Abstract
Group A Streptococcus (GAS) and other bacterial pathogens are known to interact with integrins as an initial step in a complex pathway of bacterial ingestion by host cells. Efficient GAS invasion depends on the interaction of bound fibronectin (Fn) with integrins and activation of integrin signaling. TGF-β1 regulates expression of integrins, Fn, and other extracellular matrix proteins, and positively controls the integrin signaling pathway. Therefore, we postulated that TGF-β1 levels could influence streptococcal invasion of mammalian cells. Pretreatment of HEp-2 cells with TGF-β1 increased their capacity to ingest GAS when the bacteria expressed fibronectin-binding proteins (M1 or PrtF1). Western blots revealed significant induction of α5 integrin and Fn expression by HEp-2 cells in response to TGF-β1. Increased ingestion of streptococci by these cells was blocked by a specific inhibitor of the TGF-β1 receptor I and antibodies directed against α5 integrin and Fn, indicating that increased invasion depends on TGF-β1 up-regulation of both the α5 integrin and Fn. The capacity of TGF-β1 to up-regulate integrin expression and intracellular invasion by GAS was reproduced in primary human tonsil fibroblasts, which could be a source of TGF-β1 in chronically infected tonsils. The relationship between TGF-β1 and GAS invasion was strengthened by the observation that TGF-β1 production was stimulated in GAS-infected primary human tonsil fibroblasts. These findings suggest a mechanism by which GAS induce a cascade of changes in mammalian tissue leading to elevated expression of the α5β1 receptor, enhanced invasion, and increased opportunity for survival and persistence in their human host.
Keywords: integrin, fibronectin, fibronectin binding protein, tonsils
Group A Streptococcus (GAS) infections of the throat are often recurrent. Children frequently retain streptococci in their tonsils after an exhaustive course of antibiotic therapy, accounting for the fact that as many as 30% of healthy school age children have positive throat cultures during the fall–winter months in temperate climates (1, 2). Excised tonsil tissue contains viable intra- and extracellular streptococci, suggesting that this organ is a reservoir for recurrent infection and that persistent streptococci are the instigators of chronic changes that lead to hypertrophy (3). A murine intranasal infection model confirmed that GAS has a tropism for nasal associated lymphoid tissue (4). GAS infections in children can result in a variety of serious, often chronic conditions, such as rheumatic fever, rheumatic heart disease, glomerulonephritis, psoriasis, and obsessive–compulsive behavior. However, a clear understanding of the relationship between GAS infection and these diseases is lacking (5).
GAS invasion of immortalized epithelial cells is highly efficient (10–60% of inoculum) in the presence of fibronectin (Fn) and this invasion is critically dependent on the interaction of bound Fn with cellular integrins (6–8). The bacteria can express several invasins, but the Fn-binding proteins (FnBPs), M1 and PrtF1∕Sfb1, have been studied in most detail (9–11). Engagement of α5β1 integrin with Fn bound to these proteins initiates phosphoinositide 3-kinase (10, 12) and integrin-linked kinase-dependent intracellular signals that induce cytoskeletal rearrangement and ingestion of streptococci (10). Most studies of invasion have used immortalized human cell lines; however, a connection between α5β1 integrin and Fn was also confirmed when GAS invasion was studied in primary cultures of human tonsillar cells (8).
TGF-β1 is a multifunctional cytokine that regulates a wide spectrum of biological activities including cell differentiation, angiogenesis, programmed cell death, fibrosis, and cellular immunity (13, 14). Therefore, this growth factor is associated with a variety of disease states. A well established function of TGF-β1 is regulation of extracellular matrix protein (ECM) expression, including Fn, collagen, and laminin (13, 15, 16), and a variety of integrin subunits (17). TGF-β1 is also a positive regulator of the integrin signal pathway by up-regulating integrin-linked kinase, phosphoinositide 3-kinase (PI3K), paxillin, and focal adhesion kinase (FAK) (18, 19). The capacity of GAS cell walls (SCW) to promote expression of TGF-β1 was recognized more than a decade ago (20). Lafyatis et al. (20) showed that explanted synovial tissues from patients with rheumatoid arthritis and rats with SCW-induced arthritis produced TGF-β1. Increased levels of TGF-β1 were induced in the blood of rats by oral administration of SCW and in the supernatant of cultured rat Kupffer cells exposed to SCW (21, 22). A connection between elevated levels of TGF-β1 and streptococcal human disease has also been noted. TGF-β1 was observed in all biopsies of renal tissue from patients with acute poststreptococcal glomerulonephritis (23) and in the culture supernatant of lymphocytes from patients with IgA nephropathy when the cells were stimulated with M protein (24). However, the role of TGF-β1 in GAS pathogenesis remains largely undefined. TGF-β1 was shown to increase adherence to and invasion of brain microvascular endothelial cells by Escherichia coli K1; however, the molecular basis for this change was not further explored (25). Recent studies on the involvement of integrin signaling associated with GAS invasion prompted us to postulate that exposure of epithelial cells to TGF-β1 would increase their capacity to ingest GAS (10). This hypothesis was tested with HEp-2 cells and primary human tonsil fibroblasts (PHTF). Pretreatment of these cells with TGF-β1 increased their capacity to ingest GAS, independent on exogenous Fn, and this increase was found to depend on TGF-β1 up-regulation of α5 integrin and cell-associated Fn. Consistent with previous studies of streptococcal cell walls (20–22), infection of PHTF with GAS produced a gradual increase in TGF-β1 levels in cell culture supernatants. These findings suggest a mechanism by which GAS induce a cascade of changes in mammalian cells leading to elevated expression of the α5β1 receptor, enhanced invasion, and increased opportunity for survival and persistence in their human host.
Results
TGF-β1 Enhances GAS Invasion of Epithelial Cells.
