Abstract
Ikaros transcription factors play critical functions in the control of lymphohematopoiesis and immune regulation. Family members contain multiple zinc fingers that mediate DNA binding and homooligomerization or heterooligomerization. Ikaros is abundantly expressed in pituitary mammosomatotrophs, where it deacetylates histone 3 sites on the proximal growth hormone (GH) promoter to silence gene expression. Ikaros-null mice display stunted growth with reduced circulating levels of the GH target factor insulin-like growth factor I (IGF-I). Ikaros-deficient mice have small anterior pituitary glands with a disproportionately reduced somatotroph population. Systemic administration of GH results in increased IGF-I levels and enhanced somatic growth. In contrast, reconstitution with WT lymphocytes was not sufficient to rescue the stunted growth phenotype of Ikaros-deficient mice. Ikaros was identified in mouse hypothalamic arcuate nuclei, where it colocalized with GH-releasing hormone (GHRH); in contrast, Ikaros-null mice lack GHRH immunoreactivity in the hypothalamus. Overexpression of Ikaros enhanced GHRH promoter activity and induced endogenous GHRH gene expression. These findings unmask a wider role for Ikaros in the neuroendocrine system, highlighting a critical contribution to the development of the hypothalamic–pituitary somatotrophic axis.
Keywords: growth hormone, growth hormone-releasing hormone, insulin-like growth factor I, somatotrophs
Ikaros (Ik) was initially described as a transcription factor that recognizes regulatory sequences of genes expressed in lymphoid cells (1, 2). The N terminus encodes zinc finger motifs that recognize cognate DNA-binding sites. The C terminus shared by all Ik isoforms contains the dimerization domain that is required for the formation of homodimers or heterodimers. Isoforms that lack a DNA-binding domain, such as Ik6, can act as dominant negative regulators of Ik function. The various isoforms act as activators or repressors in a functionally diverse chromatin-remodeling network (1).
We recently identified expression of Ik in the pituitary, where it is thought to play a role in the regulation of fibroblast growth factor receptor (FGFR) type 4 (FGFR4) (3). Altered expression of Ik isoforms, particularly Ik6, has been implicated in pituitary tumorigenesis through its actions on FGFR4 promoter acetylation (4, 5). We have demonstrated the expression of Ik by proopiomelanocortin-producing pituitary corticotroph cells as part of a transcriptional network governing corticotropin expression, corticotroph development, and adrenal corticosterone output (6). Ik is also expressed in pituitary mammosomatotrophs, where it deacetylates histones on the growth hormone (GH) promoter to silence this gene (7). In the current study we examined the impact of Ik on GH and its target growth factor, insulin-like growth factor I (IGF-I), by using Ik-deficient mice. Instead of a predicted GH hypersecretion, Ik-null mice exhibit a striking dwarf phenotype (8) with characteristics of pituitary GH deficiency. Here we also report the expression of Ik in the hypothalamus, where it regulates GH-releasing hormone (GHRH). These data unmask a role for this zinc finger protein in the hypothalamus, where it plays a critical role in hypothalamic–pituitary somatotroph development and function.
Results
Loss of Ik Leads to a GH-Deficient Phenotype.
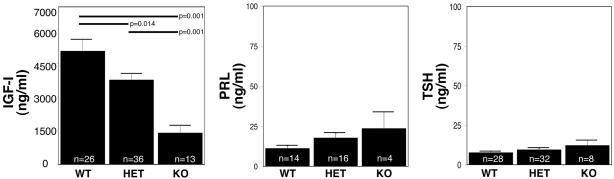
We have previously shown that WT Ik1 leads to epigenetic GH silencing through recruitment of a histone-deacetylating complex to the proximal 5′ GH promoter (7). Thus, Ik deficiency might have been expected to result in relief from a potential inhibitory mechanism, resulting in enhanced GH secretion and possible gigantism. Contrary to this prediction, however, loss of Ik in homozygote-null mice resulted in a GH-deficient phenotype characterized by postnatal growth retardation (8). All animals have a birth weight of 1.5 ± 0.4 g [WT, n = 26; heterozygote (HET) mice, n = 36; and knockout (KO) mice, n = 13], and the differing genotypes cannot be distinguished at that stage. However, postnatal growth patterns reveal progressive growth retardation (6). The genotypes start to be distinguishable at day 5, and mature Ik-deficient animals are dwarfs, with body weight reaching only ≈50% of WT littermate controls at 3 weeks of age (8). This postnatal form of dwarfism is associated with diminished GH secretion evidenced by a reduction of the GH-target IGF-I (1,404 ± 331 ng/ml compared with 5,030 ± 540 ng/ml; P = 0.014 compared with WT littermates) (Fig. 1). This GH deficiency is not a result of nonspecific generalized pituitary dysfunction, because these animals do not demonstrate reduced circulating levels of other pituitary hormones, e.g., prolactin (PRL) and thyroid-stimulating hormone (TSH) (Fig. 1). Moreover, although the animals have a corticotropin deficiency resulting in reduced circulating corticosterone and we have shown that administration of exogenous steroids prolongs life (6), this treatment alone did not restore normal growth or circulating IGF-I levels (data not shown).
Fig. 1.
