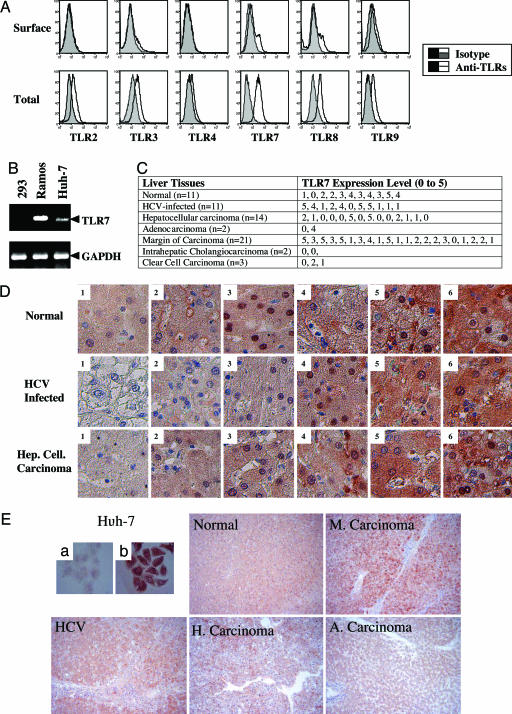
Flow cytometry revealed that several TLRs are expressed in Huh-7 hepatoma cells, albeit mostly in intracellular compartments (Fig. 1A). TLR7 expression was most prominent. Anti-TLR7 antibody specifically recognized human TLR7 stably expressed in HEK293 cells (Fig. 5, which is published as supporting information on the PNAS web site). TLR7 mRNA was also expressed in Huh-7 cells (Fig. 1B), consistent with the flow cytometry data. In addition, various levels of TLR7 were detectable by immunohistochemistry in hepatocytes of normal, HCV-infected, and carcinoma liver tissues (Fig. 1 C and D) but not detectable in fibroblasts of HCV-infected livers (Fig. 1E).
Fig. 1.
Expression of TLR7 in hepatocytes. (A) Expression of TLR7 antigens on the surface and in the cytoplasm of Huh-7 replicon cells was assessed by antibody staining and FACS, as described in Materials and Methods. (B) Extracts of Huh-7 hepatocytes, HEK293 kidney cells (negative control), and Ramos B lymphocytes (positive control) were used to amplify TLR7 specific mRNA, as described in Materials and Methods. (C and D) Human liver tissue arrays were stained with TLR7 antibody after antigen recovery. The expression levels of TLR7 were estimated in different specimens using an arbitrary 0–5 scale. (E) Differential expression of TLR7 in human hepatocytes, compared to fibroblasts, was assessed by immunohistochemistry. A representative TLR7-stained tissue from each category is presented. M, margin of carcinoma; H, hepatocellular carcinoma; HCV, HCV-infected liver; A, adenocarcinoma.
A Potent TLR7 Ligand Suppresses HCV Replication.