Our previous findings demonstrated that inactivation of integrin signaling significantly inhibited GAS invasion (10, 12). TGF-β1 is known to up-regulate integrin signaling and expression of ECM proteins; therefore, we postulated that TGF-β1 could enhance GAS invasion. HEp-2 cells were pretreated with TGF-β1 or medium alone for 24 h before invasion assays were performed with a wild type M1+ strain, 90-226, in the absence of an exogenous source of Fn. As previously shown, bacterial uptake was inefficient without exogenous Fn (7). After treatment with TGF-β1, significantly more bacteria were ingested (Fig. 1). TGF-β1 inhibits cell growth by arresting cells in late G1 phase of the cell cycle and also induces cell proliferation, depending on cell types and experimental conditions. The numbers of cells in wells treated with TGF-β1 were 15–20% lower than those treated with medium alone (data not shown). Therefore, enhanced GAS ingestion by TGF-β1 treated cells was not due to increased cell numbers.
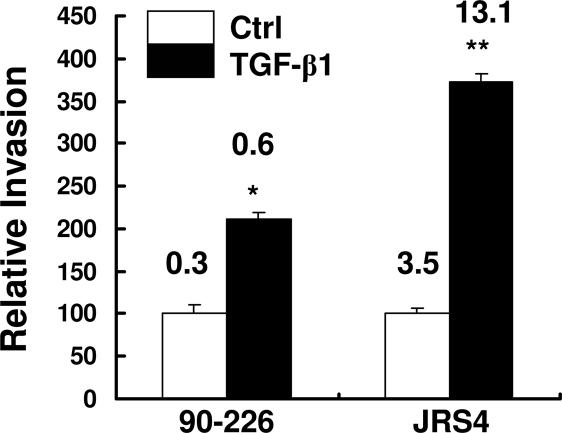
Fig. 1.
TGF-β1 enhances GAS invasion of epithelial cells. HEp-2 cells were pretreated with TGF-β1 and infected with strains 90-226 (M1+PrtF1−) and JRS4 (M6+ PrtF1+). Extracellular bacteria were killed by 2-h incubation with antibiotics. Intracellular bacteria were released by lysis buffer and quantified by colony-forming units (CFU). CFU from nontreated control cells (Ctrl) was considered as 100%. CFU from treated cells was normalized against controls and was reported as a percent of the control. Numbers shown above the columns are percentages of inoculated CFU internalized. Data are presented as means ± SD (n = 3) from a representative of at least three separate experiments. ∗, P < 0.008; ∗∗, P < 0.004 compared with Ctrl.
Strain JRS4 was previously shown to express PrtF1 protein, a FnBP that is genetically distinct from M1 protein. It has a higher affinity for Fn and promotes more efficient internalization in a Fn-, α5β1-, or αVβ3-dependent manner (11). We expected that TGF-β1 would also enhance internalization of this strain if the enhanced ingestion observed above is due to up-regulation of ECM proteins or integrins. Invasion assays with this strain showed that, under the same experimental conditions, ingestion of strain JRS4 by TGF-β1-treated HEp-2 cells was even more efficient than uptake of strain 90-226 (Fig. 1).
TGF-β1 Enhanced Invasion Depends on Fn-Binding Proteins of GAS.
The observation that TGF-β1 enhanced invasion, mediated by both M1 and PrtF1 proteins suggested dependence on FnBPs and Fn. The 90-226 mutant, which does not express M1 protein and is unable to invade epithelial cells efficiently in the presence of exogenous Fn was used to assess the requirement for M1 protein to support invasion. As shown in Fig. 2, although more M1+ 90-226 were ingested by TGF-β1-treated HEp-2 cells, the M− 90-226 mutant was still ingested at very low levels by these cells. We predicted that expression of a different FnBP, PrtF1, by this M− mutant would restore the ability to invade treated cells if TGF-β1 enhanced invasion requires the bacteria to bind Fn. The prtF1 gene was introduced into strain M1− 90-226 to generate a M1− PrtF1+ recombinant of strain 90-226. This recombinant regained the potential to invade epithelial cells efficiently in the presence of exogenous Fn (10). Invasion assays showed that expression of PrtF1 protein by strain M1− 90-226 resulted in greater uptake of bacteria by TGF-β1-treated epithelial cells without an exogenous source of Fn (Fig. 2). These results confirmed that the effect of TGF-β1 on epithelial cells for GAS uptake depends on FnBPs and suggested that TGF-β1 affected cellular molecules that are directly involved in interactions with these FnBPs.
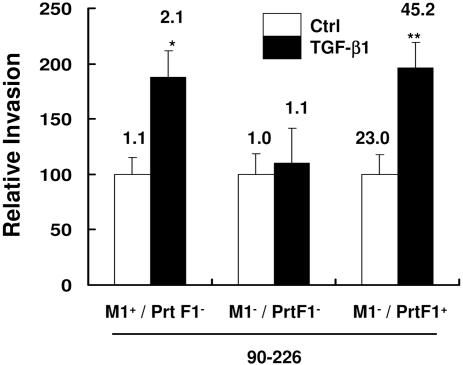
Fig. 2.
TGF-β1 enhanced invasion depends on expression of either M1 or PrtF1 Fn-binding proteins. HEp-2 cells were pretreated with TGF-β1. Invasion assays were performed with 90-226 strains: 90-226 (M1+PrtF1−), M1 deletion mutant (M1−PrtF1−), and PrtF1+ recombinant (M1−PrtF1+). Data and numbers above the columns are presented as described in Fig. 1 ∗, P < 0.03; ∗∗, P < 0.05 compared with Ctrl.
TGF-β1 Up-Regulates Expression of α5 Integrin and Fn by Epithelial Cells.