Loss of Ik leads to a somatotrophin-deficiency phenotype. At 3 weeks of age, circulating hormone levels measured by specific ELISA show a marked reduction in the GH-target hormone, IGF-I (Left), in KO mice; there is no significant difference in levels of serum PRL (Center) or TSH (Right). Values represent means ± SEM. n, the number of animals in each group; ∗, P < 0.05.
Ik HET animals display near normal somatic growth with a modest (≈15%) reduction in IGF-I levels (3,749 ± 304 ng/ml) compared with WT littermate controls (Fig. 1). Healthy homozygote Ik-null mice had the same body proportions as those of HET and WT mice. At autopsy, internal organs were proportionally equivalent across the genotypes with the exception of the contracted pituitary and adrenal glands of Ik-null mice.
GH Administration Reverses the GH-Deficient Phenotype, Whereas Bone-Marrow Reconstitution Does Not.
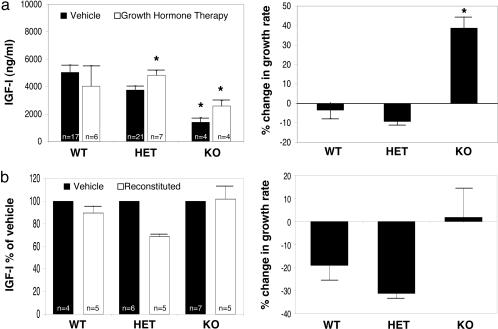
To more specifically determine whether impaired GH secretion was causally associated with the diminished postnatal somatic growth, systemic GH was administered. Because we have previously demonstrated that Ik-deficiency leads to impaired corticotroph development and adrenocortical insufficiency (6), animals received glucocorticoids as described in ref. 6 as well as GH. The combination of glucocorticoid and GH treatment resulted in enhanced circulating IGF-I levels (Fig. 2a Left). This increase in IGF-I was associated with increased postnatal somatic growth in Ik-null mice reaching ≈35% (Fig. 2a Right). On average, Ik-null mice grew at the slowest rate of 0.18 ± 0.04 g/d (n = 4), which was increased to 0.20 ± 0.03 g/d (n = 5) with dexamethasone treatment alone but significantly enhanced to 0.30 ± 0.03 g/d (n = 4; P < 0.05) with combined dexamethasone and GH treatment. By comparison, vehicle-treated HET animals grew at a rate of 0.38 ± 0.06 g/d (n = 21), which declined to 0.30 ± 0.19 g/d (n = 8) with dexamethasone alone and returned to 0.39 ± 0.07 g/d (n = 7) with combined dexamethasone and GH. WT animals treated with vehicle alone grew at a rate of 0.40 ± 0.05 g/d (n = 17); dexamethasone administration resulted in a growth rate of 0.35 ± 0.05 g/d (n = 3), whereas dexamethasone and GH resulted in a growth rate of 0.38 ± 0.06 g/d (n = 6). These data from WT and HET mice prove that the doses of hormone replacement were not supraphysiological.
Fig. 2.
Growth hormone treatment, but not lymphocyte reconstitution, restores normal growth of Ik-deficient mice. (a) Systemic GH administration increases circulating IGF-I levels (Left) and rate of weight gain (Right) in Ik-deficient mice. Ik-null mice experienced a 35% gain in weight with this treatment. (b) In contrast, lymphocyte reconstitution with WT marrow cells at birth failed to increase IGF-I levels (Left) and did not significantly alter growth (Right) compared with vehicle-treated KO mice at 5 weeks after injection. All values represent means ± SEM. ∗, P < 0.05.
Because Ik is known to be required for the development of lymphocytes whose cytokine production may alter pituitary hormone secretion (9, 10), we examined the impact of lymphocyte reconstitution on the stunted growth phenotype of Ik-deficient mice. Reconstitution with WT marrow cells at birth in homozygote Ik-deficient animals failed to stimulate IGF-I production or reverse the GH-deficient phenotype (Fig. 2b). Thus, these reconstitution experiments are consistent with a lymphocyte-independent mechanism of Ik regulation of the somatotrophic axis.
Loss of Ik Impairs Somatotroph Development.
