Abstract
SLC12A cation/Cl− cotransporters are mutated in human disease, are targets of diuretics, and are collectively involved in the regulation of cell volume, neuronal excitability, and blood pressure. This gene family has two major branches with different physiological functions and inverse regulation: K-Cl cotransporters (KCC1–KCC4) mediate cellular Cl− efflux, are inhibited by phosphorylation, and are activated by dephosphorylation; Na-(K)-Cl cotransporters (NCC and NKCC1/2) mediate cellular Cl− influx and are activated by phosphorylation. A single kinase/phosphatase pathway is thought to coordinate the activities of these cotransporters in a given cell; however, the mechanisms involved are as yet unknown. We previously demonstrated that WNK3, a paralog of serine-threonine kinases mutated in hereditary hypertension, is coexpressed with several cation/Cl− cotransporters and regulates their activity. Here, we show that WNK3 completely prevents the cell swelling-induced activation of KCC1–KCC4 in Xenopus oocytes. In contrast, catalytically inactive WNK3 abolishes the cell shrinkage-induced inhibition of KCC1–KCC4, resulting in a >100-fold stimulation of K-Cl cotransport during conditions in which transport is normally inactive. This activation is completely abolished by calyculin A and cyclosporine A, inhibitors of protein phosphatase 1 and 2B, respectively. Wild-type WNK3 activates Na-(K)-Cl cotransporters by increasing their phosphorylation, and catalytically inactive kinase inhibits Na-(K)-Cl cotransporters by decreasing their phosphorylation, such that our data suggest that WNK3 is a crucial component of the kinase/phosphatase signaling pathway that coordinately regulates the Cl− influx and efflux branches of the SLC12A cotransporter family.
Keywords: ion transport, protein serine-threonine kinases, hypertension, cell volume regulation
Phylogenetic analysis of the electroneutral cation/Cl−-cotransporter family (SLC12A) reveals two branches: the K-Cl cotransporter branch, composed of four members (KCC1, KCC2, KCC3, and KCC4) that exhibit ≈70% identity, and the Na-(K)-Cl cotransporter branch, composed of three members (NCC, NKCC1, and NKCC2) that exhibit ≈50% identity. Because of the gradient of the accompanying cation, K-Cl cotransporters mediate Cl− efflux, whereas Na-(K)-Cl cotransporters mediate Cl− influx. These cotransporters are necessary for several fundamental physiological processes, including the regulation of cell volume, neuronal excitability, transepithelial NaCl transport, and arterial blood pressure (1).
The activities of SLC12A cotransporters are regulated by phosphorylation/dephosphorylation processes (for review, see refs. 1–5). Dephosphorylation activates K-Cl cotransporters and inhibits Na-(K)-Cl cotransporters, whereas phosphorylation inhibits K-Cl cotransporters and activates Na-(K)-Cl cotransporters. It has been proposed that these cotransporters share a common regulatory pathway, because stimuli that activate the K-Cl cotransporters inhibit the Na-(K)-Cl cotransporters and vice versa. Thus cell swelling, high intracellular Cl−, and protein phosphatases (PPs) stimulate K-Cl cotransporters but inhibit Na-(K)-Cl cotransporters; in contrast, cell shrinkage, low intracellular Cl−, and PP inhibitors have the opposite effect on these two transport systems. Although several kinases have been proposed to regulate the phosphorylation of the basolateral isoform of the Na-K-2Cl cotransporter NKCC1 (3, 6), a member of the pathway that coordinates the opposing actions of Na-(K)-Cl and K-Cl cotransport has yet to be identified.
The WNK kinases (WNK, with no lysine = K), a family of four novel serine-threonine kinases, are emerging as key regulatory proteins for the SLC12A family (7). Sequence analysis reveals that WNK kinases contain an amino-terminal kinase domain and a carboxyl-terminal regulatory domain. Mutations in WNK1 and WNK4 cause pseudohypoaldosteronism type II, a Mendelian disease that features hypertension and hyperkalemia (8). WNK4 has been shown to be a regulatory kinase for ion channels, transporters, and tight junction proteins, indicating that WNK4 possesses the properties of a multifunctional regulator of diverse ion transport pathways (7). Although the mechanism of WNK4’s regulation of these transport pathways is unknown, two recent studies have demonstrated that WNK4 lies upstream of the STE20 kinases, SPAK and OSR1, that are known to regulate cation/Cl− cotransport (9–11). Furthermore, WNK1 modulates the effect of WNK4 on the Na-Cl cotransporter NCC (12, 13) and of SGK1 (serum and glucocorticoid-induced protein kinase) on the epithelial Na+ channel ENaC (14).