TGF-β1 positively regulates expression of ECM proteins (15, 16); therefore, we tested the hypothesis that TGF-β1 enhances GAS invasion by up-regulating expression of Fn, α5β1 integrins or both. First, to determine whether a soluble or membrane associated factor accounts for enhanced invasion, supernatants from TGF-β1-treated HEp-2 cells were replaced by serum∕Fn free medium before bacterial infection. Enhanced streptococcal uptake by TGF-β1-treated cells was still observed (data not shown), indicating that cell-associated molecules are responsible for the effect. Cell lysates were analyzed by Western blots to define changes in cell associated proteins. Significantly more α5 integrin and Fn were detected in TGF-β1-treated cells than in cells treated with medium alone by 4 h and continuously detected thereafter (Fig. 3A). Enhanced bacterial invasion was observed when HEp-2 cells were pretreated with TGF-β1 for 24 h (Figs. 1 and 2). No significant increases of β1 integrin were observed in extracts from TGF-β1 treated cells (Fig. 3A), suggesting that this integrin subunit was not limiting invasion of untreated cells.
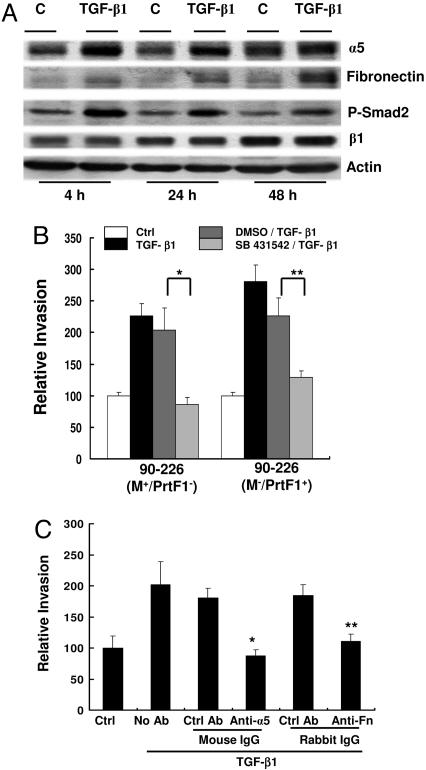
Fig. 3.
TGF-β1 up-regulates expression of α5 integrin and Fn by epithelial cells. (A) HEp-2 cells were pretreated with TGF-β1 and lysed in RIPA buffer (Supporting Text, which is published as supporting information on the PNAS web site) at indicated time. Equal amounts of protein were separated by SDS∕PAGE and blotted with anti-α5, anti-Fn, anti-phospho-smad2 (P-Smad 2), anti-β1, or anti-actin. (B) HEp-2 cells were not treated (Ctrl) or treated with TGF-β1 for 24 h after pretreatment with the inhibitor of TGF-β1 type I receptor SB 431542 (10 μM), (SB 431542∕TGF-β1) or DMS (10 μM) (inhibitor solvent control), (DMSO∕TGF-β1) or medium alone (TGF-β1) for 60 min, then infected with streptococci. Data are presented as described in Fig. 1. ∗, P < 0.02; ∗∗, P < 0.04. (C) HEp-2 cells were not treated (Ctrl) or pretreated with TGF-β1 and then incubated with anti-α5 integrin and anti-Fn and indicated corresponding control antibodies for 30 min before infection with bacteria. ∗, P < 0.02; ∗∗, P < 0.04 compared with Ctrl.
TGF-β1 signals through classical Smad-dependent and through Smad-independent pathways (14, 26, 27). Interaction with its receptors leads to activation and phosphorylation of Smad2, which then translocates from the cytoplasm to the nucleus and induces gene expression. Up-regulation of integrins by TGF-β depends on the Smad signaling pathway (28), which accounts for increased levels of phosphorylated Smad2 in extracts of HEp2 cells treated with TGF-β1 (Fig. 3A). To confirm that enhanced invasion is TGF-β1 specific, HEp-2 cells were pretreated with a specific inhibitor (SB 431542) of the TGF-β1 type I receptor before exposure to TGF-β1. This inhibitor has been shown to block induction of integrin and ECM proteins by TGF-β1 (29). As shown in Fig. 3B, the inhibitor prevented TGF-β1-enhanced entry of both M1+ PrtF1− and M1−PrtF1+ strains. Data shown in Fig. 3A suggested that enhanced invasion is due to increased expression of α5 integrin, cell-associated Fn, or both. This suggestion was confirmed by antibody blocking assays. After pretreatment with TGF-β1, HEp-2 cells were incubated with neutralizing anti-α5 integrin or anti-Fn antibody before infection with bacteria. As predicted, fewer bacteria were ingested by cells treated with TGF-β1 and anti-α5 integrin or with TGF-β1 and anti-Fn antibody than by cells treated with TGF-β1 and control antibodies (Fig. 3C). Collectively, these results demonstrated that TGF-β1 enhanced GAS invasion depends on up-regulation of α5 integrin and Fn.
TGF-β1 Enhances Expression of α5 Integrin and Invasion of PHTF.