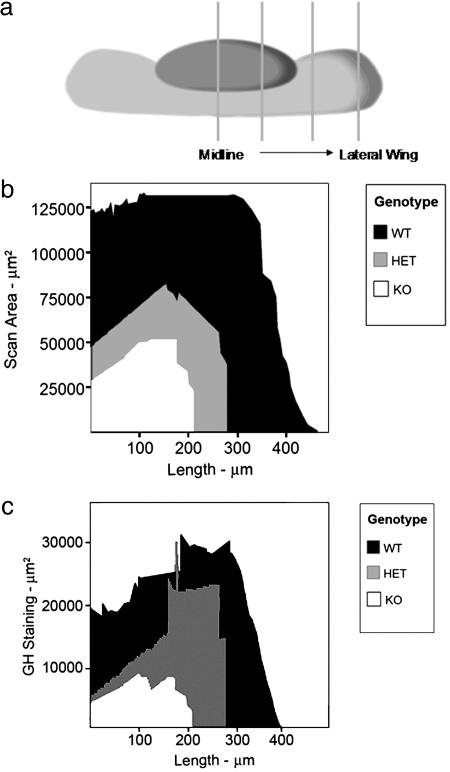
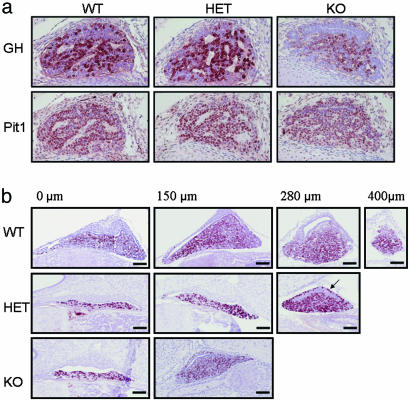
To examine the impact of Ik on pituitary somatotroph development, this cell population was quantified across the various Ik genotypes during embryonic and early postnatal life. Morphometric evaluation of pituitary size was estimated by the summation of areas of serial cross sections starting from the midline to the lateral wing as shown in Fig. 3a. Somatotrophs were present in all genotypes, but the glands were smaller in HET animals than in WT mice and even smaller in homozygote Ik-deficient mice (Fig. 3b). The intensity of GH reactivity was reduced in Ik-deficient mice (Fig. 4a). Pit1 staining, however, revealed no loss of expression of this GH activator in somatotrophs of homozygote KO mice (Fig. 4a). GH positivity persisted in the intermediate lobe, likely attributable to the lack of melanocorticotrope expansion (6) that normally replaces intermediate lobe somatotropes during pituitary development (Fig. 4b). GH staining areas reflecting the magnitude of contraction of this cell population are shown in Fig. 4b, and quantitative summation of the analysis is illustrated in Fig. 3c. There was a disproportionate reduction in GH staining in Ik-null mice. Other pituitary cell types were not affected; the number and area of immunoreactive thyrotrophs and gonadotrophs were not disproportionately different from those of WT littermates (data not shown).
Fig. 3.
Loss of Ik impairs normal pituitary development. (a) Morphometric evaluation of pituitary size was estimated by the summation of areas of serial cross sections starting from the midline to the lateral wing. The summation yields a curve equivalent to half the pituitary as shown in b. (b) Pituitaries of 2-week-old, Ik-null (KO) mice are smaller than those of their WT controls, and the HET mice are an intermediate size. (c) Quantification with image analysis software yields a result equivalent to half the pituitary mass. Note the disproportional change in the GH-positive area of HET animals compared with pituitary size (see b) that may explain the lack of a dwarf phenotype. In contrast, KO mice have markedly reduced pituitary GH-positive mass.
Fig. 4.
Loss of Ik impairs pituitary somatotroph development. (a) Immunostaining of fetal (embryonic day 16) mouse pituitary demonstrates diminished GH staining despite intact Pit1 expression in Ik KO mice. (b) Pituitaries of 2-week-old mice were stained for GH. The sections illustrated were obtained at the distances from the midline indicated as 0, 150, 280, and 400 μm. (Top) WT mice have the largest glands with the most numerous GH-positive cells. (Middle) HET mice have glands of intermediate size with persistent GH-immunoreactivity in the intermediate lobe (arrow). (Bottom) KO mice have small pituitary glands with fewer GH-immunoreactive cells and reduced intensity of staining.
Ik Localizes in Hypothalamic GHRH-Producing Neurons.
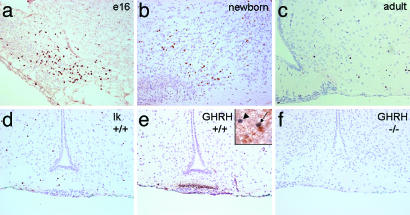
The apparent discrepancy between the in vitro-based predictions of the effect of Ik on the GH promoter and endogenous gene (7) and the current in vivo findings prompted us to search for an additional level of Ik-mediated control of the somatotrophic axis. We found convincing evidence for Ik expression in the mouse hypothalamus (Fig. 5). The staining was intense, and the number of positive neurons was largest in the fetal hypothalamus at embryonic days 16–18 (Fig. 5a), was reduced already at birth (Fig. 5b), and was found only in scattered neurons in the adult (Fig. 5c). Ik staining in WT mice was detected in the arcuate nucleus (Fig. 5d), where it colocalized with GHRH in cell bodies (Fig. 5e). Not all Ik-positive neurons contained GHRH, and reactivity was found outside the arcuate nucleus in other regions of the hypothalamus; however, the majority of GHRH-positive neurons contained nuclear Ik reactivity.
Fig. 5.
Ik is expressed in the hypothalamus, colocalizes with GHRH in hypothalamic neurons, and is lost in Ik-null mice. (a) Ik immunoreactivity is identified in the fetal hypothalamus where the nuclei of neurons in the arcuate nucleus exhibit strong positivity at embryonic day 16 (e16). (b and c) Staining is reduced in the newborn brain (b) and in the adult (c); only a few scattered cells exhibit Ik reactivity. (d) In 2 week-old mice, Ik staining is identified in individual cells of the arcuate nucleus. (e) At 2 weeks, GHRH expression is detected mainly in the median eminence of WT mice where it colocalizes with Ik in individual cells of the arcuate nucleus. (Inset) Note a neuron (arrow) that exhibits nuclear Ik (blue) and cytoplasmic GHRH (brown) and an adjacent neuron that exhibits nuclear Ik but no cytoplasmic GHRH (arrowhead); in the neuropil, there are axons that also stain for GHRH. (f) GHRH is undetectable in the hypothalamus of homozygote Ik-null mice.