We investigated whether activation of different TLRs could inhibit replication of HCV in the Huh-7 replicon system (20). Treatment with prototype pharmacologic activators of TLR2, TLR3, TLR4, TLR5, TLR7, and TLR9 did not reduce the level of HCV protein (NS5A) as measured by Western blotting (Fig. 2A). A TLR2 ligand, pam3cys, actually enhanced the level of NS5A (Fig. 2A). Various 8-hydroxyadenine analogs were reported recently to be potent inducers of type I IFNs (21). By genetic complementation in HEK293 cells, we identified SM360320 as a specific TLR7 ligand (Fig. 2B). Accordingly, SM360320 induced typical TLR7-mediated cytokines in human PBL (22), such as IFN-α, but little IL-6, IL-10, or IL-12, whereas R848, a ligand for TLR7 and TLR8 in humans, induced the latter cytokines in large quantities (Table 1, which is published as supporting information on the PNAS web site). However, in mouse splenocytes, where TLR8 is silent (23), SM360320 was at least 10 times more potent than R848 in induction of cytokines (Fig. 6, which is published as supporting information on the PNAS web site). Conditioned medium from SM360320- or R848-activated human PBL significantly inhibited HCV replication (Fig. 2C), consistent with the high levels of type I IFN in the medium. However, SM360320 by itself inhibited HCV replication in Huh-7 cells in a dose-dependent manner (Fig. 2D). In contrast to the PBL-conditioned medium derived from SM360320-stimulated cells, the direct inhibition of HCV replication by SM360320 in Huh-7 cells was not mediated by extracellular type I IFN because (i) it was not inhibited by neutralizing anti-IFN-α receptor antibodies, and (ii) STAT-1 was not activated after drug treatment (Fig. 3A). However, the antiviral effect was TLR7 dependent, because chloroquine, an inhibitor of endosomal maturation (9), blocked the SM360320 anti-HCV activity (Fig. 3B). Treatment of Huh-7 cells with SM360320 or IFN-α induced several antiviral proteins, of which IRF-7 was prominent (Fig. 3C). However, NF-κB was activated only by SM360320 as expected, and MxA, an IFN-α target gene, was not induced by SM360320 (Fig. 3C). SM360320 reduced the level of HCV RNA in Huh-7 cells by >60% after overnight incubation, and by almost 80% after 2 weeks of treatment (Fig. 3D).
Fig. 2.
Suppression of HCV replication by a TLR7 ligand, SM360320. (A) Huh-7-replicon cells were stimulated with the indicated TLR ligands or with IFN-α (1 ng/ml) for 24 h, and the level of the HCV NS5A protein or IFN-inducible phospho-STAT-1 was measured by Western blotting. [pam3Cys at 5 μg/ml, p(I:C) at 5 μg/ml, LPS at 100 ng/ml, flagellin at 10 ng/ml, TOG at 100 μM, and CpG at 5 μg/ml.] (B) HEK293 cells were transfected with vectors encoding the indicated TLRs, plus a NF-κB luciferase reporter, and then were stimulated with SM360320 (2 μM) or the indicated control ligands for 8 h. Luciferase activities were normalized to β-galactosidase activities in the same cells, as described (9). (C) Conditioned medium (CM) from PBL stimulated with the indicated drugs or untreated medium (C, 10 μl) was applied to Huh-7 cells carrying the HCV replicon. NS5A or STAT-1 levels were measured by Western blotting after 24 h. (D) Huh-7 cells were treated with the indicated concentrations of SM360320 for 24 h, and the levels of NS5A, STAT-1, or actin were measured by Western blotting.
Fig. 3.
Suppression of HCV replication by SM360320 through IFN dependent and independent pathways. (A) (Left) Conditioned medium (CM) was obtained from cells treated with R848 (R) or SM360320 (S), or unstimulated cells (C), and was applied to Huh-7 cells carrying HCV replicon. NS5A and phospho-STAT-1 levels were measured by Western blotting. Huh-7 cells carrying HCV replicon were pretreated with anti- IFN-α receptor (IFN-αR) antibody for 30 min followed by treatment with conditioned medium. (Right) Huh-7 cells were pretreated with neutralizing anti- IFN-αR antibody for 30 min followed by treatment with SM360320 (10 μM) or IFN-α (10 ng) for 24 h. The levels of NS5A and STAT1 were measured by Western blotting. The antibody blocked the IFN effect, but not the effect of SM360320. (B) Huh-7 cells were pretreated with the endosomal maturation inhibitor chloroquine (CQ, 10 μg/ml) for 30 min followed by addition of SM360320 (10 μM) or IFN-α (1 ng/ml). The levels of NS5A, STAT-1, or actin were measured by Western blotting after 24 h. (C) Huh-7 HCV replicon cells were treated with SM360320 (10 μM) or IFN-α (1 ng/ml) for the indicated time periods, and the levels of IRF-7, OAS-1, PKR, and MxA were measured by Western blotting. NF-κB actvation was measured by EMSA. (D) Huh-7 cells were treated with SM360320 (1 or 10 μM) for 24 h or 2 weeks, and the relative levels of HCV RNA were quantified by real-time PCR. For the 2-week assay, cells were treated every other day and were split every third day. The results are the average of two independent experiments.