In recent studies, we observed that coexpression of any of the three Na-(K)-Cl cotransporters (NCC, NKCC1, and NKCC2) with WNK3 resulted in a significant increase in 22Na+ (NCC) or 86Rb+ (NKCC1/2) uptake in Xenopus oocytes (15, 16). WNK3-induced activation of NKCC1 or NKCC2 was associated with increased phosphorylation of two amino-terminal threonine residues necessary for full cotransporter activation; this effect was present under hypotonic conditions, in which NKCC1 is normally dephosphorylated and, therefore, inactivated. In contrast, coexpression of the K-Cl cotransporters KCC1 or KCC2 with WNK3 resulted in a complete inhibition of cotransporter activity, even when oocytes were exposed to hypotonic conditions in which K-Cl cotransporters are otherwise maximally active. Strikingly, coexpression of these cotransporters with the WNK3 catalytically inactive mutant (WNK3-D294A) resulted in the opposite effects: the Na-(K)-Cl cotransporters were inhibited, whereas KCC1 and KCC2 were activated. Immunolocalization studies with a specific WNK3 antibody demonstrated that WNK3 is coexpressed with KCC2 and NKCC1 in several regions of the central nervous system, and with KCCs, NCC and NKCC2 in the kidney. Thus, WNK3 possesses the expected properties of a regulator that is able to coordinate the function of SLC12A family members. Here, we extended our observations to include the effects of WNK3 on all four K-Cl cotransporter isoforms and analyze the mechanism by which WNK3-D294A induces activation of the K-Cl cotransporters in osmolarities that normally inhibit these cotransporters.
Results and Discussion
WNK3 Prevents Hypotonic Activation of KCCs.
The K-Cl cotransporter was first described as a swelling-activated K+ efflux pathway in erythrocytes (17). In the oocyte expression system, all four K-Cl cotransporter isoforms are activated by incubation of oocytes in hypotonic medium (18–21). In isotonic conditions, KCC2 is the only isoform that exhibits significant transport activity in both oocytes and HEK-293 cells (20, 22, 23)
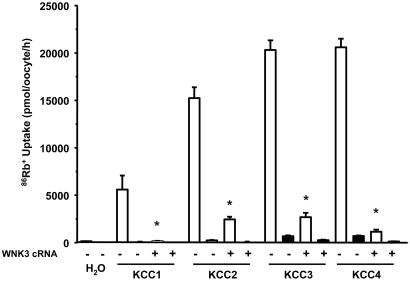
In the present study, we tested the effects of WNK3 on the activity of K-Cl cotransporters in both isotonic and hypotonic conditions. 86Rb+ uptake observed for KCC1 (163 ± 16 pmol·oocyte−1·h−1), KCC3 (199 ± 26 pmol·oocyte−1·h−1), and KCC4 (229 ± 50 pmol·oocyte−1·h−1) was not significantly different from water-injected oocytes in isotonic medium (91 ± 9 pmol·oocyte−1·h−1), and WNK3 had no effect on the observed 86Rb+ uptakes (data not shown). In these experimental conditions, KCC2 exhibited significant activity (937 ± 223 pmol·oocyte−1·h−1, P > 0.01) over water-injected control oocytes, and most of this increased 86Rb+ uptake was prevented by coexpression with WNK3 (206 ± 21 pmol·oocyte−1·h−1, P < 0.01 vs. KCC2 alone). Fig. 1 shows the results of experiments performed when oocytes were incubated in hypotonic conditions, which resulted in a significant activation of all four K-Cl cotransporters. WNK3 inhibited KCC activity in hypotonic medium because 86Rb+ uptake in all groups coexpressing WNK3 was 80% to 98% lower compared to oocytes expressing KCCs alone. Thus, WNK3 prevents the hypotonic activation of all four KCCs and also inhibits KCC2 activity in isotonic conditions.
Fig. 1.
Effect of wild-type WNK3 on K-Cl cotransport induced by cell swelling under hypotonic conditions. X. laevis oocytes were injected with water or 0.2 μg/μl each of the KCCs cRNA alone or together with WNK3 cRNA. 86Rb+ uptake was assessed 4 days later in hypotonic conditions (110 mOsm/kg) in the presence (open bars) or absence (filled bars) of Cl− in the uptake medium. A representative experiment from a single frog, with the mean ± SEM of 15 oocytes for each group, is shown. Similar results have been observed in at least five experiments, each experiment using oocytes from a different frog. ∗, significantly different from the uptake observed in the corresponding control (absence of WNK3, P < 0.01).
Kinase-Dead WNK3 Activates KCCs in Isotonic Conditions.