In contrast to the intense GHRH reactivity in the arcuate nucleus and median eminence, in Ik-deficient mice, GHRH reactivity was undetectable in the arcuate nucleus and median eminence (Fig. 5f).
Ik Regulates GHRH Gene Expression.
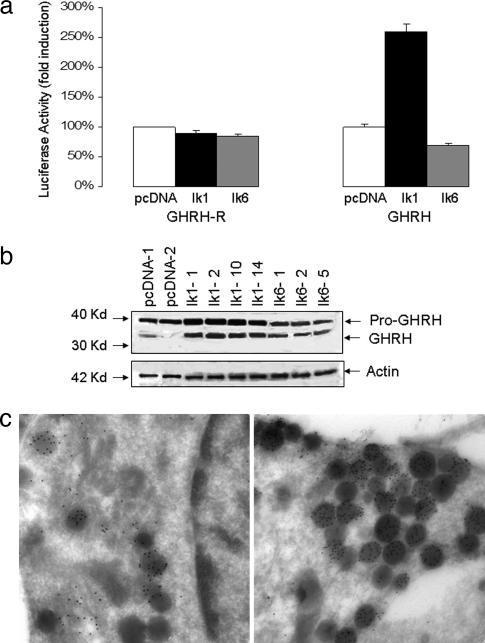
To examine the potential impact of Ik on GHRH synthesis, we tested the ability of Ik to modulate the GHRH promoter or that of the GHRH receptor (GHRH-R). Fig. 6a demonstrates the ability of Ik1 to induce the 5′ GHRH promoter. In contrast, Ik had no measurable impact on the GHRH-R promoter (Fig. 6a). The effect of Ik1 on GHRH was not shared with the dominant negative (dn) Ik6 isoform, which resulted in modest reduction of GHRH promoter activity.
Fig. 6.
Ik induces GHRH gene expression. (a) GH4 pituitary cells stably expressing Ik1, dn Ik6, or their empty vector control (pcDNA) were transiently transfected with the 5′ GHRH-R or GHRH promoter as indicated. Note the lack of appreciable effect of Ik on GHRH-R promoter activity compared with the selective stimulation of GHRH by Ik1. The effect on GHRH was not shared with dn Ik6, which resulted in modest attenuation of promoter activity. All transfections included corresponding empty control vectors along with 20 ng of pCMV-βgal to normalize for transfection efficiency. Data are presented as the mean luciferase activity adjusted for β-gal activity (±SD) and were compared with control wells of three separate experiments, each performed in triplicate (P < 0.005). (b) Mouse hypothalamic N3 cells endogenously expressing GHRH protein were stably transfected with Ik1, dn Ik6, or empty control vector (pcDNA3.1) and examined by Western blotting for GHRH or actin as indicated. Each lane represents an independent, transfected clone. Ik1 consistently increased GHRH expression in four independent clones as determined by densitometry (pro-GHRH/actin, 2.2 ± 0.1, and GHRH/actin, 3.0 ± 0.2, relative to pcDNA). (c) Detection of GHRH expression by electron microscopy in N3 cells confirms that, compared with empty vector-transfected cells (Left), cells transfected with Ik1 (Right) contain an increased number of secretory granules with increased immunogold localization of GHRH.
To determine the effect of Ik on the endogenous GHRH gene, we examined the impact of stable Ik transfection in GHRH-expressing N3 hypothalamic cells (11). Multiple independent clones demonstrated a positive effect of Ik1 on endogenous GHRH as determined by Western immunoblotting (Fig. 6b). The effect of Ik on GHRH was not shared by Ik6. Ik-transfected N3 cells also were examined by electron microscopy, which technique confirmed the presence of increased numbers of GHRH-containing secretory granules in cells overexpressing Ik1 (Fig. 6c).
Discussion
Gene-targeting experiments have firmly established that nuclear factors encoded by the Ik gene are essential for normal lymphoid development (12). Mice homozygous for a null mutation in the Ik gene display a severe lack of B and T cell differentiation (13, 14). Thus, Ik is primarily recognized to be an essential regulator of early lymphocyte differentiation. However, we have identified Ik expression in the pituitary (3, 4) and have shown that it plays an important role in pituitary melanocorticotroph development and function (6).