IMPDH Inhibitors Enhance the Anti-HCV Activity of TLR7 Ligands.
Two IMPDH inhibitors, ribavirin and mizoribine base, were screened for inhibition of HCV replication, but had no activity at nontoxic concentrations (Fig. 7A, which is published as supporting information on the PNAS web site). However, the addition of an IMPDH inhibitor to SM360320 augmented the anti-HCV activity of the TLR7 ligand (Fig. 4A). Whereas IFN-α completely eliminated HCV RNA in 2 weeks, SM360320 treated cells still harbored 10–20% of HCV RNA at the end of the experiment (data not shown). To elucidate how IMPDH blockade might increase the consequences of TLR7 activation, we tested whether the enzyme inhibitors could stimulate and/or enhance signaling pathways induced by SM360320. The IMPDH inhibitors enhanced TLR7-induced activation of NF-κB in bone marrow-derived macrophages (BMDM) (Fig. 4B), increased the protein levels of IRF-1 and IRF-7 (Fig. 4C), and induced translocation of IRF-1 and IRF-3 to the nucleus (Fig. 7B). IRF-7 undergoes ubiquitination when activated by a TLR ligand (24) in addition to phosphorylation. Both TLR7 ligands and IMPDH inhibitors independently induced ubiquitination of IRF-7, which was further enhanced by the drugs in combination (Fig. 4D). The enzymes DNA-PK (14, 15) and IKKi/ε (11, 25) have been reported to play a role in the phosphorylation of IRFs (11, 13). Both TLR7 ligands and IMPDH inhibitors activated DNA-PK and IKKi/ε (Fig. 7 C and D).
Fig. 4.
Enhancement of TLR7-mediated anti-HCV activity by an IMPDH inhibitor. (A) Huh-7 cells were treated with SM360320 (1 or 10 μM), in some cases supplemented with mizoribine base (Mb, 1 μM) for 24 h or 2 weeks. The relative levels of HCV RNA were quantified by real-time PCR. The results are the average of two independent experiments. (B) BMDM were stimulated for the indicated time periods with the TLR7 ligand TOG (100 μM) or the TLR7/8 ligand R848 (1 μM) in the absence or presence of an IMPDH inhibitor [mizoribine (Mb, 10 μM), mycophenolic acid (MPA, 1 μM), or ribavirin (Rb, 100 μM)], and NF-κB activation was measured by EMSA. (C) BMDM were stimulated with Mb (10 μM) or TOG (100 μM) for the indicated time periods, and the levels of IRF-1, -3, and -7 were measured by Western blotting. (D) IMPDH inhibition enhances IRF-7 ubiquitination. BMDM were stimulated as indicated, and the cytosolic extracts were subjected to immunoprecipitation with anti-ubiquitin antibody, followed by SDS/PAGE and immunoblotting with anti-IRF-7 antibody.
Discussion
Synthetic activators of certain TLRs induce type I IFNs, which are known to inhibit HCV replication. Here, we tested whether TLR stimulation can also exert an IFN-independent antiviral effect, using the Huh-7 HCV replicon system (20). The results showed that a synthetic TLR7 activator, SM360320, reduced HCV mRNA and protein levels in isolated Huh-7 hepatocytes, whereas other activators of TLRs were ineffective. The anti-HCV action of SM360320 was associated with stimulation of antiviral genes such as IFN response factor-7 (IRF-7), but not with activation of the IFN-responsive STAT-1 transcription factor. Moreover, anti-IFN-α receptor antibodies did not neutralize the antiviral effect of the drug. Collectively, these results suggest that potent synthetic TLR7 ligands may inhibit HCV replication not only by stimulation of IFN production, but also by direct activation of antiviral mechanisms in hepatocytes.
