Abstract
Cyclic nucleotides can relax smooth muscle without a change in [Ca2+]i, a phenomenon termed Ca2+ desensitization, contributing to vasodilation, gastrointestinal motility, and airway resistance. The physiological importance of telokin, a 17-kDa smooth muscle-specific protein and target for cyclic nucleotide-induced Ca2+ desensitization, was determined in telokin null mice bred to a congenic background. Telokin null ileal smooth muscle homogenates compared to wild type exhibited an ≈30% decrease in myosin light-chain phosphatase (MLCP) activity, which was reflected in a significant leftward shift (up to 2-fold at pCa 6.3) of the Ca2+ force relationship accompanied by an increase in myosin light-chain phosphorylation. No difference in the Ca2+ force relationship occurred in telokin WT and knockout (KO) aortas, presumably reflecting the normally ≈5-fold lower telokin content in aorta vs. ileum smooth muscle. Ca2+ desensitization of contractile force by 8-Br-cGMP was attenuated by 50% in telokin KO intestinal smooth muscle. The rate of force relaxation reflecting MLCP activity, in the presence of 50 μM 8-Br-cGMP, was also significantly slowed in telokin KO vs. WT ileum and was rescued by recombinant telokin. Normal thick filaments in telokin KO smooth muscles indicate that telokin is not required for filament formation or stability. Results indicate that a primary role of telokin is to modulate force through increasing MLCP activity and that this effect is further potentiated through phosphorylation by cGMP in telokin-rich smooth tissues.
Smooth muscle (SM) myosin II ATPase activity and associated contraction is activated by actin only when Ser-19 of the myosin regulatory light chain (RLC20) is phosphorylated. The extent of RLC20 phosphorylation is a reflection of the balance between Ca2+ calmodulin-dependent myosin light-chain kinase (MLCK) and myosin light-chain phosphatase (MLCP) activities. Although the major mechanism for initiating contraction is the rise in [Ca2+]i, force can be further increased or decreased through signaling pathways that modulate MLCK and/or MLCP activities giving rise to processes termed Ca2+ sensitization or -desensitization (reviewed in ref. 1). Ca2+ sensitization due to inhibition of MLCP activity is mediated by an agonist G protein-coupled, Ca2+-independent process that activates RhoA/Rho-kinase, phosphorylates the myosin regulatory subunit of MLCP (MYPT1), and leads to increased force (1). On the other hand, cyclic nucleotide-activated kinases, in addition to decreasing cytosolic Ca2+, make a significant contribution to dephosphorylation of RLC20 and relaxation through Ca2+-desensitization processes (2–5) and can reverse G protein-coupled Ca2+ sensitization (2, 3). Interaction between the leucine zipper motifs of protein kinase G and MYPT1 leads to direct stimulation of MLCP (6). A role for this interaction in Ca2+ desensitization is supported by the observation that chicken gizzard MYPT1 lacking the leucine zipper motifs is resistant to 8-Br-cGMP-induced dephosphorylation of myosin II (7). cGMP-induced inhibition of Ca2+ sensitization mediated by RhoA/Rho-kinase signaling may occur through PKG-mediated phosphorylation of RhoA at Ser-188, preventing its binding to Rho-kinase and other effectors, and this phosphorylation may also play a significant role in Ca2+ desensitization and relaxation of SM (8) under some conditions (reviewed in ref. 1).
Telokin is another target for cGMP-induced Ca2+ desensitization and is abundantly phosphorylated at Ser-13 in SM in response to 8-Br-cGMP or forskolin (3, 9, 10). Depletion of endogenous telokin from permeabilized SM correlates with a loss of 8-Br-cGMP-induced relaxation, which is rescued by the addition of recombinant telokin and further enhanced by PKG (2, 3, 10). Site-specific mutation of Ser-13 to an acidic, Asp residue to mimic phosphorylation, but not to a nonphosphorylatable Ala, enhances the Ca2+-desensitizing effect on force. Telokin is a small highly acidic protein (11) whose sequence is identical to the COOH terminus of SM MLCK and is independently expressed in SMs through a promoter located within an intron of the MYLK gene (12, 13). Telokin has neither the kinase domain nor the CaM-binding domain of MLCK and its physiological function in vivo has been unclear. In vitro, telokin binds to the S1/S2 region of unphosphorylated SM myosin (14), stabilizes unphosphorylated myosin filaments (14–16), modulates the oligomerization of MLCK (17), and inhibits the phosphorylation of myosin by MLCK (14, 17) through competitive inhibition with MLCK for binding to myosin (16).