The current studies extend the role of Ik beyond the hematopoietic system and pituitary gland, unmasking a critical role for this factor in the hypothalamus, where it regulates GHRH expression. We have previously demonstrated Ik expression in pituitary somatotrophs (7), where it inhibits the GH gene through a histone deacetylation-dependent mechanism (7). Thus, loss of Ik would have been expected to relieve an inhibitory signal, resulting in unrestrained GH secretion with possible gigantism. Contrary to this prediction, loss of Ik is associated with a dwarf phenotype. That this dwarfism is on the basis of a neuroendocrine mechanism is supported here by multiple lines of evidence. First, we identified contraction of the pituitary somatotroph population in Ik-null mice, providing evidence for Ik involvement in anterior pituitary development. Second, the reduction in circulating IGF-I levels and somatic growth in Ik-deficient mice was reversed by systemic GH administration. Moreover, reconstitution with WT lymphocytes in Ik-null mice failed to restore IGF-I levels, excluding the possibility of impaired lymphopoiesis and cytokine production as a significant contributing mechanism to the dwarf phenotype. Third, we identified expression of Ik in hypothalamic GHRH-producing neurons, where it regulates GHRH expression, and Ik-deficient mice lack hypothalamic GHRH. Given the well established effects of GHRH on somatotroph mass and GH production (15), these findings provide a mechanism for Ik to mediate growth through GHRH-stimulated somatotroph development and function.
We have previously demonstrated that Ik targets the proximal 5′ promoter of the FGFR4 gene. FGFR4 likely plays a role, along with other FGFRs (16), in normal pituitary development, which is particularly relevant in the case of somatotrophs, in which Ik is abundantly expressed (7). Loss of Ik signaling is shown here to result in significant contraction of the pituitary somatotroph population. This cell population contraction was evident from detailed morphometric analyses and was confirmed by significant reduction in circulating IGF-I concentrations. However, in all likelihood FGFR4 is not the only target of Ik in pituitary somatotrophs, because an FGFR4-null mouse model has not shown evidence of pituitary dwarfism (17). This finding is not surprising, given the marked redundancy of FGFRs and the fact that the pituitary gland expresses FGFR1, FGFR2, and FGFR3 (18).
Other examples of pituitary dwarf phenotypes associated with somatotroph deficiency include mutations in the Pit1 transcription factor or its regulator, PROP1 (prophet of Pit1) (19–21). These mutations result in combined loss of all Pit1-target hormones, GH, PRL, and TSH. In contrast, inactivating mutations of the GHRH receptor (22) or its signaling CREB (cAMP response element-binding protein) (23) result in a more restricted somatotroph defect. These somatotroph-specific pituitary phenotypes bear closer resemblance to the morphologic changes observed in Ik-deficient mice.
Although GHRH is known to play a pivotal role in the regulation of the GH–IGF-I axis and somatotroph expansion (19), the molecular mechanisms governing GHRH gene expression are less well understood. The homeobox transcriptional factor Gsh-1 positively regulates the GHRH promoter in cooperation with CREB-binding protein (24). Homozygous inactivation of Gsh-1 leads to a postnatal dwarf phenotype with diminished GHRH expression (25), reminiscent of the Ik-null mouse. Interestingly, another lymphocyte-derived transcription factor, NFAT (nuclear factor of activated T lymphocytes), has also been shown to facilitate transcriptional activation of the GHRH promoter (26).
Relatively little is known about the transcriptional mechanisms involved in regulation of the GHRH-R promoter. It is known that thyroid hormone and glucocorticoids positively regulate this gene (27). Hence, loss of glucocorticoids in Ik-null mice may have contributed indirectly to impaired GHRH-R expression; however, administration of glucocorticoids to these mice does not restore normal growth (6) or IGF-I levels. There is also evidence confirming the importance of Pit-1-dependent activation of the GHRH-R (27, 28). However, we found no evidence of a role for Ik in regulating the GHRH-R promoter.
Although Ik appears to play a major role in development, its expression is reduced in mature lymphocytes, in the anterior pituitary gland and, as we now show, in the hypothalamus. However, Ik is also expressed in human neoplasia along with alternatively spliced dn isoforms (4). Overexpression of a dn non-DNA-binding isoform Ik6 has been identified in approximately one-third of the cases of B cell acute leukemia (29). Animals heterozygous for this dn Ik isoform develop T cell disorders mimicking T lymphoblastic leukemia or lymphoma (30). In nearly one-third of human pituitary tumors, the Ik gene undergoes alternative splicing to generate Ik6 (4). In pituitary cells, Ik6 alters the normal interaction of Ik with the 5′ FGFR4 promoter, creating an environment that favors utilization of a cryptic downstream intronic site (5). Alternative utilization of this intron 4 promoter leads to the transcription of a distinct truncated tumor-derived receptor isoform, ptd-FGFR4 (5). Taken together, these findings suggest that Ik may serve as a key regulator of growth-modulating genes. It would be interesting to know the extent to which Ik-related transcriptional networks share similarities in lymphocytes, the anterior pituitary gland, and selected hypothalamic nuclei.
The current identification of Ik as a factor implicated in the regulation of the somatotrophic axis builds on our previous description of Ik in the pituitary–adrenocortical axis (6). Data implicating Ik as a mediator of early and late lymphopoietic cell differentiation in concert with the effects of Ik on melanocorticotroph and now hypothalamic–pituitary–somatotroph function suggests a wider role for this zinc finger protein in orchestrating multiple neuroendocrine functions.
Materials and Methods
Cell Culture.
Rat mammsomatroph GH4 cells were propagated in DMEM (Life Technologies, Grand Island, NY) medium with high glucose supplemented with 10% FBS (Sigma)/2 mM glutamine/100 units/ml penicillin/100 μg/ml of streptomycin. The mouse N3 hypothalamic GHRH-expressing cell line was kindly provided by D. Belsham (University of Toronto) and propagated as described in ref. 11.