Pharmacologic inhibitors of the enzyme IMPDH enhanced IRF activity in Huh-7 cells, and similarly potentiated the anti-HCV activity of TLR7 stimulants. The IMPDH inhibitors also increased TLR activation of mouse BMDM cells and human peripheral blood leukocytes. The effect was not compound specific, because it was observed with different inhibitors of IMPDH and was abrogated by replenishment of guanine nucleotides (data not shown). Nucleotide pool imbalances are known to induce DNA-PK, which can subsequently activate IRF-3 (14). IRF-3 and IRF-7 may cooperate to regulate IFN production and responses (26).
In humans, the expression of TLR7 (and TLR9) is mainly confined to plasmacytoid dendritic cells and B lymphocytes. Recently, low levels of TLR7 and/or TLR9 have been reported in other cell types, including hepatocytes, particularly in the setting of chronic inflammation (27). The level of certain TLRs is up-regulated at sites of inflammation (28), such as hepatocytes (29). Expression pattern of TLR2 and TLR4 changes to become focal and irregular in HCV-infected hepatocytes (30). We detected TLR7 mRNA and protein in Huh-7 cells, and TLR7 antigen in normal and HCV-infected human liver. To determine whether the TLR7 was functional, Huh-7 cells were preincubated with chloroquine, which inhibits the maturation of endosomes, and hence the proper subcellular localization of TLR7 (9). Chloroquine blocked the anti-HCV effects of SM360320, without altering the antiviral activity of IFN-α. Thus, although one cannot rule out other off-target effects of SM360320, its anti-HCV activity requires endosomal TLR7.
The pattern of TLR7 expression in liver may explain why SM360320 exerted direct anti-HCV effects, whereas six other TLR activators did not, including R848 or 7-thia-8-oxo-guanosine, a TLR7 ligand in clinical trials in HCV-infected patients (31). Although immunoreactive TLR7 was detectable in HCV-infected hepatocytes, the levels of the antigen were much lower than those found in infiltrating mononuclear cells in the same specimens (data not shown). In cells with low TLR7 concentrations, only a high-affinity ligand may be able to achieve maximal signal transduction. The mouse system, where TLR8 is not functional, allows a direct comparison of TLR7 ligands, and we found SM360320 was 10-fold more active than R848 (resiquimod) (Fig. 6), the most potent TLR7 stimulant studied to date (9, 23).
Hepatocytes normally express TLR3, which can be activated by the double-stranded RNA produced during HCV replication. Circumstantial evidence supporting a potential antiviral role of TLR activation in hepatocytes is the observation that HCV encodes a protease (NS3/4A) capable of cleaving the TLR3 adapter protein TRIF (32). However, TLR7 signaling does not require TRIF, but instead depends on the adapter protein MyD88, which is not specifically cleaved by the HCV protease. Accordingly, potent pharmacologic activators of TLR7 may be able to circumvent the HCV encoded protease that blocks TLR3.
In summary, our data provide evidence that TLR7-mediated immunity against HCV involves at least two different mechanisms: one depends on type I IFN production by leukocytes, and the other is mediated by TLR7 expressed by virally infected hepatocytes. Suppression of HCV replication via either mechanism is augmented by IMPDH inhibitors that prime the target cell through activation of one or more IRFs. Therefore, the combination of a TLR7 ligand and an IMPDH inhibitor may provide an orally active approach to HCV therapy.
Materials and Methods
Reagents.
Deoxy-Guanosine (dG), deoxy-Cytosine (dC), deoxy-Adenosine (dA), and deoxy-Thymidine (dT) and mizoribine were purchased from Sigma. 7-Thia-8-oxo-G (TOG) was synthesized as described (33). R848 [also called resiquimod, 4-amino-2-ethoxymethyl-α, α-dimethyl-1H-imidazo(4, 5-c)quinoline-1-ethanol] was from GL Synthesis (Worchester, MA). Ribavirin was a gift from ICN. Mizoribine base (5-hydroxy-1H-imidazole-4-carboxamide) was synthesized according to U.S. Patent no. 4, 503, 235 (P. D. Cook, 1985). Mizoribine base was used for most of the studies, because it was found to be equivalent to mizoribine in all biological systems, but was easier and less costly to prepare. SM360320 was synthesized as described (34). l-Alanosine was obtained from the National Cancer Institute (NSC 153353). The source of the other TLR ligands has been described (9). HEK293 cells stably expressing human TLR7 were purchased from InvivoGen (San Diego, CA).