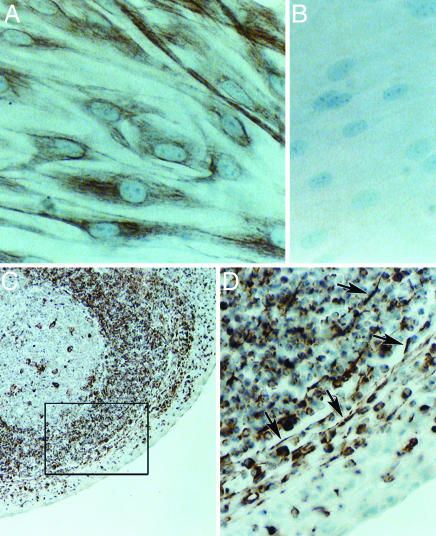
Recurrent tonsillitis due to both endogenous and exogenous sources of GAS is a common problem. We postulate that fibroblasts or lymphocytes associated with small numbers of streptococci within this tissue are chronic sources of TGF-β1, which up-regulates integrin receptors on the tonsil epithelium and increases susceptibility to infection. In earlier studies, we reported the potential of GAS to invade human primary tonsil epithelial cells (PHTF) in vitro (8). As we have continued to work with tonsil explants, we have identified considerable variability in the populations of primary cells that can be grown out (30). For these studies, we deliberately focused on PHTF as a potential source of TGF-β1 because PHTF can be propagated in larger numbers to support biochemical analyses. PHTF were immunostained with antibody directed against vimentin, as a marker for fibroblasts. This antibody displayed extensive staining of these cells (Fig. 4A); whereas there was no detectable staining with an antibody directed against cytokeratin, an epithelial cell marker (Fig. 4B). Immunostaining of tonsil sections revealed elongated vimentin-positive cells in close proximity to the stratified squamous epithelium covering the external surface of tonsils (Fig. 4C). A subset of tonsil lymphocytes also stained positively, but these round cells are easily distinguished from the spindle-like fibroblasts (arrow, Fig. 4D).
Fig. 4.
Immunocytochemical detection of vimentin-positive cells. Monolayers of PHTF and 5-μm sections of normal human palatine tonsil were processed for routine immunocytochemistry using a monoclonal antibody against vimentin and a peroxidase-conjugated secondary antibody as described (30). A positive signal is indicated by the brown stain; cell nuclei were counterstained with hematoxylin. (Original magnification: ×400 in A, B, and D and ×100 in C.) (A) PHTF monolayer stained with antivimentin antibody. (B) PHTF monolayer stained with pan-cytokeratin specific antibody. (C) Tonsil section stained with anti-vimentin antibody. (D) Enlargement of area enclosed within the box in C.
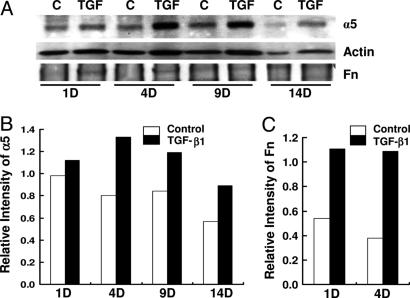
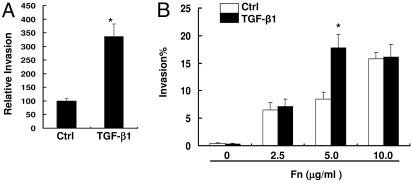
Experiments were then performed to test whether TGF-β1 up-regulates integrin receptors on PHTF and also increases their susceptibility to invasion. As anticipated from experiments with HEp-2 cells, Western blots showed that treatment of PHTF with TGF-β1 significantly increased expression of α5 protein, which peaked by 4 days (Fig. 5A and B). Compared to HEp-2 cells, steady state levels of Fn associated with PHTF were low, but a greater increase in Fn expression by these cells was observed on days 1 and 4 in response to TGF-β1 treatment (Fig. 5 A and C). However, the increased levels of Fn were not as dramatic as that of α5 integrin. Invasion assays showed that in the absence of exogenous Fn, TGF-β1-treated PHTF ingested more JRS4 (M6+ PrtF1+) streptococci than nontreated cells (Fig. 6A); whereas strain 90-226 (M1+ PrtF1−) invasion of these cells was very low under the same condition and no increase was observed after TGF-β1 treatment (Fig. 6B). The slight increase of cell-associated Fn upon exposure to TGF-β1 may be insufficient to promote invasion by this strain because M1 protein has a relatively low affinity for Fn compared with PrtF1. This consideration prompted us to test whether TGF-β1 up-regulation of α5 integrin would enhance invasion by strain 90-226 (M1+ PrtF1−) in the presence of exogenous Fn. Invasion assays were performed with various concentrations of Fn. Internalization of streptococci was not efficient in the absence of Fn even with TGF-β1 but substantially increased in the presence of Fn in a dose-dependent manner above 5.0 μg∕ml Fn and TGF-β1 significantly enhanced invasion when 5.0 μg∕ml Fn was provided. This finding suggests that insufficient Fn (<5.0 μg∕ml) limits M1-mediated invasion when integrins are in excess. At 10.0 μg∕ml of Fn, invasion was highly efficient; however, no increase was observed in TGF-β1-treated cells. It is possible that, at high Fn concentrations, more complex mechanisms are involved, including aggregation of the bacteria by Fn or use of additional receptors. In general, the above experiments replicated our findings with HEp-2 cells in primary human tonsil cells with one notable difference; maximal expression of α5 integrin after TGF-β1 induction required considerably longer incubation times with primary cells than with HEp-2 cells.
Fig. 5.
TGF-β1 enhances expression of α5 integrin and invasion of PHTF. (A) PHTF were treated with TGF-β1 and analyzed as described in Fig. 3A. The bands of α5, Fn, and actin were quantified with densitometry. (B and C) Each column shows the ratio of α5 to actin (B) or the ratio of Fn to actin (C). D, days of cells incubated with TGF-β1 before preparation of cell lysate.
Fig. 6.
TGF-β1 enhances invasion of PHTF. (A) PHTF were pretreated with TGF-β1. Invasion assays were performed with strain JRS4, (M6+ PrtF1+). Ctrl indicates invasion of cells that were not treated with TGF-β1. Data were normalized and presented as described in Fig. 1. ∗, P < 0.02. (B) PHTF were pretreated with TGF-β1. Cells were infected with strain 90-226 (M1+ PrtF1−) in media containing the indicated concentrations of Fn. Percentage invasion equals internalized CFU divided by total CFU inoculated into wells, multiplied by 100. ∗, P < 0.003 compared with Ctrl.
GAS-Induced Production of TGF-β1 by PHTH.