Because many of the studies on telokin have used permeabilized SMs to manipulate telokin content and this permeabilization may give rise to loss of additional diffusible proteins, we have generated telokin null mice to more directly explore the in vivo importance of telokin in Ca2+ desensitization and relaxation of SM. Because telokin is a SM-specific protein, the effects of the telokin deletion is specific to SM. Our results demonstrate the importance of telokin in regulating the Ca2+ sensitivity through activation of MLCP and in regulating the cGMP-induced relaxation of phasic SM of the gastrointestinal, airway, and reproductive tracts.
Results
Generation of Telokin Null Mice on a Congenic Background.
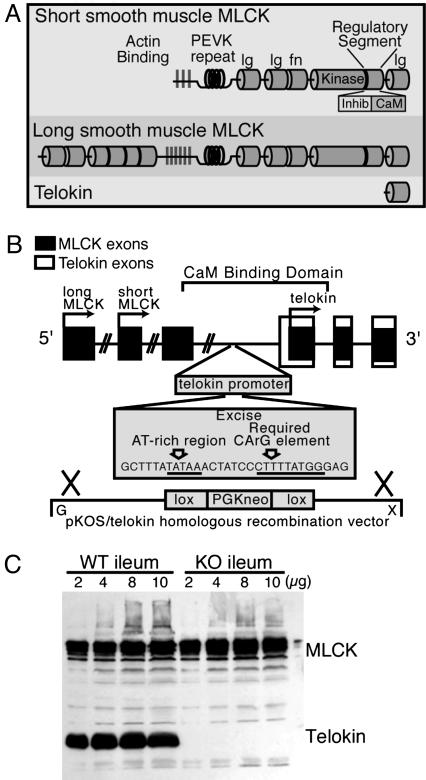
The 130-kDa and 220-kDa MLCK isoforms and telokin are products of a single gene, the MYLK gene. Telokin, a protein without enzymatic activity, is an independently regulated gene embedded within the larger MYLK gene (Fig. 1). Deletion of the a 30-nt fragment containing the AT-rich region and CArG box, which have been shown to be critical for promoter activity (19), resulted in a complete loss (Fig. 1C) of telokin protein in mutant animals, with an occasional animal showing a trace of telokin ≈200-fold lower in the knockout (KO) compared to the WT SM tissues. The content of MLCK protein expression did not differ in the telokin KO and WT ileum SM. Using the speed congenic approach, it was found that by the 5th backcross generation, no residual 129-derived microsatellite alleles could be detected except in the regions surrounding MLCK on chromosome 16. Thus, functional differences observed in the telokin KO and WT animals are due to the deletion of telokin and not strain-mediated variability in the rest of the genome. The telokin null animals appeared outwardly normal, were fertile, and showed a normal Mendelian inheritance ratio with an average normal litter size of nine pups.
Fig. 1.
Generation of the telokin null mouse. (A) The structure and functional domains of 130-kDa and 220-kDa MLCK (modified from ref. 18). (B) Schematic representation of the MYLK gene and the telokin deletion strategy. The genomic DNA with the MLCK exons is represented by shaded boxes (not all exons are indicated), telokin exons are represented by open boxes, and transcriptional start sites are indicated by arrows. The start of coding of long and short MLCK and telokin begin, respectively, in exons 1, 15, and 29. The telokin promoter and the start of telokin exon 1 are in a region that is intronic for MLCK. Plasmid pKOS/UV1/29.1 was used as the KO vector with an overall length of ≈9 kb. The floxed PGKneo cassette was inserted in such a way that the sequence shown comprising an AT-rich region and critical CArG element of the telokin promoter were deleted during homologous recombination in ES cells. (C) Western blot illustrating normal MLCK content in telokin KO and WT ileum and the absence of telokin.
Increased Ca2+ Sensitivity of Force in Telokin Null Ileal Smooth Muscle.