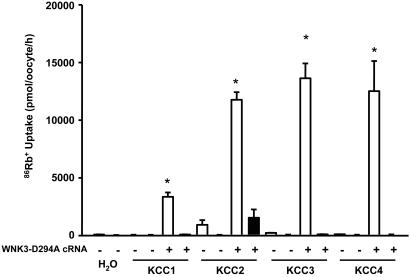
Kinase-dead WNK3-D294A exhibits exactly the opposite effects on KCCs compared to those observed with wild-type WNK3. When exposed to isotonic conditions, each KCC had a several hundred-fold higher activity when coexpressed with WNK3-D294A compared to oocytes not expressing the WNK3 mutant (Fig. 2). The uptakes observed in all KCC/WNK3-D294A coexpressing oocytes were Cl−-dependent, indicating that 86Rb+ uptake occurred specifically through the K-Cl cotransporters. We recently demonstrated that WNK3-D294A failed to inhibit K-Cl cotransport by KCC1 or KCC2 in hypotonic conditions but instead induced a further increase in 86Rb+ uptake (15). In the present study, we confirmed these observations for KCC1 and KCC2 but did not initially see the effect of WNK3-D294A on swelling-activated KCC3 and KCC4. When uptakes were performed in hypotonic conditions in oocytes injected with cRNA concentration at 0.2 μg/μl, the values for each KCC in the absence or presence of WNK3-D294A were as follows: KCC1, 4,079 ± 570 vs. 6,991 ± 896 pmol·oocyte−1·h−1 (P < 0.01); KCC2, 12,908 ± 1,034 vs. 18,645 ± 630 pmol·oocyte−1·h−1 (P > 0.01); KCC3, 19,214 ± 2,023 vs. 18,720 ± 1,546 pmol·oocyte−1·h−1 [P = not significant (NS)]; KCC4, 21,762 ± 1,476 vs. 21,541 ± 2,205 pmol·oocyte−1·h−1 (P = NS). Thus coexpression of KCC1 and KCC2 with WNK3-D294A resulted in a further increase in 86Rb+ uptake in hypotonic conditions, whereas coinjection with KCC3 and KCC4 had no effect on 86Rb+ uptake. Because we always see higher expression of KCC3 and KCC4 relative to KCC1 and KCC2 when similar amounts of each KCC cRNA are injected (0.2 μg/μl), an explanation for the differences observed with the effect of WNK3-D294A could be that KCC1/KCC2 may not be fully active, whereas KCC3/4 are functioning at their Vmax. In support of this hypothesis, Fig. 6, which is published as supporting information on the PNAS web site, shows the results of an experiment in which oocytes were injected with 50 nl of KCC4 cRNA at 0.05 or 0.2 μg/μl, alone or together with WNK3-D294A cRNA. 86Rb+ uptake in which oocytes were injected with KCC4 at 0.05 μg/μl was 13,010 ± 728 pmol·oocyte−1·h−1, whereas in oocytes injected with the same concentration of KCC4 cRNA plus WNK3-D294A cRNA, 86Rb+ uptake was 19,931 ± 1,223 pmol·oocyte−1·h−1 (P < 0.001). In contrast, in oocytes injected with KCC4 cRNA at 0.2 μg/μl, the 86Rb+ uptake was similar in the absence or presence of WNK3-D294A (24,066 ± 439 vs. 24,237 ± 1,170 pmol·oocyte−1·h−1, P = NS). It is evident, therefore, that WNK3-D294A induces the opposite effects to those of wild-type WNK3 on the KCCs. In isotonic conditions, WNK3-D294A activates all four K-Cl cotransporters to levels seen previously only in their usual hypotonic “activating” medium. In addition, in hypotonic conditions, WNK3-D294A further activates swelling-activated K-Cl cotransport.
Fig. 2.
Effect of catalytically inactive WNK3-D294A on K-Cl cotransport under isotonic conditions. X. laevis oocytes were injected with water or 0.2 μg/μl each of the KCCs cRNAs alone or together with WNK3-DA cRNA. 86Rb+ uptake was assessed 4 days later in isotonic conditions (220 mOsm/kg) in the presence (open bars) or absence (filled bars) of Cl− in the uptake medium. A representative experiment from a single frog, with the mean ± SEM of 15 oocytes for each group, is shown. Similar results have been observed in at least five different experiments, each experiment using oocytes from a different frog. ∗, significantly different from the uptake observed in the corresponding control (absence of WNK3-DA, P < 0.01).
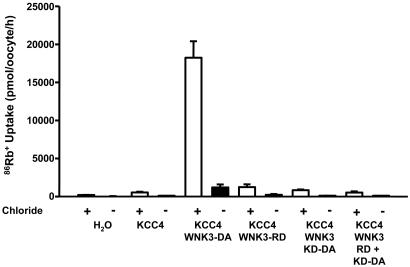
In recent studies (15, 16), we showed that WNK3 and WNK3-D294A have inverse effects on the activity of Na-(K)-Cl and K-Cl cotransporters. For both NKCC1 and KCCs, WNK3 bypasses the normal requirements of hypertonicity and hypotonicity, respectively, for cotransporter activation. In addition, catalytically inactive WNK3 prevents the activity of the Na-(K)-Cl cotransporters and, in the absence of cell swelling, allows K-Cl cotransporters to be active. If there is an endogenous WNK3 activity in oocytes, then one possible explanation for the WNK3-D294A-induced activation of KCCs would be a dominant negative effect in which the catalytically inactive WNK3-D294A will render the endogenous WNK3 inactive and, thus, prevent the “endogenous” WNK3-induced phosphorylation and inhibition of the cotransporter in isotonic conditions, with the consequent activation of KCCs. To test this possibility, we injected oocytes with KCC4 cRNA alone or together with cRNA in vitro transcribed from the full-length WNK3-D294A cDNA or from the regulatory domain or the kinase domain harboring the D294A mutation cDNAs only. A fifth group of oocytes was coinjected with KCC4 together with the regulatory and the kinase-D294A domain cRNAs. As shown in Fig. 3, coinjection of oocytes with KCC4 and WNK3-D294A resulted in several hundred-fold activation of KCC4 in isotonic conditions. In contrast, coinjection of KCC4 with the WNK3 regulatory domain cRNA only, the WNK3 kinase-D294A domain cRNA only, or both WNK3 domains on separate cRNAs had no effect upon KCC4 activation. Thus, the observation that neither the kinase domain nor the regulatory domain alone were able to activate the KCCs suggests that the effect of the full-length WNK3-D294A upon KCC4 transport activity is unlikely to be due to a dominant negative effect.