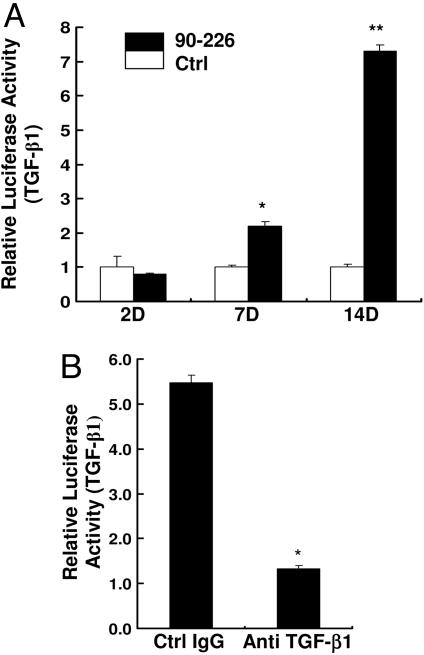
Both animal and human studies have shown that GAS or their products have the potential to induce TGF-β1 (20, 21, 24, 31). Tonsils are a common target of acute streptococcal infections, where bacteria are retained after antibiotic treatment to become a reservoir for recurrent pharyngitis (3). We tested a prediction of our model that GAS can induce TGF-β1 expression by PHTF (see Fig. 8). TGF-β1 is known to be secreted as a latent form and requires activation to exert its biological effects (13). A well characterized bioassay was used to quantitate TGF-β1, because antibodies assays do not distinguish active from inactive forms of TGF-β1 and mRNA measurements may misrepresent levels of secreted protein. In this assay, the activity of a TGF-β1 responsive gene is measured by luciferase activity from a reporter cell line (Mv1Lu) (32, 33). PHTFs were incubated with medium or live 90-226 streptococci under conditions that do not support multiplication of extracellular bacteria, but the bacteria remain viable for several days. Supernatants from treated cells were collected at different times, and aliquots were incubated with Mv1Lu reporter cells. Luciferase assays showed higher activity in samples from PHTF treated with bacteria than in cells treated with medium alone at days 7 and 14 of treatment (Fig. 7A). Exposure of reporter cells to streptococci did not induce TGF-β1. To confirm that the functional activity in the supernatant was caused by TGF-β1, supernatant samples were pretreated with a neutralizing anti-TGF-β1 antibody before incubation with Mv1Lu reporter cells. As shown in Fig. 7B, TGF-β1 antibody-treated samples induced ≈4-fold less luciferase activity than those treated with a control antibody (Fig. 7B), indicating that the luciferase-inducing activity in supernatants was indeed functional TGF-β1.
Fig. 8.
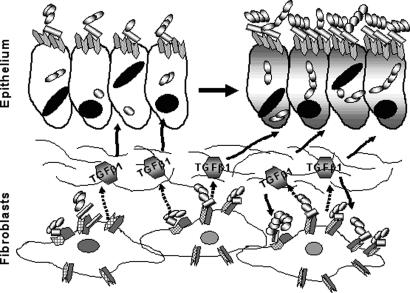
TGF-β1 activation of group A streptococcal ingestion in the tonsils. Interaction of streptococci (chains of banded spheres) with integrin-bound fibronectin or other cellular molecules leads to induction of TGF-β1 from fibroblasts. TGF-β1 then up-regulates expression of α5 integrin (gray tabs) and Fn (open tabs) on epithelial cells and also fibroblasts with formation of a higher density of α5β1 receptors and more efficient ingestion of streptococci. Hatched tab, β1 integrin; solid line, interstitium; dashed arrow, expression of TGF-β1; solid arrow, effect of TGF-β1.
Fig. 7.
GAS-induced production of TGF-β1 by PHTF. (A) Cell-free supernatants from PHTF, uninfected, and infected cells with viable strain 90-226 (M1+PrtF1−) at a MOI of 1:5 were collected at days 2, 7, and 14 (2D, 7D, and 14D) after infection and heat-activated (90°C, 5 min). Total TGF-β1 activity (active and latent forms) was measured by incubation of supernatants with Mv1Lu reporter cells overnight at 37°C. Cells were then washed and lysed, and luciferase activity in lysates was measured. ∗, P < 0.007; ∗∗, P < 0.004 compared with Ctrl. (B) Supernatants were preincubated with mouse anti-TGF-β1 antibody or nonimmune antibody of the same isotype before addition to Mv1Lu reporter cells. Data are presented as average luciferase activity of triplicate samples ± SD. ∗, P < 0.0001 compared with Ctrl IgG.
Discussion
FnBPs, such as M1 and PrtF1, are crucial for GAS adherence to and subsequent entry into host cells. The capacity to bind Fn renders these bacteria capable of employing integrins as receptors for efficient internalization. Extensive studies established that α5β1 integrin is the primary receptor for M1-mediated (8, 34) and PrtF1-mediated GAS invasion of epithelial cells (9, 11). Integrin signaling required for Streptococcus internalization has only recently been investigated (10, 12, 35). The activities of at least two kinases, integrin-linked kinase and phosphoinositide 3-kinase, in the integrin signaling pathway are essential for efficient uptake of GAS by epithelial cells (10, 12). Moreover, paxillin and focal adhesion kinase are required for intracellular infection by M1+ strain 90-226 (unpublished data) and the PrtF1+ strain JRS4 (35). The fact that integrin signaling is in part controlled by TGF-β1 (17) suggested the hypothesis that TGF-β1 could influence GAS invasion of host cells. To test this hypothesis, we examined the effects of TGF-β1 on GAS internalization by HEp2 cells and PHTF. Our study demonstrates that culture of HEp-2 cells with TGF-β1 leads to increased bacterial uptake in a FnBP-dependent manner. We showed that TGF-β1 enhanced GAS invasion of epithelial cells without an exogenous source of Fn and that increased invasion correlated with increased expression of α5 integrin and cell associated Fn. As anticipated, a specific inhibitor of TGF-β1 receptor I, or antibodies directed against α5 integrin or Fn, neutralized this activity of TGF-β1. The capacity of TGF-β1 to up-regulate integrin expression and intracellular invasion by GAS was also reproduced in PHTF. The relationship between TGF-β1 enhanced invasion and streptococcal disease was also strengthened by the observation that streptococcal infection induces PHTF to produce measurable quantities of TGF-β1.