Antibodies.
The following antibodies were used; antibodies to IKKβ, IKKi/ε, IRF-1, IRF-3, IRF-7, PKR, and JNK1 (Santa Cruz Biotechnology); antibodies to STAT-1, pSTAT-1, pERK, and p-p38 (Cell Signaling Technologies, Beverly, MA); anti-NS5A (Maine Biotechnology Services, Portland, ME); anti-DNA-PK (NeoMarkers, Fremont, CA); and anti-β-actin antibody (Sigma). Anti-OAS-1 antibody was purchased from Abgent (San Diego, CA). Anti-human TLR2, TLR3, and TLR4 antibodies were purchased from eBiosciences (San Diego, CA), and TLR7, TLR8, and TLR9 antibodies were purchased from Imgenex (San Diego, CA). Anti-human IFN-α receptor β-chain neutralizing antibody (clone MMHAR-2) was purchased from Research Diagnostics. Anti-MxA antibody was obtained from Othmar G. Engelhardt (Oxford University, Oxford).
Mice.
C57BL/6 female mice, 6–8 weeks old, were purchased from The Jackson Laboratory. All experimental procedures were conducted in accordance with institutional guidelines for animal care and use. Murine splenocytes were isolated from the mice and cultured in RPMI medium 1640 supplemented with 10% FBS, as described (9). BMDM were isolated and cultured from the long bones of C57BL/6 mice for 7 days in L929 cell-conditioned medium (35).
Signaling Assays.
Preparation of nuclear extracts and activation of NF-κB was measured by EMSA as described (36). Levels of different IRFs in the nuclei were measured before and after stimulation by using nuclear extracts of BMDM. IRFs were detected by Western blotting using specific anti-IRF-1, -IRF-3, and -IRF-7 antibodies. Activation of ERK, STAT-1, and p38 mitogen-activated protein kinase was assayed with antibodies specific to respective phosphorylated proteins. β-Actin levels were used for normalization of protein loading. The kinase activities of JNK (Jun N-terminal kinase), IKKβ (IκB kinase β), IKKi/ε (IκB kinase i/ε), and DNA-PK (DNA-dependent kinase catalytic subunit) were measured by an in vitro kinase assay using the respective recombinant proteins GST-cJun, GST-IκBα, and GST-p53 as substrates (36). Each enzyme was immunoprecipitated, and immune complexes were incubated with substrate and [γ-32P]ATP for 30 min. The reaction samples were then electrophoresed (SDS/PAGE) and analyzed by autoradiography.
Activation of Human Peripheral Blood Leukocytes (PBL).
Human blood samples were obtained from the San Diego Blood Bank. PBL were isolated from the blood samples by using Ficoll-Plaque Plus (Amersham Pharmacia), as described (9). PBL were washed twice with 50 ml of RPMI medium 1640, resuspended in 10 ml of RPMI medium 1640 with 10% FBS, and stimulated for 24 h with the indicated drugs. The cytokines in the supernatants were measured by using the Luminex multiple ELISA (Austin, TX), according to the manufacturer’s instructions.
Studies on Huh-7 Cells with HCV Subgenome.