Fig. 3.
An intact WNK3-294A molecule is necessary for the regulation of K-Cl cotransport under isotonic conditions. 86Rb+ uptake was measured in X. laevis oocytes microinjected with water, KCC4 cRNA alone, or KCC4 cRNA together with cRNA transcribed from each of the following cDNAs: WNK3-D294A (containing kinase-inactive WNK3), WNK3-RD (containing the sequence of the WNK3 regulatory domain only), WNK3-KD-D294A (containing the sequence of the WNK3 kinase domain only, with the D294A mutation), and WNK3-RD + KD-D294A (containing the WNK3 regulatory domain and the kinase domain DA together, but as separate transcripts). Uptakes were performed in isotonic medium (220 mOsm/kg) in the presence (open bars) or absence (filled bars) of extracellular Cl− in the uptake medium. Each bar represents the mean ± SEM of 20 oocytes extracted from two different frogs.
WNK3 Regulates KCCs by Modulating Phosphatases.
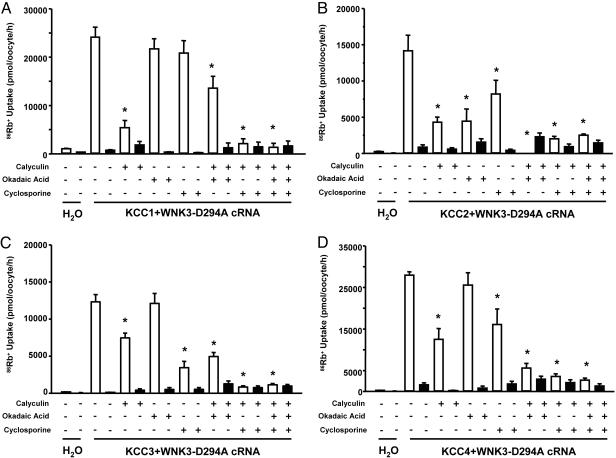
Inhibition of specific PP is associated with activation of NKCC1 and inhibition of KCCs (24–26) due to increased phosphorylation of cotransporters (27, 28). We observed effects of WNK3-D294A on electroneutral cotransporters that would be expected with activation of PPs, suggesting that WNK3-D294A is functionally interacting with both the cotransporter proteins and the PPs. To test this possibility, we assessed the effects of three different PP inhibitors on the WNK3-D294A-induced activation of K-Cl cotransporters in oocytes exposed to isotonic conditions (Fig. 4). Parallel experiments were performed in which uptakes were assessed in oocytes expressing the corresponding KCC cotransporter alone in hypotonic conditions (Fig. 7, which is published as supporting information on the PNAS web site). Calyculin A at 100 nM inhibits the function of PP1 > PP2A, okadaic acid at 1 nM inhibits only PP2A, and cyclosporine A at 25 μM inhibits the function of the PP2B.
Fig. 4.
WNK3-D294A activates KCC1, KCC2, KCC3, and KCC4 via a phosphatase-dependent mechanism. Effect of protein phosphatase inhibitors upon 86Rb+ uptake in oocytes injected with KCC1 (A), KCC2 (B), KCC3 (C), and KCC4 (D). All groups were injected with cRNA concentration at 0.2 μg/μl, except for KCC1 (0.4 μg/μl). All groups were coinjected with WNK3-D294A cRNA at 0.1 μg/μl. Uptakes were performed in isotonic conditions in oocytes that were exposed to Cl−-containing medium (open bars) or Cl−-depleted medium (filled bars). Each bar represents the mean ± SEM of 20 oocytes from two different frogs. Protein phosphatase inhibitors used were as follows: calyculin A (at 100 nM), okadaic acid (at 1 nM), and cyclosporine A (at 25 μM). ∗, significantly different from the uptake observed in the corresponding control (absence of protein phosphatase inhibitors, P < 0.01).
As shown in Fig. 4A, isotonic incubation of oocytes injected with KCC1+WNK3-D294A resulted in a significant increase in KCC1 activity (uptake in KCC1 cRNA alone injected oocytes in similar conditions was 394 ± 30 pmol·oocyte−1·h−1). 86Rb+ uptake was significantly reduced by calyculin A but not by okadaic acid. Cyclosporine induced a small, but nonsignificant, reduction in 86Rb+ uptake. However, when two or three PP inhibitors were used together, KCC1 activity in the presence of WNK3-D294A was completely prevented. These observations suggest that WNK3-D294A-induced activation of KCC1 in isotonic conditions depends on the activity of the protein phosphatases, particularly PP1 and the PP2B, because inhibition of these two proteins by the combination of calyculin A and cyclosporine A completely prevented the KCC1 activation by WNK3-D294A. Fig. 7 shows that in the parallel experiment, oocytes injected with KCC1 cRNA alone exhibited increased 86Rb+ uptake when incubated in hypotonic conditions, and the effect of phosphatases inhibitors was similar to that observed in KCC1+WNK3-D294A-injected oocytes.