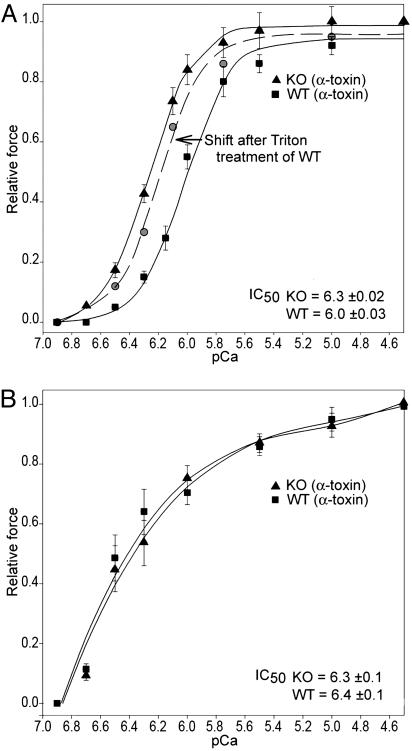
The pCa force curve was significantly left shifted by 0.3 pCa units, over the physiological range of [Ca2+] (Fig. 2A), in α-toxin permeabilized ileal SM from the telokin KO animals compared with WT (EC50: KO = 6.3 ± 0.05, WT = 6.0 ± 0.06, P < 0.02 by using the two-tailed t test, df = 9 pairs, t = 2.9). No difference in the Ca2+-force relationship was observed for aorta SM from telokin WT and KO animals (Fig. 2B). Note that the Ca2+ sensitivity of the aorta samples is similar to the ileum KO tissue. Permeabilization with 0.5% Triton X-100, a protocol that leads to depletion of endogenous telokin (10), was used on ileal SM from telokin WT mice. The pCa tension curve was significantly leftward shifted (Fig. 2A) after loss of endogenous telokin. In another series of experiments, the addition of 15 μM recombinant S13D telokin, which mimics phosphotelokin, to telokin KO ileal SM permeabilized with Triton X-100, markedly right shifted the pCa force curve, mimicking the WT curve but, however, did not reach the WT position (Fig. 8, which is published as supporting information on the PNAS web site). Thus recombinant telokin rescued the pCa force phenotype in telokin-depleted SM, and depletion of telokin in WT SM recapitulated the KO pCa force phenotype.
Fig. 2.
Ca2+-force relationship in phasic ileum SM (A) and the tonic aorta (B) from telokin KO and WT mice. The pCa-force relationship is significantly shifted to the left in the α-toxin permeabilized telokin null ileal SM. Heavy permeabilization of WT ileal SM, with 0.5% Triton X-100 resulting in depletion of endogenous telokin, also induced a leftward shift of the pCa tension curve of WT animals similar to that observed in the telokin KO muscles. No significant difference was found in the pCa-force relationship between telokin KO and WT abdominal aorta preparations (IC50 = pCa 6.3 ± 0.1 and 6.4 ± 0.1, respectively) (B).
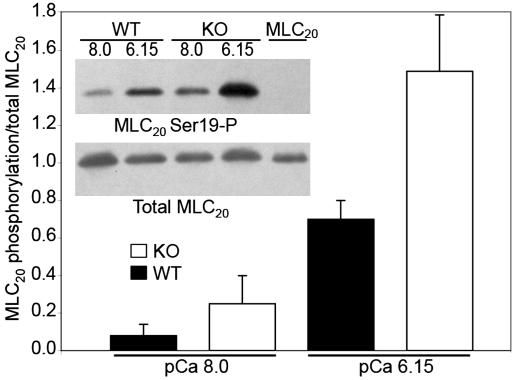
Maximal force per cross sectional area at pCa 4.5 was not significantly different in KO and WT SM. The leftward shift of the pCa force curve for the telokin KO ileum compared to the WT was correlated with a significant increase in RLC20 phosphorylation measured at the midpoint (pCa 6.15) of the KO pCa force curve (paired t test, P < 0.05; Fig. 3), suggesting either an increase in MLCK or decrease in MLCP activity. Note that RLC20 phosphorylation is slightly higher at resting force, pCa 8.0 in the telokin KO than in the WT ileum, consistent with a known basal kinase activity (20), although this phosphorylation did not reach statistical significance.
Fig. 3.
The leftward shift of the pCa-force curve in telokin KO vs. WT ileal SM is accompanied by an increase in RLC20 phosphorylation.
Telokin-Deficient Ileum Smooth Muscles Exhibit Reduced 8-Br-cGMP Induced Relaxation of Ca2+ Force.