Previous studies showed that either anti-α5 or anti-β1 monoclonal antibody alone efficiently block GAS invasion of epithelial cells, indicating that both proteins of the α5β1 heterodimer serve as a receptor for GAS entry (7, 8). However, TGF-β1-increased GAS entry was not accompanied by increased expression of the β1 integrin, a finding previously reported (36), indicating that availability of β1 does not limit ingestion of streptococci by untreated cells. The β1 subunit can pair with at least 10 different α subunits to form heterodimeric receptors that display different ligand specificities (27). Thus, it is possible that increased levels of the α5 subunit compete with other α subunits for pairing with a constant amount of β1 subunits, leading to formation of more α5β1 receptors, thereby facilitating GAS entry.
GAS invasion of cultured epithelial cells has been extensively studied with in vitro assays, in which invasion of epithelial cell lines by GAS is not efficient unless exogenous Fn (serum or purified Fn) is supplied. We demonstrate here that TGF-β1-increased GAS entry is mediated by M1 or PrtF1 proteins in the absence of Fn by up-regulating expression of α5 integrin and Fn associated with HEp-2 cells. TGF-β1 also enhanced PrtF1+-mediated invasion (strain JRS4) of PHTF without a supply of exogenous Fn; however, it did not enhance M1-mediated invasion (strain 90-226) of these PHTF unless an optimal amount of soluble Fn was supplied. The difference between these strains with regard to invasion of PHTF is most likely explained by the much higher affinity of PrtF1 than M1 protein for Fn. On the other hand, αVβ3 integrin is also up-regulated by TGF-β1 (37) and is known to be another receptor to support PrtF1-mediated invasion of epithelial cells (11). This integrin is not known to support M1-mediated invasion. Other gene products that distinguish these strains could also account for the failure of strain 90-226 to efficiently invade TGF-β1-treated PHTF. These differences may have clinical implications because antibiotic treatment failures were reported to be more frequent when the infecting strain expressed PrtF1 (38).
Although TGF-β1 was detected in GAS-treated PHTF (Fig. 7) but was not detected in supernatants from GAS-treated HEp2, increased expression of α5 integrin occurred in both cell types in response to TGF-β1. Our experiments are consistent with the model shown in Fig. 8 and provide an explanation for the clinical observation that some individuals experience recurrent episodes of GAS tonsillitis. We propose that streptococci can be retained in or near fibroblasts located beneath the tonsillar epithelium after antibiotic therapy. Small numbers of bacteria may be sufficient to induce these underlying fibroblasts to chronically secrete TGF-β1, which in turn up-regulates receptors on the epithelium, permitting increased adherence of streptococci, integrin clustering, and downstream signaling that leads to efficient ingestion of the bacteria, thus sensitizing the tonsil to subsequent superinfection. Our experiments do not indicate whether engagement of the integrin by streptococci is required to induce TGF-β1 or whether bacteria must first be internalized. Nor can we conclude from these experiments that increased ingestion of streptococci by fibroblasts contributes directly to recurrent infection; rather, the infected fibroblast may just be the primary source of TGF-β1. Local increases in TGF-β1 would have the potential to up-regulate α5 integrin and Fn expression on other types of surrounding cells, making these cells a better target for streptococci. Immunostaining of human tonsil sections revealed fibroblasts located in close proximity to both the epithelium covering the external surface of the tonsil (Fig. 4C and D) and the reticulated epithelium, lining tonsillar crypts (data not shown). Persistence of even small numbers of streptococci with chronic, localized expression of TGF-β1 could also lead to the fibrosis and hypertrophic changes that often require surgical removal of palatine tonsils from children (3). Moreover, because TGF-β1 has the potential to increase the array of integrins and ECM proteins displayed by cells, it may also significantly increase susceptibility of specific tissues to a variety of other bacterial pathogens, known to exploit ECM proteins or integrins to reach an intracellular compartment.
Materials and Methods
Bacterial Strains and Culture Conditions.
GAS strain 90-226 is a serotype of M1 strain (7). The M1− deletion variant of strain 90-226 (90-226Δemm1) that carries a complete nonpolar deletion of emm1, is sensitive to phagocytosis, and produces no M1 antigen or peptide fragment (10). The PrtF1-expressing variant (90-226 M1−PrtF1+) of strain 90-226 was constructed by introducing plasmid pPTF8, which contains the prtF1 gene (39) into strain 90-226Δemm1. Strain 90-226 M1−PrtF1+ was cultured in medium containing Kanamycin (500 μg∕ml). GAS strain JRS4 (emm6.1) (PrtF+ and M6+) was a gift from M. Caparon (Washington University School of Medicine, St. Louis) (25). GAS strains were maintained on sheep blood agar and grown in THY-Neo medium at 37°C for invasion assays.
Primary Culture of PHTF.