Huh-7 cells, which contain an HCV replicon, were established and maintained as described (20, 37, 38). HCV-specific RNA levels in cell extracts were determined by real-time RT-PCR amplification with primers specific for the HCV untranslated region: 5′-GAG TGT CGT GCA GCC TCC AG-3′ (sense, 10 μM), 5′-CACTCGCAAGCACCCTATCA-3′ (antisense, 10 μM), and 5′-FAM (carboxyfluorescein) CCCGCAAGACTGCTAGCCGAGTAGTGTGG-TAMRA-3′ (probe, 10 μM; Biosearch, Novato, CA). RT reaction mixtures were incubated for 50 min at 60°C, followed by inactivation of the reverse transcriptase coupled with activation of Taq polymerase for 5 min at 95°C. Forty cycles of PCR were performed with cycling conditions of 15 s at 94°C, 10 s at 55°C, and 1 min at 69°C. The real-time PCR signals were normalized to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Applied Biosystems). To investigate the effect of SM36020 and mizoribine base on HCV replication for 2 weeks, Huh-7 cells carrying replicon were treated with the indicated drugs every 2 days and cells were split every 3 days. HCV replication was also assessed by Western blotting using anti-NS5A antibody, and the relative changes in the level of NS5A or other proteins were measured with the software imagej. To assess TLR expression, the cells were detached with 20 mmol/liter EDTA in PBS. Cells were fixed and permeabilized by using CytoFix/CytoPerm (BD Biosciences). Cells were incubated in PBS containing 2% BSA for 30 min on ice and incubated for 1 h with either anti-hTLRs or isotype control at 1:200 dilution. For TLR7, TLR8, and TLR9, cells were washed in PBS/BSA twice and incubated with anti-rabbit IgG-FITC for TLR7 and TLR8 and anti-mouse IgG1-PE (BD PharMingen) for TLR9 for 30 min on ice. Antibody binding was detected by using a FACSCalibur (BD PharMingen). The following primers were used to amplify TLR7 message in human cell lines: Forward primer 5′-AGT GTC TAA AGA ACC TGG-3′ and reverse primer 5′-CTT GGC CTT ACA GAA ATG-3′. The annealing temperature was 55°C.
Immunohistochemistry of Human Liver Tissues.
Pathologically confirmed human liver tissue arrays were obtained from U.S. Biomax (Rockville, MD). Deparafinization, inactivation of endogenous peroxidases, and antigen recovery were performed as described (39). Tissues were incubated with polyclonal anti-TLR7 antibody (1:25) overnight at 4°C, followed by anti-rabbit IgG-biotin (1:100) for 30 min and streptavidin–HRP (1:500) for 30 min at room temperature. The samples were developed with AEC kit (Vector Laboratories) and counterstained with Mayer’s hematoxylin. The slides were graded for the intensity of hepatocytes staining by an arbitrary 0–5 scale.
Supplementary Material
Acknowledgments
This work is supported by National Institutes of Health Grants AI56453 and AI57436 (to D.A.C.) and AI57709 (to E.R.).
Abbreviations
- HCV
hepatitis C virus
- TLR
Toll-like receptor
- IKK
IκB kinase
- IRF
IFN regulatory factor
- DNA-PK
DNA-dependent protein kinase
- SM360320
9-benzyl-8-hydroxy-2-(2-methoxyethoxy)adenine
- IMPDH
inosine monophosphate dehydrogenase
- BMDM
bone marrow-derived macrophages
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Kontorinis N., Agarwal K., Dieterich D. T. Rev. Gastroenterol. Disord. 2004;4(Suppl. 1):S39–S47. [PubMed] [Google Scholar]
- 2.Weiss U. Nature. 2005;436:929. [Google Scholar]