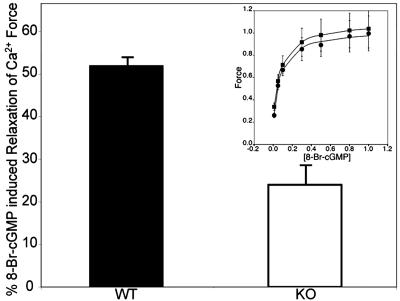
Telokin is markedly phosphorylated in SM stimulated by cyclic nucleotides (2, 3). The ability of 8-Br-cGMP to induce relaxation of Ca2+-induced force was significantly (P < 0.0005) decreased in α-toxin permeabilized telokin KO ileal SM precontracted with pCa 6.3 compared to WT muscles (Fig. 4): Magnitudes were 24 ± 3%, n = 5, and 52 ± 1%, n = 7, for KO and WT ileum muscles, respectively. The sensitivity of SM to 8-Br-cGMP did not differ for telokin KO and WT ileal SM, after telokin was depleted from WT animals by triton permeabilization, suggesting that cGMP targets other than telokin are not altered in the telokin KO SM (Fig. 4 Inset).
Fig. 4.
8-Br-cGMP-induced relaxation of Ca2+-induced force was significantly attenuated by >50% in ileal SM from telokin KO compared to WT mice. (Inset) Dose–response curves showing no change in sensitivity for 8-Br-cGMP induced relaxation upon depletion of telokin, indicating that activation of c-GMP targets are not altered in the telokin KO muscles.
Telokin Activates in Vitro and in Vivo MLCP Activity.
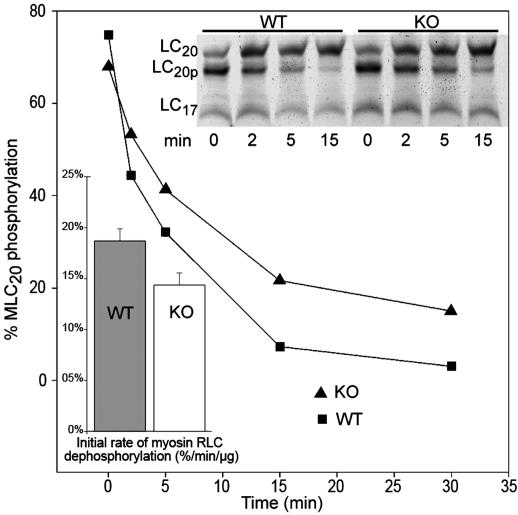
Whole homogenates of ileal SM from telokin WT and KO mice were assayed for MLCP activity to directly test the hypothesis that telokin activates MLCP activity. The content of MYPT1 quantitated from Western blots and normalized to GAPDH or myosin RCL20 content did not significantly differ in WT and telokin KO samples. For example, the [MYPT1]:[RLC20] was 0.23 and 0.26 OD in WT and KO and the [MYPT1]:[GAPDH] was 0.10 and 0.10 OD in WT and KO, respectively. Therefore, MLCP protein expression was not altered by deletion of telokin and, thus, the differences in measured MLCP activity is not due to changes in content of MLCP or MLCK (Fig. 1). MLCP activity was measured in WT and KO whole ileum homogenates (Fig. 5). Initial rates of MLC dephosphorylation were 30% lower in telokin KO mice as compared to WT (15 ± 1% as compared to 19 ± 2% of change in RLC20 phosphorylation· min−1·μg−1 of protein (n = 3, P < 0.04), respectively. The observed difference in MLCP activity is not due to inhibition of MLCK in these experiments because the assay was carried out in the presence of EDTA, thus eliminating MgATP and Ca2+ required for MLCK activity.
Fig. 5.
WT compared with KO homogenates of ileal SM exhibit a greater MLCP activity. The time course of RLC20 dephosphorylation induced by mixing phosphorylated SM myosin with ileum SMs homogenates. UREA-PAGE gel demonstrating different rates of dephosphorylation of phosphorylated SM myosin samples by homogenates from WT and telokin KO ileum SMs. Summary of the initial rates of dephosphorylation of the prephosphorylated SM myosin by telokin WT and KO homogenates. The rates are expressed as a percentage of RLC20 phosphorylation level decrease· min−1·μg−1 of homogenate protein (P < 0.03).