PHTF were cultured from palatine tonsil tissue obtained from otherwise normal healthy individuals undergoing routine tonsillectomy at the Department of Surgery, University of Minnesota. Segments of tonsil were rinsed in complete growth medium (RPMI medium 1640 containing 10% heat-inactivated FCS, penicillin, streptomycin, and fungizone), and then the tissue was mechanically disrupted. Single-cell, mixed populations of cells were cultured in standard tissue culture flasks (≈2–5 × 107 cells per T75 flask). Microcolonies of adherent cells were first visible within 4–8 days. Proliferating populations of large elongated cells spread throughout the flasks to establish confluent monolayers within 20–30 days. Adherent cell populations were readily released by trypsinization and were further expanded by routine passaging procedures for adherent cells. All experiments were performed with cells that were cultured for no more than 6–12 weeks from the time of tissue disruption. Expanded primary cell populations were determined to be fibroblasts by strong positive staining with antibodies directed against vimentin. No detectable staining with antibodies directed against cytokeratins was observed (30).
Invasion Assay.
Invasion assays were performed as described (7, 10, 34) with some modifications. Briefly, streptococci grown to stationary phase were washed with PBS and resuspended in DMEM. HEp-2 cells were cultured in 24-well plates to confluence, starved with DMEM without serum and antibiotics overnight, and infected with bacteria at a MOI of 1:5 in DMEM in the presence of fibronectin (10 μg∕ml). After 2-h incubation, unbound bacteria were removed by washing wells with PBS and extracellular bacteria were killed by 2-h incubation with DMEM containing gentamicin and penicillin. After washing off antibiotics with PBS, intracellular bacteria were released by lysing cells with 0.025% Triton X-100 in distilled H2O and quantified by colony-forming units (CFU). Bacterial invasion per well is CFU that survived antibiotic treatment and expressed as a percentage of the inoculum CFU. To investigate TGF-β1 effects on GAS invasion, cells were seeded and grown for 16 h in complete medium, and then treated with TGF-β1 (10 ng∕ml) in serum∕antibiotic-free medium for 24 h before invasion assays were performed. Controls included infected cells without TGF-β1 treatment. Treated cells were normalized against controls that were considered 100% invasion. For invasion blocking assays, cells were treated with neutralizing anti-α5 integrin, anti-Fn, or the appropriate control nonimmune antibodies for 30 min after TGF-β1 treatment and before infection with streptococci.
Mv1Lu Luciferase Assay for TGF-β1 Bioactivity.
Mv1Lu reporter cells (105 per ml) used for quantitation of TGF-β1 were grown overnight in a 96-well plate (Costar) at 37°C. Cell supernatants (100 μl) from GAS-infected or uninfected PHTF were heat activated at 90°C for 5 min and then incubated with Mv1Lu reporter cells at 37°C overnight. Reporter cells were washed and lysed, and luciferase activity was measured by using a Luciferase Assay System (Promega). To verify specificity, supernatants were preincubated with anti-TGF-β1 or control antibody (10 μg∕ml) at room temperature for 1 h. Data are reported as the mean luciferase activity of triplicate wells ± SD. For further details, see Supporting Text
Supplementary Material
Acknowledgments
We thank Tim Leonard for figure preparation and Ryan Yurecko for technical assistance. This work was supported by Public Health Service Grant AI34503.
Glossary
ABBREVIATIONS:
- GAS
group A Streptococcus
- Fn
fibronectin
- FnBP
Fn-binding protein
- ECM
extracellular matrix
- PHTF
primary human tonsil fibroblast.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Kaplan E. L., Johnson D. R. Pediatrics. 2001;108:1180–1186. doi: 10.1542/peds.108.5.1180. [DOI] [PubMed] [Google Scholar]
- 2.Gerber M. A., Tanz R. R., Kabat W., Bell G. L., Siddiqui B., Lerer T. J., Lepow M. L., Kaplan E. L., Shulman S. T. Pediatrics. 1999;104:911–917. doi: 10.1542/peds.104.4.911. [DOI] [PubMed] [Google Scholar]
- 3.Osterlund A., Popa R., Nikkila T., Scheynius A., Engstrand L. Laryngoscope. 1997;107:640–647. doi: 10.1097/00005537-199705000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Park H. S., Francis K. P., Yu J., Cleary P. P. J. Immunol. 2003;171:2532–2537. doi: 10.4049/jimmunol.171.5.2532. [DOI] [PubMed] [Google Scholar]
- 5.Bisno A. L., Brito M. O., Collins C. M. Lancet Infect. Dis. 2003;3:191–200. doi: 10.1016/s1473-3099(03)00576-0. [DOI] [PubMed] [Google Scholar]
- 6.LaPenta D., Rubens C., Chi E., Cleary P. P. Proc. Natl. Acad. Sci. USA. 1994;91:12115–12119. doi: 10.1073/pnas.91.25.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cue D., Dombek P. E., Lam H., Cleary P. P. Infect. Immun. 1998;66:4593–4601. doi: 10.1128/iai.66.10.4593-4601.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cue D., Southern S. O., Southern P. J., Prabhakar J., Lorelli W., Smallheer J. M., Mousa S. A., Cleary P. P. Proc. Natl. Acad. Sci. USA. 2000;97:2858–2863. doi: 10.1073/pnas.050587897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molinari G., Rohde M., Guzman C. A., Chhatwal G. S. Cell Microbiol. 2000;2:145–154. doi: 10.1046/j.1462-5822.2000.00040.x. [DOI] [PubMed] [Google Scholar]