Plasmids.
The expression vectors encoding full-length Ik1 (CDM8-Ik1) and dn Ik6 (CDM8-Ik6) were generously provided by K. Georgopoulos (Harvard Medical School, Boston). The orientation and sequence of all constructs was confirmed by restriction digestion and nucleotide sequencing. GHRH and GHRH-R promoter activity were analyzed with luciferase reporter constructs containing the 543-bp fragment of the mouse GHRH promoter (kindly provided by Y. Iwasaki, Nagoya University, Nagoya, Japan) and the 1,456-bp of the human GHRH-R promoter (kindly provided by S. Petersenn, University of Essen, Germany).
Transfection and Luciferase Assays.
Plasmid reporters were prepared by column chromatography (Qiagen, Missisauga, ON, Canada) for sequencing and transfections. Cells were transfected by the Lipofectamine method (Invitrogen) according to the manufacturer’s protocol. Cells were plated into six-well cluster dishes (7 × 105 cells per well), transfected the following day with 3 μl of Lipofectamine per well and 1 μg of DNA per well. The total amount of transfected DNA was kept constant by adding empty vector. To normalize for transfection efficiency variation within and between experiments, simultaneous cotransfection was performed with a β-galactosidase expression plasmid CMV-βgal (20 ng per well, Promega) . Forty-eight hours after transfection, cells were lysed in buffer containing 25 mM glycylglycine, 15 mM MgSO4, 4 mM EGTA, 1% Triton X-100, and 1 mM dithio-dl-threitol. Luciferase activity was measured for 20 s in a luminometer. The results were normalized to β-galactosidase activity. Promoter activity of each construct was expressed as firefly luciferase/β-galactosidase activity. Each experiment was performed in triplicate and repeated on three separate occasions.
Hormonal Regulation.
Expression of endogenous GHRH was determined by Western blotting of cell extracts from transfected and control N3 cells and normalized to actin. The polyclonal antiserum to GHRH (Phoenix Pharmaceuticals, San Carlos, CA) was used at a dilution of 1:100. Relative concentrations of GHRH and actin were determined by densitometric analysis of autoradiographs.
Ik-Deficient Mice.
Ik-null mice were generated by Georgopoulos and colleagues (13). Deletion of the exon-7-encoded Ik dimerization domain results in an unstable protein (13). Mice were propagated on the original C57BL/6 background. Germline allelic transmission was verified by PCR analysis using tail DNA as described in ref. 13. Samples for blood and tissue analyses were obtained from embryos at various times during gestation and from adult mice as indicated. The care of animals was approved by the Institutional Animal Care Facilities at the Ontario Cancer Institute, where the animals were housed.
Morphologic and Immunohistochemical Studies.
Fetal and neonatal brains were fixed in neutral buffered formalin and embedded in paraffin or freshly frozen and sectioned coronally. Sections of hypothalamus were stained with hematoxylin and eosin for localization of individual hypothalamic nuclei, and serial sections were used for immunolocalization studies. Immunohistochemistry for GHRH used a polyclonal antiserum from Santa Cruz Laboratories at a concentration of 1:300. For Ik localization, a polyclonal antibody (1:20,000; kindly provided by S. Smale, University of California, Los Angeles) that recognizes the C-terminal fragment of Ik proteins was applied.
Fetal and neonatal pituitaries were localized by sagittal sections of the intact head using the cartilage of the sphenoid bone as a landmark as described in refs. 31 and 32. Consecutive serial sections from the midline to the lateral aspect of the pituitary were collected. Pituitary size was determined by the summation of the scan area of cross sections starting from the midline portion through to the distal lateral wing of the pituitary. Serial sections were stained with hematoxylin and eosin and immunohistochemistry. GH and Pit-1 were localized by using polyclonal antisera from the National Institute of Diabetes and Digestive and Kidney Diseases (Bethesda) and Babco (Berkeley, CA), respectively, as described in refs. 31 and 33.
Immunolocalization was detected with the streptavidin–biotin–peroxidase complex technique and 3,3′-diaminobenzidine. Colocalization studies were performed as described in ref. 31.
For ultrastructural analysis, harvested N3 cells were pelleted and fixed in 2.5% glutaraldehyde, postfixed in 1% osmium tetroxide, and embedded in a polymerized plastic. Ultrathin sections mounted on grids were stained with uranyl acetate and lead citrate. Samples from both cell lines were also fixed in 4% paraformaldehyde containing 0.1% glutaraldehyde and embedded in gelatin before being infused with 2.3 M sucrose. Specimens were then frozen in liquid nitrogen. Ultrathin cryosections were then prepared and mounted on formvar-coated nickel grids. Sections were then labeled with Ik monoclonal antibody 4E9, which recognizes the C terminus (kindly provided by K. Georgopoulos) was used at a dilution of 1:100 and detected with 5-nm-gold-labeled goat–anti-mouse IgG complexes. A polyclonal antibody against GHRH (Santa Cruz Biotechnology) at a concentration of 1:50 was detected with 10-nm-gold-labeled goat–anti-rabbit complexes. Both single and double labeling was done. Sections were then stabilized in a thin film of methyl cellulose containing uranyl acetate. Results were imaged with a transmission electron microscope and a charge-coupled device digital camera (Advanced Microscopy Techniques, Danvers, MA).