Fig. 4 B–D show results obtained with KCC2, KCC3, and KCC4, respectively, when coinjected with WNK3-D294A. Increased 86Rb+ uptake in isotonic conditions was observed in all three groups. In these K-Cl cotransporters, addition of either calyculin A or cyclosporine A alone partially prevented the WNK3-D294A-induced activation, and in all cases, full inhibition was observed when combinations of phosphatase inhibitors were used. Similar observations were obtained in oocytes injected with each of the KCCs cRNA alone and incubated in hypotonic conditions (Fig. 7).
Our observations are consistent with the hypothesis that K-Cl cotransporters need to be dephosphorylated to be activated and suggest that during cell swelling, PP1 is the major protein phosphatase involved. However, the fact that combinations of PP inhibitors further decrease the activity of KCCs indicates that at least two PPs are required for full activation. Because the most consistent observation in this study was that a combination of calyculin A and cyclosporine A completely prevented the cell swelling-induced activation of all four KCCs, it is possible that PP1 and PP2B are the major protein phosphatases responsible. Thus, our observations strongly suggest that WNK3-D294A induces dephosphorylation of the KCCs by activating protein phosphatases.
WNK kinases regulate ion transport proteins through catalytic and/or physical interaction with their targets. For example, the effects of WNK4 on NCC and claudins, but not the K+ channel ROMK, requires its kinase activity (29–31). In addition, the effect of WNK1 on ENaC does not require WNK catalytic activity (32). It is believed that actions not requiring phosphorylation reside in the WNK carboxyl-terminal domain of WNKs. The observation in this study that WNK3 and WNK3-D294A bypass the normal regulation of the K-Cl cotransporters by extracellular osmolarity has parallelism with the loss of volume sensitivity that was recently reported for NKCC1 with catalytically active and inactive SPAK/OSR1 kinases in conjunction with WNK4 (10, 11). However, unlike SPAK/OSR1/WNK4, WNK3 and WNK3-D294A are able to accomplish their dramatic effects on the cotransporters without requiring coinjection with other kinases (SPAK/OSR1), suggesting that WNK3 may be downstream of SPAK/OSR1 or represents an alternative pathway. Thus, we propose that the effects of both wild-type WNK3 and WNK3-D294A are normal biochemical actions of WNK3 in vivo. In this regard, WNK3-D294A mimics the kinase inactive form of wild-type WNK3, which could occur via auto inhibition (33) or by interacting with an accessory protein. In our previous studies (15, 16), we observed that increased activity of NKCC1 or NKCC2 induced by wild-type WNK3 is associated with increased immunodetection of NKCC1 or NKCC2 by the R5 phosphoantibody that recognizes phosphorylated amino-terminal threonine residues T184 and T189 of NKCC1 or T96 and T101 of NKCC2; these phosphorylation events have previously been demonstrated to play an important role in activation of both isoforms of the Na-K-2Cl cotransporters, NKCC1 and NKCC2 (34, 35). In addition, we observed that decreased activity of NKCC1 or NKCC2 induced by WNK3-D294A, even in hypertonic conditions, was associated with dephosphorylation of these same threonines. In this study, we show that the activation of KCC1–KCC4 by WNK3-D294A is executed via a mechanism that requires activation of protein phosphatases, because inhibition of PP1 and PP2B prevents the effect of WNK3-D294A on all KCCs. These observations suggest that WNK3 could be acting as, or interacting with, a regulatory subunit of the protein phosphatases.
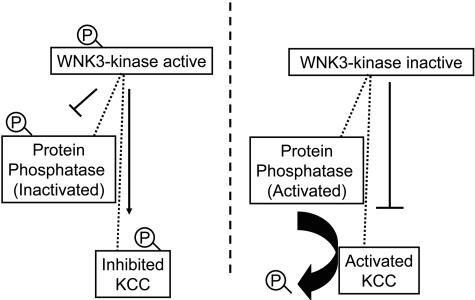
Specificity of a protein phosphatase catalytic domain (PPc)1 for a target protein is thought to be determined by its association with one or two regulatory subunits that modulates PP1 activity and/or brings the phosphatase in close proximity to its substrate. More than 45 proteins that act as regulatory subunits of PP1c have been identified and classified in two groups based on their mechanism of action (36, 37). One group is composed of proteins that regulate the activity of PP1c; that is, the interaction between PP1c and the regulatory protein results in activation or inhibition of the dephosphorylating activity of PP1c toward all substrates (38). The second group of regulatory proteins binds both the PP1c and one or two substrates or subcellular structures. These regulatory proteins work as scaffolding proteins by forming complexes between the PP1c and specific substrates and, at the same time, can regulate the activity of the PP1c. Our observations suggest a model shown in Fig. 5 in which WNK3 is itself a PP regulatory protein, or through interaction with a regulatory protein, bring together the cation/Cl− cotransporters with PP1c and/or PP2Bc. In its active form, WNK3 induces phosphorylation of the cotransporter and probably also of PP1c, which inhibits PP1 phosphatase activity. These combined effects would increase phosphorylation of the K-Cl cotransporters and, thus, inhibition. In contrast, WNK3-D294A may also bind to the cotransporter and PP1c, but the lack of catalytic activity precludes their phosphorylation, thus increasing the dephosphorylating activity of PP1c on the K-Cl cotransporters and, thus, activation. The fact that PP inhibitors prevent KCCs activation by WNK3-D294A suggest that in isotonic conditions, KCCs are still phosphorylated by other pathways different from WNK3. It is known that WNKs possess autophosphorylation activity that is required for their catalytic activity; this autophosphorylation is under negative control by a WNK kinase autoinhibitory domain. Xu et al. (33) demonstrated that two phenylalanine residues in this domain are critical for its inhibitory activity. If these residues are eliminated, the autoinhibitory properties of WNK1 are decreased, with the consequent increase in autophosphorylation and ability to phosphorylate the substrate myelin basic protein (33). Thus, it is possible that under certain circumstances (e.g., cell shrinkage and/or decreased intracellular chloride), the autoinhibitory activity is prevented, activating WNK3 autophosphorylation with the resulting effect of increased phosphorylation of the cotransporters. As a consequence, NCC and NKCCs are activated, whereas KCCs are inhibited. In contrast, during cell swelling and/or increased intracellular chloride concentration, the autoinhibitory domain might prevent the autophosphorylation of WNK3, therefore producing catalytically inactive WNK3. Because scaffolding activity is probably not required for kinase activity, the lack of inhibition of PP1c would allow this protein to dephosphorylate the cotransporters, resulting in inhibition of NCC/NKCCs and activation of KCCs.