- 3.Gao B., Hong F., Radaeva S. Hepatology. 2004;39:880–890. doi: 10.1002/hep.20139. [DOI] [PubMed] [Google Scholar]
- 4.Chisari F. V. Nature. 2005;436:930–932. doi: 10.1038/nature04076. [DOI] [PubMed] [Google Scholar]
- 5.Feld J. J., Hoofnagle J. H. Nature. 2005;436:967–972. doi: 10.1038/nature04082. [DOI] [PubMed] [Google Scholar]
- 6.De Francesco R., Migliaccio G. Nature. 2005;436:953–960. doi: 10.1038/nature04080. [DOI] [PubMed] [Google Scholar]
- 7.Brassard D. L., Grace M. J., Bordens R. W. J. Leukoc. Biol. 2002;71:565–581. [PubMed] [Google Scholar]
- 8.Smee D. F., Alaghamandan H. A., Cottam H. B., Sharma B. S., Jolley W. B., Robins R. K. Antimicrob. Agents Chemother. 1989;33:1487–1492. doi: 10.1128/aac.33.9.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J., Chuang T. H., Redecke V., She L., Pitha P. M., Carson D. A., Raz E., Cottam H. B. Proc. Natl. Acad. Sci. USA. 2003;100:6646–6651. doi: 10.1073/pnas.0631696100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akira S., Takeda K. Nat. Rev. Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald K. A., McWhirter S. M., Faia K. L., Rowe D. C., Latz E., Golenbock D. T., Coyle A. J., Liao S. M., Maniatis T. Nat. Immunol. 2003;4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 12.Shi S., Nathan C., Schnappinger D., Drenkow J., Fuortes M., Block E., Ding A., Gingeras T. R., Schoolnik G., Akira S., et al. J. Exp. Med. 2003;198:987–997. doi: 10.1084/jem.20030603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perry A. K., Chow E. K., Goodnough J. B., Yeh W. C., Cheng G. J. Exp. Med. 2004;199:1651–1658. doi: 10.1084/jem.20040528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karpova A. Y., Trost M., Murray J. M., Cantley L. C., Howley P. M. Proc. Natl. Acad. Sci. USA. 2002;99:2818–2823. doi: 10.1073/pnas.052713899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katakura K., Lee J., Rachmilewitz D., Li G., Eckmann L., Raz E. J. Clin. Invest. 2005;115:695–702. doi: 10.1172/JCI22996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foy E., Li K., Wang C., Sumpter R., Jr., Ikeda M., Lemon S. M., Gale M., Jr. Science. 2003;300:1145–1148. doi: 10.1126/science.1082604. [DOI] [PubMed] [Google Scholar]
- 17.Zhang T., Lin R. T., Li Y., Douglas S. D., Maxcey C., Ho C., Lai J. P., Wang Y. J., Wan Q., Ho W. Z. Hepatology. 2005;42:819–827. doi: 10.1002/hep.20854. [DOI] [PubMed] [Google Scholar]
- 18.Honda K., Yanai H., Negishi H., Asagiri M., Sato M., Mizutani T., Shimada N., Ohba Y., Takaoka A., Yoshida N., et al. Nature. 2005;434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 19.Levy D. E., Marie I., Smith E., Prakash A. J. Interferon Cytokine Res. 2002;22:87–93. doi: 10.1089/107999002753452692. [DOI] [PubMed] [Google Scholar]
- 20.Lee K. J., Choi J., Ou J. H., Lai M. M. J. Virol. 2004;78:3797–3802. doi: 10.1128/JVI.78.7.3797-3802.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurimoto A., Ogino T., Ichii S., Isobe Y., Tobe M., Ogita H., Takaku H., Sajiki H., Hirota K., Kawakami H. Bioorg. Med. Chem. 2003;11:5501–5508. doi: 10.1016/j.bmc.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 22.Gorden K. B., Gorski K. S., Gibson S. J., Kedl R. M., Kieper W. C., Qiu X., Tomai M. A., Alkan S. S., Vasilakos J. P. J. Immunol. 2005;174:1259–1268. doi: 10.4049/jimmunol.174.3.1259. [DOI] [PubMed] [Google Scholar]