For negative controls, primary antiserum or antibody was replaced with nonimmune serum or normal mouse ascites, respectively, and, to verify the specificity of the reaction, primary antibody or antiserum was preabsorbed with purified antigen.
Morphometric Analysis.
Quantitative morphology of the pituitary was assessed by using a light microscope (Leica) and microcomputer imaging software (Imaging Research, St. Catherine’s, ON, Canada). Slides were analyzed to determine the total adenohypophyseal area and hormone content, estimated as the total area of immunopositive cells and as the percentage of total adenohypophyseal area.
Hormone Measurements and Administration.
To reduce sampling fluctuations due to the rhythmic release of pituitary GH, we measured its downstream target, IGF-I, by using a mouse enzyme-linked immunoassay (Diagnostic Systems Laboratories, Webster, TX). The other circulating anterior pituitary hormones measured with sensitive enzyme-linked immunoassay by commercial assays were PRL (Amersham Pharmacia) and TSH (Biotrak, Del Mar, CA). Hormone treatment consisted of GH (Eli Lilly) administered at a dose of 0.25 mg/kg every 2 d i.p. for 6 weeks. This dose was selected based on titration studies that avoided IGF-I increases in WT animals. Because Ik deficiency is associated with proopiomelanocortin and adrenocortical insufficiency (6), animals also received dexamethasone 0.05 mg/kg every 2 d throughout these experiments.
Hematopoietic Cell Repopulation.
Bone marrow was prepared from 6-week-old Ik+/+ littermates as described in ref. 6. Red blood cells were lysed, and the resulting cell suspension was filtered through a 70-μm cell strainer. Of these cells, 106 were injected intrahepatically in newborn Ik-deficient and sufficient animals. Bone marrow recipients were euthanized 5 weeks after reconstitution for examination of pituitary morphology and circulating hormone measurements. Lymphocyte reconstitution was assessed in the spleen and thymus of bone marrow recipients by multicolor cell surface immunofluorescence and FACS analysis as described in ref. 6.
Acknowledgments
We thank Dr. K. Georgopoulos for generously providing the Ik-null mice and Ik antibody, Dr. S. Smale for Ik antiserum, Dr. P. Poussier for advice on the lymphocyte reconstitution experiments and technical assistance, and Mr. K. So for advice on morphologic studies. This work was supported by The Canadian Institutes of Health Research and Toronto Medical Laboratories.
Glossary
Abbreviations:
- GH
growth hormone
- GHRH
GH-releasing hormone
- GHRH-R
GHRH receptor
- Ik
Ikaros
- dn
dominant negative
- HET
heterozygote
- KO
knockout
- IGF-I
insulin-like growth factor I
- FGFR
fibroblast growth factor receptor
- PRL
prolactin
- TSH
thyroid-stimulating hormone.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Molnar A., Wu P., Largespada D. A., Vortkamp A., Scherer S., Copeland N. G., Jenkins N. A., Bruns G., Georgopoulos K. J. Immunol. 1996;156:585–592. [PubMed] [Google Scholar]
- 2.Georgopoulos K., Winandy S., Avitahl N. Annu. Rev. Immunol. 1997;15:155–176. doi: 10.1146/annurev.immunol.15.1.155. [DOI] [PubMed] [Google Scholar]
- 3.Yu S., Asa S. L., Ezzat S. Mol. Endocrinol. 2002;16:1069–1078. doi: 10.1210/mend.16.5.0832. [DOI] [PubMed] [Google Scholar]
- 4.Ezzat S., Yu S., Asa S. L. Am. J. Pathol. 2003;163:1177–1184. doi: 10.1016/S0002-9440(10)63477-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu S., Asa S. L., Weigel R. J., Ezzat S. J. Biol. Chem. 2003;278:19597–19602. doi: 10.1074/jbc.M212432200. [DOI] [PubMed] [Google Scholar]
- 6.Ezzat S., Mader R., Yu S., Ning T., Poussier P., Asa S. L. J. Clin. Invest. 2005;115:1021–1029. doi: 10.1172/JCI22486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ezzat S., Yu S., Asa S. L. Mol. Endocrinol. 2005;19:1004–1011. doi: 10.1210/me.2004-0432. [DOI] [PubMed] [Google Scholar]
- 8.Georgopoulos K., Bigby M., Wang J. H., Molnar A., Wu P., Winandy S., Sharpe A. Cell. 1994;79:143–156. doi: 10.1016/0092-8674(94)90407-3. [DOI] [PubMed] [Google Scholar]