Fig. 5.
Model of KCC regulation by active and inactive WNK3. See text for details.
In summary, WNK3 and WNK3-D294A have the ability to bypass the normal regulation of the electroneutral cotransporters by extracellular osmolarity. In the presence of active or inactive WNK3 kinase, the changes in cell volume that are required to activate or inhibit the cotransporters of the SLC12 family are no longer necessary. The active WNK3 seems to induce phosphorylation (15, 16), whereas the inactive WNK3 seems to induce dephosphorylation of the cotransporters. Therefore, WNK3 possesses properties of the proposed NKCC1/KCC regulatory kinase that is responsive to intracellular Cl− concentrations and/or changes in cell volume (28, 39).
Materials and Methods
Xenopus laevis Oocyte Preparation.
Adult female X. laevis frogs were purchased from Nasco (Fort Atkinson, MI) and maintained at the animal facility under control of room temperature and humidity at 16°C and 65%, respectively. Oocytes were surgically collected from anesthetized animals under 0.17% tricaine and incubated for 1 h with vigorous shaking in frog Ringer ND96 solution (96 mM NaCl/2 mM KCl/1.8 mM CaCl2/1 mM MgCl/5 mM Hepes/Tris, pH 7.4) in the presence of 2 mg/ml collagenase B. Oocytes were then washed four times in ND96, manually defolliculated, and incubated overnight in ND96 at 18°C. The next day, stage V-VI oocytes (40) were injected with 50 nl of water alone or containing 0.2–0.4 μg/μl cRNA in vitro transcribed by using the T7 RNA polymerase mMESSAGE kit (Ambion, Austin, TX) from rabbit KCC1 (19), human KCC2 (20), human KCC3 (21), or mouse KCC4 (19) cDNA. Transcription product integrity was confirmed on agarose gels, and concentration was determined by absorbance reading at 260 nm (DU 640; Beckman). For coinjection experiments, the same amount of KCC cRNA was maintained, and 0.1 μg/μl cRNA transcribed from WNK3 or WNK3-D294A was added. Oocytes were incubated at 18°C for 4 days in ND96 supplemented with 2.5 mM sodium pyruvate and 5 mg/100 ml of gentamicin. Oocytes were switched to Cl−-free ND96 (96 mM Na+ isethionate/2 mM K+-gluconate/6.0 mM Ca2+ gluconate/1.0 mM Mg2+ gluconate/5 mM Hepes/2.5 mM sodium pyruvate,/5 mg/100 ml gentamicin, pH 7.4) 2 h before the uptake assay.
Assessment of the K-Cl Cotransporter Function.
K-Cl cotransport was assessed by measuring tracer 86Rb+ uptake (New England Nuclear) in experimental groups of at least 15 oocytes. 86Rb+ uptake was assessed in oocytes exposed to two different osmolar conditions. For uptake in hypotonic conditions, oocytes were incubated for a 30-min period in a hypotonic K+- and Cl−-free medium [50 mM N-methyl-d-glucamine (NMDG) gluconate/4.6 mM Ca2+ gluconate/1.0 mM Mg2+ gluconate/5 mM Hepes/Tris, pH 7.4/110 mOsm/kg H2O] with 1 mM ouabain, followed by a 60-min uptake period in a hypotonic Na+-free medium containing 10 mM KCl and 40 mM NMDG-Cl. The uptake solution also contained 1.8 mM CaCl2, 1 mM MgCl2, and 5 mM Hepes (pH 7.4) and was supplemented with 1 mM ouabain/2.0 μCi of 86Rb+ (1 Ci = 37 GBq). Parallel groups of oocytes were exposed to Cl−-free uptake solution in which Cl− was substituted with gluconate. For uptake in isotonic conditions, we used the same solutions but supplemented with 3.5 g/100 ml sucrose to reach isosmolar conditions for oocytes (≈210 mOsm/kg). Ouabain was added to prevent 86Rb+ uptake via the Na-K-ATPase. The absence of extracellular Na+ and the hypotonicity of the uptake medium prevented 86Rb+ uptake via the oocyte’s endogenous Na-K-2Cl cotransporter (19).