- 23.Jurk M., Heil F., Vollmer J., Schetter C., Krieg A. M., Wagner H., Lipford G., Bauer S. Nat. Immunol. 2002;3:499. doi: 10.1038/ni0602-499. [DOI] [PubMed] [Google Scholar]
- 24.Kawai T., Sato S., Ishii K. J., Coban C., Hemmi H., Yamamoto M., Terai K., Matsuda M., Inoue J., Uematsu S., et al. Nat. Immunol. 2004;5:1061–1068. doi: 10.1038/ni1118. [DOI] [PubMed] [Google Scholar]
- 25.Sharma S., tenOever B. R., Grandvaux N., Zhou G. P., Lin R., Hiscott J. Science. 2003;300:1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- 26.Yang H., Ma G., Lin C. H., Orr M., Wathelet M. G. Eur. J. Biochem. 2004;271:3693–3703. doi: 10.1111/j.1432-1033.2004.04310.x. [DOI] [PubMed] [Google Scholar]
- 27.Takii Y., Nakamura M., Ito M., Yokoyama T., Komori A., Shimizu-Yoshida Y., Nakao R., Kusumoto K., Nagaoka S., Yano K., et al. Lab. Invest. 2005;85:908–920. doi: 10.1038/labinvest.3700285. [DOI] [PubMed] [Google Scholar]
- 28.Matsumura T., Ito A., Takii T., Hayashi H., Onozaki K. J. Interferon Cytokine Res. 2000;20:915–921. doi: 10.1089/10799900050163299. [DOI] [PubMed] [Google Scholar]
- 29.Ojaniemi M., Liljeroos M., Harju K., Sormunen R., Vuolteenaho R., Hallman M. Immunol. Lett. 2005;102:158–168. doi: 10.1016/j.imlet.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Mozer-Lisewska I., Sluzewski W., Kaczmarek M., Jenek R., Szczepanski M., Figlerowicz M., Kowala-Piaskowska A., Zeromski J. Scand. J. Immunol. 2005;62:407–412. doi: 10.1111/j.1365-3083.2005.01670.x. [DOI] [PubMed] [Google Scholar]
- 31.Horsmans Y., Berg T., Desager J. P., Mueller T., Schott E., Fletcher S. P., Steffy K. R., Bauman L. A., Kerr B. M., Averett D. R. Hepatology. 2005;42:724–731. doi: 10.1002/hep.20839. [DOI] [PubMed] [Google Scholar]
- 32.Li K., Foy E., Ferreon J. C., Nakamura M., Ferreon A. C., Ikeda M., Ray S. C., Gale M., Jr., Lemon S. M. Proc. Natl. Acad. Sci. USA. 2005;102:2992–2997. doi: 10.1073/pnas.0408824102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagahara K., Anderson J. D., Kini G. D., Dalley N. K., Larson S. B., Smee D. F., Jin A., Sharma B. S., Jolley W. B., Robins R. K., et al. J. Med. Chem. 1990;33:407–415. doi: 10.1021/jm00163a064. [DOI] [PubMed] [Google Scholar]
- 34.Kurimoto A., Ogino T., Ichii S., Isobe Y., Tobe M., Ogita H., Takaku H., Sajiki H., Hirota K., Kawakami H. Bioorg. Med. Chem. 2004;12:1091–1099. doi: 10.1016/j.bmc.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Chu W., Gong X., Li Z., Takabayashi K., Ouyang H., Chen Y., Lois A., Chen D. J., Li G. C., Karin M., Raz E. Cell. 2000;103:909–918. doi: 10.1016/s0092-8674(00)00194-x. [DOI] [PubMed] [Google Scholar]
- 36.Lee J., Mira-Arbibe L., Ulevitch R. J. J. Leukoc. Biol. 2000;68:909–915. [PubMed] [Google Scholar]
- 37.Shi S. T., Lee K. J., Aizaki H., Hwang S. B., Lai M. M. J. Virol. 2003;77:4160–4168. doi: 10.1128/JVI.77.7.4160-4168.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Machida K., Cheng K. T., Sung V. M., Lee K. J., Levine A. M., Lai M. M. J. Virol. 2004;78:8835–8843. doi: 10.1128/JVI.78.16.8835-8843.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martins A. R., Dias M. M., Vasconcelos T. M., Caldo H., Costa M. C., Chimelli L., Larson R. E. J. Neurosci. Methods. 1999;92:25–29. doi: 10.1016/s0165-0270(99)00090-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.