- 9.Chrousos G. P. N. Engl. J. Med. 1995;332:1351–1362. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 10.Turnbull A. V., Rivier C. L. Physiol. Rev. 1999;79:1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- 11.Belsham D. D., Cai F., Cui H., Smukler S. R., Salapatek A. M., Shkreta L. Endocrinology. 2004;145:393–400. doi: 10.1210/en.2003-0946. [DOI] [PubMed] [Google Scholar]
- 12.Cortes M., Wong E., Koipally J., Georgopoulos K. Curr. Opin. Immunol. 1999;11:167–171. doi: 10.1016/s0952-7915(99)80028-4. [DOI] [PubMed] [Google Scholar]
- 13.Wang J. H., Nichogiannopoulou A., Wu L., Sun L., Sharpe A. H., Bigby M., Georgopoulos K. Immunity. 1996;5:537–549. doi: 10.1016/s1074-7613(00)80269-1. [DOI] [PubMed] [Google Scholar]
- 14.Winandy S., Wu L., Wang J. H., Georgopoulos K. J. Exp. Med. 1999;190:1039–1048. doi: 10.1084/jem.190.8.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sano T., Asa S. L., Kovacs K. Endocr. Rev. 1988;9:357–373. doi: 10.1210/edrv-9-3-357. [DOI] [PubMed] [Google Scholar]
- 16.Scully K. M., Rosenfeld M. G. Science. 2002;295:2231–2235. doi: 10.1126/science.1062736. [DOI] [PubMed] [Google Scholar]
- 17.Weinstein M., Xu X., Ohyama K., Deng C. X. Development (Cambridge, U.K.) 1998;125:3615–3623. doi: 10.1242/dev.125.18.3615. [DOI] [PubMed] [Google Scholar]
- 18.Abbass S. A. A., Asa S. L., Ezzat S. J. Clin. Endocrinol. Metab. 1997;82:1160–1166. doi: 10.1210/jcem.82.4.3896. [DOI] [PubMed] [Google Scholar]
- 19.Asa S. L., Ezzat S. Endocr. Rev. 1998;19:798–827. doi: 10.1210/edrv.19.6.0350. [DOI] [PubMed] [Google Scholar]
- 20.Asa S. L., Ezzat S. Pituitary. 1999;1:159–168. doi: 10.1023/a:1009948813587. [DOI] [PubMed] [Google Scholar]
- 21.Asa S. L., Ezzat S. Front Horm. Res. 2004;32:1–19. doi: 10.1159/000079035. [DOI] [PubMed] [Google Scholar]
- 22.Godfrey P., Rahal J., Beamer W., Copeland N., Jenkins N., Mayo K. Nat. Genet. 1993;4:227–232. doi: 10.1038/ng0793-227. [DOI] [PubMed] [Google Scholar]
- 23.Struthers R. S., Vale W. W., Arias C., Sawchenko P. E., Montminy M. R. Nature. 1991;350:622–624. doi: 10.1038/350622a0. [DOI] [PubMed] [Google Scholar]
- 24.Mutsuga N., Iwasaki Y., Morishita M., Nomura A., Yamamori E., Yoshida M., Asai M., Ozaki N., Kambe F., Seo H., et al. Mol. Endocrinol. 2001;15:2149–2156. doi: 10.1210/mend.15.12.0747. [DOI] [PubMed] [Google Scholar]
- 25.Li H., Zeitler P. S., Valerius M. T., Small K., Potter S. S. EMBO J. 1996;15:714–724. [PMC free article] [PubMed] [Google Scholar]
- 26.Asai M., Iwasaki Y., Yoshida M., Mutsuga-Nakayama N., Arima H., Ito M., Takano K., Oiso Y. Mol. Endocrinol. 2004;18:3011–3019. doi: 10.1210/me.2003-0471. [DOI] [PubMed] [Google Scholar]
- 27.Petersenn S., Rasch A. C., Heyens M., Schulte H. M. Mol. Endocrinol. 1998;12:233–247. doi: 10.1210/mend.12.2.0057. [DOI] [PubMed] [Google Scholar]
- 28.Iguchi G., Okimura Y., Takahashi T., Mizuno I., Fumoto M., Takahashi Y., Kaji H., Abe H., Chihara K. J. Biol. Chem. 1999;274:12108–12114. doi: 10.1074/jbc.274.17.12108. [DOI] [PubMed] [Google Scholar]
- 29.Nakase K., Ishimaru F., Avitahl N., Dansako H., Matsuo K., Fujii K., Sezaki N., Nakayama H., Yano T., Fukuda S., et al. Cancer Res. 2000;60:4062–4065. [PubMed] [Google Scholar]
- 30.Winandy S., Wu P., Georgopoulos K. Cell. 1995;83:289–299. doi: 10.1016/0092-8674(95)90170-1. [DOI] [PubMed] [Google Scholar]
- 31.Bennani-Bäiti I. M., Asa S. L., Song D., Iratni R., Liebhaber S. A., Cooke N. E. Proc. Natl. Acad. Sci. USA. 1998;95:10655–10660. doi: 10.1073/pnas.95.18.10655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shewchuk B. M., Asa S. L., Cooke N. E., Liebhaber S. A. J. Biol. Chem. 1999;274:35725–35733. doi: 10.1074/jbc.274.50.35725. [DOI] [PubMed] [Google Scholar]
- 33.Asa S. L., Coschigano K. T., Bellush L., Kopchick J. J., Ezzat S. Am. J. Pathol. 2000;156:1009–1015. doi: 10.1016/S0002-9440(10)64968-1. [DOI] [PMC free article] [PubMed] [Google Scholar]