- 10.Wang B., Yurecko R. S., Dedhar S., Cleary P. P. Cell Microbiol. 2006;8:257–266. doi: 10.1111/j.1462-5822.2005.00618.x. [DOI] [PubMed] [Google Scholar]
- 11.Ozeri V., Rosenshine I., Mosher D. F., Fässler R., Hanski E. Mol. Microbiol. 1998;30:625–637. doi: 10.1046/j.1365-2958.1998.01097.x. [DOI] [PubMed] [Google Scholar]
- 12.Purushothaman S. S., Wang B., Cleary P. P. Infect. Immun. 2003;71:5823–5830. doi: 10.1128/IAI.71.10.5823-5830.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leask A., Abraham D. J. FASEB J. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 14.Shi Y., Massague J. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura T., Miller D., Ruoslahti E., Border W. A. Kidney Int. 1992;41:1213–1221. doi: 10.1038/ki.1992.183. [DOI] [PubMed] [Google Scholar]
- 16.Ziyadeh F. N., Sharma K., Ericksen M., Wolf G. J. Clin. Invest. 1994;93:536–542. doi: 10.1172/JCI117004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai T., Lei Q. Y., Wang L. Y., Zha X. L. Biochem. Biophys. Res. Commun. 2000;274:519–525. doi: 10.1006/bbrc.2000.3177. [DOI] [PubMed] [Google Scholar]
- 18.Xu Z., Ma D. Z., Wang L. Y., Su J. M., Zha X. L. Biochem. Biophys. Res. Commun. 2003;312:388–396. doi: 10.1016/j.bbrc.2003.10.130. [DOI] [PubMed] [Google Scholar]
- 19.Han X., Stewart J. E., Jr, Bellis S. L., Benveniste E. N., Ding Q., Tachibana K., Grammer J. R., Gladson C. L. Oncogene. 2001;20:7976–7986. doi: 10.1038/sj.onc.1204996. [DOI] [PubMed] [Google Scholar]
- 20.Lafyatis R., Thompson N. L., Remmers E. F., Flanders K. C., Roche N. S., Kim S. J., Case J. P., Sporn M. B., Roberts A. B., Wilder R. L. J. Immunol. 1989;143:1142–1148. [PubMed] [Google Scholar]
- 21.Chen W., Jin W., Cook M., Weiner H. L., Wahl S. M. J. Immunol. 1998;161:6297–6304. [PubMed] [Google Scholar]
- 22.Manthey C. L., Kossmann T., Allen J. B., Corcoran M. L., Brandes M. E., Wahl S. M. Am. J. Pathol. 1992;140:1205–1214. [PMC free article] [PubMed] [Google Scholar]
- 23.Mezzano S., Burgos M. E., Olavarria F., Caorsi I. J. Am. Soc. Nephrol. 1997;8:234–241. doi: 10.1681/ASN.V82234. [DOI] [PubMed] [Google Scholar]
- 24.Nishikawa Y., Shibata R., Ozono Y., Ichinose H., Miyazaki M., Harada T., Kohno S. Nephrol. Dial. Transplant. 2000;15:772–777. doi: 10.1093/ndt/15.6.772. [DOI] [PubMed] [Google Scholar]
- 25.Zhang W. G., Khan A. N., Kim K. J., Stins M., Kim K. S. Cell Tissue Res. 2002;309:281–286. doi: 10.1007/s00441-002-0549-4. [DOI] [PubMed] [Google Scholar]
- 26.Derynck R., Zhang Y. E. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 27.van der Flier A., Sonnenberg A. Cell Tissue Res. 2001;305:285–298. doi: 10.1007/s004410100417. [DOI] [PubMed] [Google Scholar]
- 28.Lai C. F., Feng X., Nishimura R., Teitelbaum S. L., Avioli L. V., Ross F. P., Cheng S. L. J. Biol. Chem. 2000;275:36400–36406. doi: 10.1074/jbc.M002131200. [DOI] [PubMed] [Google Scholar]
- 29.Inman G. J., Nicolas F. J., Callahan J. F., Harling J. D., Gaster L. M., Reith A. D., Laping N. J., Hill C. S. Mol. Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- 30.Maher D. M., Zhang Z. Q., Schacker T. W., Southern P. J. J. Histochem. Cytochem. 2005;53:631–642. doi: 10.1369/jhc.4A6534.2005. [DOI] [PubMed] [Google Scholar]
- 31.Wahl S. M., Hunt D. A., Bansal G., McCartney-Francis N., Ellingsworth L., Allen J. B. J. Exp. Med. 1988;168:1403–1417. doi: 10.1084/jem.168.4.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mimuro J., Sawdey M., Hattori M., Luskutoff D. J. Nucleic Acids Res. 1989;17:8872. doi: 10.1093/nar/17.21.8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jurukovski V., Dabovic B., Todorovic V., Chen Y., Rifkin D. B. Methods Mol. Med. 2005;117:161–175. doi: 10.1385/1-59259-940-0:161. [DOI] [PubMed] [Google Scholar]
- 34.Dombek P. E., Cue D., Sedgewick J., Lam H., Finlay B. B., Cleary P. P. Mol. Microbiol. 1999;31:859–870. doi: 10.1046/j.1365-2958.1999.01223.x. [DOI] [PubMed] [Google Scholar]
- 35.Ozeri V., Rosenshine I., Ben-Ze’Ev A., Bokoch G. M., Jou T. S., Hanski E. Mol. Microbiol. 2001;41:561–573. doi: 10.1046/j.1365-2958.2001.02535.x. [DOI] [PubMed] [Google Scholar]
- 36.Nesti L. J., Caterson E. J., Wang M., Chang R., Chapovsky F., Hoek J. B., Tuan R. S. J. Orthop. Res. 2002;20:1042–1049. doi: 10.1016/S0736-0266(02)00020-7. [DOI] [PubMed] [Google Scholar]
- 37.Platten M., Wick W., Wild-Bode C., Aulwurm S., Dichgans J., Weller M. Biochem. Biophys. Res. Commun. 2000;268:607–611. doi: 10.1006/bbrc.2000.2176. [DOI] [PubMed] [Google Scholar]
- 38.Sela S., Neeman R., Keller N., Barzilai A. J. Med. Microbiol. 2000;49:499–502. doi: 10.1099/0022-1317-49-6-499. [DOI] [PubMed] [Google Scholar]
- 39.Hanski E., Caparon M. Proc. Natl. Acad. Sci. USA. 1992;89:6172–6176. doi: 10.1073/pnas.89.13.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.