All uptakes were performed at 32°C. At the end of the uptake period, oocytes were washed five times in ice-cold uptake solution and dissolved in 10% SDS, and tracer activity was determined for each oocyte by β-scintillation counting.
cDNA Constructs.
Wild-type pGH19-WNK3, or pGH19-WNK3 harboring the kinase-inactivating D294A, were described in ref. 16. Wild-type WNK3 was subdivided into the kinase domain (KD, bases 1 to 1,252) and the regulatory domain (RD, bases 1,253 to 5,229). Each domain was subcloned into pGH19 with 5′ EcoRI and 3′ Xho restriction sites.
Statistical Analysis.
Statistical significance is defined as two-tailed, with P < 0.05, and the results are presented as mean ± SEM. The significance of the differences between groups was tested by one-way ANOVA with multiple comparisons by using Bonferroni correction.
Supplementary Material
Acknowledgments
This work was supported, in part, by National Institutes of Health (NIH) Specialized Center of Research Grant in Hypertension (to R.P.L.); NIH Grants DK-36803 (to S.C.H. and G.G.), DK-64635 (to G.G.), and DK-57708 (to D.B.M.); and Wellcome Trust Grant GR070159MA (to G.G). K.T.K. is a trainee of the NIH Medical Scientist Training Program. P.d.l.H. is supported by a Consejo Nacional de Ciencia y Technología Grant. R.P.L. is an Investigator of the Howard Hughes Medical Institute.
Abbreviations
- KCC
K-Cl cotransporter
- PP
protein phosphatase
- PPc
protein phosphatase catalytic domain
- WNK
with no lysine = K.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Gamba G. Physiol. Rev. 2005;85:423–493. doi: 10.1152/physrev.00011.2004. [DOI] [PubMed] [Google Scholar]
- 2.Hebert S. C., Mount D. B., Gamba G. Pflugers Arch. 2004;447:580–593. doi: 10.1007/s00424-003-1066-3. [DOI] [PubMed] [Google Scholar]
- 3.Flatman P. W. Biochim. Biophys. Acta. 2002;1566:140–151. doi: 10.1016/s0005-2736(02)00586-2. [DOI] [PubMed] [Google Scholar]
- 4.Adragna N. C., Fulvio M. D., Lauf P. K. J. Membr. Biol. 2004;201:109–137. doi: 10.1007/s00232-004-0695-6. [DOI] [PubMed] [Google Scholar]
- 5.Lauf P. K., Adragna N. C. Cell Physiol. Biochem. 2000;10:341–354. doi: 10.1159/000016357. [DOI] [PubMed] [Google Scholar]
- 6.Haas M., Forbush B., III Annu. Rev. Physiol. 2000;62:515–534. doi: 10.1146/annurev.physiol.62.1.515. [DOI] [PubMed] [Google Scholar]
- 7.Gamba G. Am. J. Physiol. 2005;288:F245–F252. doi: 10.1152/ajprenal.00311.2004. [DOI] [PubMed] [Google Scholar]
- 8.Wilson F. H., Disse-Nicodeme S., Choate K. A., Ishikawa K., Nelson-Williams C., Desitter I., Gunel M., Milford D. V., Lipkin G. W., Achard J. M., et al. Science. 2001;293:1107–1112. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- 9.Gamba G. Biochem. J. 2005;391:e1–e3. doi: 10.1042/BJ20051345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gagnon K. B., England R., Delpire E. Am. J. Physiol. 2005;290:C134–C142. doi: 10.1152/ajpcell.00037.2005. [DOI] [PubMed] [Google Scholar]
- 11.Vitari A. C., Deak M., Morrice N. A., Alessi D. R. Biochem. J. 2005;391:17–24. doi: 10.1042/BJ20051180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang C. L., Angell J., Mitchell R., Ellison D. H. J. Clin. Invest. 2003;111:1039–1045. doi: 10.1172/JCI17443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang C. L., Zhu X., Wang Z., Subramanya A. R., Ellison D. H. J. Clin. Invest. 2005;115:1379–1387. doi: 10.1172/JCI22452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu B.-e., Stippec S., Chu P.-Y., Lazrak A., Li X.-J., Lee B.-H., English J. M., Ortega B., Huang C.-L., Cobb M. H. Proc. Natl. Acad. Sci. USA. 2005;102:10315–10320. doi: 10.1073/pnas.0504422102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahle K. T., Rinehart J., De Los H. P., Louvi A., Meade P., Vazquez N., Hebert S. C., Gamba G., Gimenez I., Lifton R. P. Proc. Natl. Acad. Sci. USA. 2005;102:16783–16788. doi: 10.1073/pnas.0508307102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rinehart J., Kahle K. T., de los Heros P., Vazquez N., Meade P., Wilson F. H., Hebert S. C., Gimenez I., Gamba G., Lifton R. P. Proc. Natl. Acad. Sci. USA. 2005;102:16777–16782. doi: 10.1073/pnas.0508303102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lauf P. K., Bauer J., Adragna N. C., Fujise H., Zade-Oppen A. M. M., Ryu K. H., Delpire E. Am. J. Physiol. 1992;263:C917–C932. doi: 10.1152/ajpcell.1992.263.5.C917. [DOI] [PubMed] [Google Scholar]
- 18.Mount D. B., Mercado A., Song L., Xu J., George A. L., Jr, Delpire E., Gamba G. J. Biol. Chem. 1999;274:16355–16362. doi: 10.1074/jbc.274.23.16355. [DOI] [PubMed] [Google Scholar]
- 19.Mercado A., Song L., Vazquez N., Mount D. B., Gamba G. J. Biol. Chem. 2000;275:30326–30334. doi: 10.1074/jbc.M003112200. [DOI] [PubMed] [Google Scholar]
- 20.Song L., Mercado A., Vazquez N., Xie Q., Desai R., George A. L., Gamba G., Mount D. B. Brain Res. Mol. Brain Res. 2002;103:91–105. doi: 10.1016/s0169-328x(02)00190-0. [DOI] [PubMed] [Google Scholar]
- 21.Mercado A., Vazquez N., Song L., Cortes R., Enck A. H., Welch R., Delpire E., Gamba G., Mount D. B. Am. J. Physiol. 2005;289:F1246–F1261. doi: 10.1152/ajprenal.00464.2004. [DOI] [PubMed] [Google Scholar]
- 22.Strange K., Singer T. D., Morrison R., Delpire E. Am. J. Physiol. 2000;279:C860–C867. doi: 10.1152/ajpcell.2000.279.3.C860. [DOI] [PubMed] [Google Scholar]
- 23.Payne J. A. Am. J. Physiol. 1997;273:C1516–C1525. doi: 10.1152/ajpcell.1997.273.5.C1516. [DOI] [PubMed] [Google Scholar]
- 24.Jennings M. L., Schulz R. K. J Gen. Physiol. 1991;97:799–817. doi: 10.1085/jgp.97.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Starke L. C., Jennings M. L. Am. J. Physiol. 1993;264:C118–C124. doi: 10.1152/ajpcell.1993.264.1.C118. [DOI] [PubMed] [Google Scholar]
- 26.Darman R. B., Flemmer A., Forbush B. J. Biol. Chem. 2001;276:34359–34362. doi: 10.1074/jbc.C100368200. [DOI] [PubMed] [Google Scholar]
- 27.Lytle C. J. Biol. Chem. 1997;272:15069–15077. doi: 10.1074/jbc.272.24.15069. [DOI] [PubMed] [Google Scholar]
- 28.Lytle C., McManus T. Am. J. Physiol. 2002;283:C1422–C1431. doi: 10.1152/ajpcell.00130.2002. [DOI] [PubMed] [Google Scholar]
- 29.Wilson F. H., Kahle K. T., Sabath E., Lalioti M. D., Rapson A. K., Hoover R. S., Hebert S. C., Gamba G., Lifton R. P. Proc. Natl. Acad. Sci. USA. 2003;100:680–684. doi: 10.1073/pnas.242735399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kahle K. T., MacGregor G. G., Wilson F. H., Van Hoek A. N., Brown D., Ardito T., Kashgarian M., Giebisch G., Hebert S. C., Boulpaep E. L., Lifton R. P. Proc. Natl. Acad. Sci. USA. 2004;101:14877–14882. doi: 10.1073/pnas.0406172101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kahle K. T., Wilson F. H., Leng Q., Lalioti M. D., O’Connell A. D., Dong K., Rapson A. K., MacGregor G. G., Giebisch G., Hebert S. C., et al. Nat. Genet. 2003;35:372–376. doi: 10.1038/ng1271. [DOI] [PubMed] [Google Scholar]
- 32.Xu B. E., Stippec S., Lazrak A., Huang C. L., Cobb M. H. J. Biol. Chem. 2005;280:34218–34223. doi: 10.1074/jbc.M505735200. [DOI] [PubMed] [Google Scholar]
- 33.Xu B. E., Min X., Stippec S., Lee B. H., Goldsmith E. J., Cobb M. H. J. Biol. Chem. 2002;277:48456–48462. doi: 10.1074/jbc.M207917200. [DOI] [PubMed] [Google Scholar]
- 34.Darman R. B., Forbush B. J. Biol. Chem. 2002;277:37542–37550. doi: 10.1074/jbc.M206293200. [DOI] [PubMed] [Google Scholar]
- 35.Gimenez I., Forbush B. J. Biol. Chem. 2003;278:26946–26951. doi: 10.1074/jbc.M303435200. [DOI] [PubMed] [Google Scholar]
- 36.Bollen M. Trends Biochem. Sci. 2001;26:426–431. doi: 10.1016/s0968-0004(01)01836-9. [DOI] [PubMed] [Google Scholar]
- 37.Cohen P. T. J. Cell Sci. 2002;115:241–256. doi: 10.1242/jcs.115.2.241. [DOI] [PubMed] [Google Scholar]
- 38.Connor J. H., Kleeman T., Barik S., Honkanen R. E., Shenolikar S. J. Biol. Chem. 1999;274:22366–22372. doi: 10.1074/jbc.274.32.22366. [DOI] [PubMed] [Google Scholar]
- 39.Lytle C. Am. J. Physiol. 1998;274:C1002–C1010. doi: 10.1152/ajpcell.1998.274.4.C1002. [DOI] [PubMed] [Google Scholar]
- 40.Dumont J. N. J. Morphol. 1970;136:153–180. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.