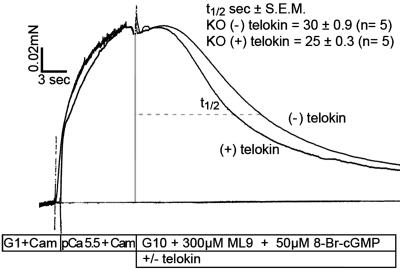
In vivo MLCP activity was also assayed by using a previously established (21) MLCP activity assay in which the rate of relaxation of Ca2+-activated force measured after removal of Ca2+, by the addition 10 mM EGTA plus 300 μM ML9 to inhibit MLCK activity, is taken as an index of in vivo MLCP activity. The assay is carried out at 10°C to slow the rate of RLC20 dephosphorylation based on the high Q10 value of 5.1 for MLCP (22) and, thus, make it the rate-limiting step. Using this MLCP activity assay, which includes the addition of 10 μM 8-Br-cGMP to activate telokin, the slowed rate of 8-Br-cGMP-induced force relaxation in the telokin null ileum SM could be rescued by the addition of S13D recombinant telokin added at the time that inhibition of MLCK activity was initiated (Fig. 6). The t1/2 of relaxation in telokin null ileal SM, 30 ± 0.9 sec (n = 5) was significantly shortened, 25 ± 0.3 sec (n = 5), in the presence of recombinant telokin P < 0.005. Thus the rate of relaxation reflecting in situ MLCP activity in telokin−/− SM is accelerated by the addition of the phospho-mimic recombinant telokin S13D.
Fig. 6.
8-Br-cGMP-induced relaxation of Ca2+-induced force was significantly accelerated in telokin KO ileal SM upon addition of 10 μM S13D recombinant telokin. At the plateau of the Ca2+-induced force trace, MLCK activity was inhibited by the addition of 300 μM ML9 and by removal of calcium (10 mM EGTA solution, G10) at 10°C. The rate of relaxation is taken as index of in vivo MLCP activity, which is accelerated by the addition of the phospho-mimic recombinant telokin S13D (P < 0.005).
Ca2+-Sensitized Force in Telokin Null and WT Smooth Muscle.
Ca2+-sensitized force induced by GTP-γS in α-toxin permeabilized telokin KO and WT mice ileal SM contracted with Ca2+ (50% of maximal force) did not differ. IC50 = 1.2 ± 0.1 μM and 1.3 ± 0.1 μM, n = 5 for WT and KO ileal SM, respectively. Thus endogenous telokin does not appear to influence the RhoA/Rho-kinase-induced phosphorylation pathway, which leads to inhibition of MYPT1.
Morphology of Smooth Muscle from Telokin Null and WT Smooth Muscle.
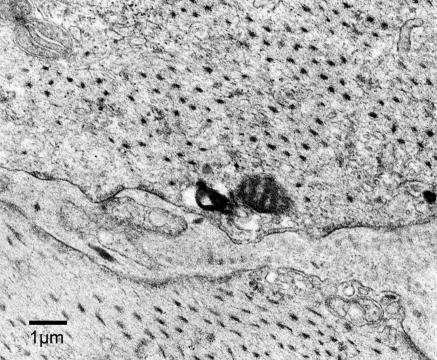
Electron microscopic evaluation of transverse sections of the longitudinal muscle of the ileum showed a normal array of myosin filaments throughout the cells in both the telokin KO (Fig. 7) and WT muscles. Longitudinal sections from the carotid artery also exhibited myosin filaments with no obvious difference due to deletion of telokin. Thus, unlike the in vitro findings demonstrating that telokin stabilizes myosin filaments (14–16), telokin is not necessary for filament formation and stability in the absence of telokin.
Fig. 7.
Electron micrograph illustrating a normal number and distribution of myosin filaments in a transverse section of longitudinal ileum SM from a telokin null mouse.
Discussion
The major previously undescribed findings of this study were as follows: (i) that telokin null C57B16 congenic mice survive normally and show no obvious developmental or maturational defects and (ii) exhibit normal expression of MLCK but marked decrease in myosin phosphatase activity, a significant leftward shift of the Ca2+-force relationship, and an increase in myosin RLC20 phosphorylation as compared to WT mice. In addition, Ca2+ desensitization of contractile force by 8-Br-cGMP is reduced by 50% in telokin null intestinal SM and myosin assembled into normal stable thick filaments in the absence of telokin. The Ca2+ sensitivity of WT aortic SM was unaffected by the deletion of telokin, likely reflecting its low endogenous telokin content. Taken together, these results, and those of our previous studies (2, 3, 23), support the hypothesis that telokin directly or indirectly increases myosin phosphatase activity and, thereby, contributes to the Ca2+ desensitization of force and cyclic nucleotide-induced relaxation in telokin-rich SMs, such as in the gut, bladder, portal vein, resistance vessels, and trachealis.
The differences observed with the deletion of telokin are not due to variable genetic backgrounds in the telokin KO mice because the mice were bred to a congenic C57BL/6 background. Furthermore, the region of the telokin promoter selected for excision and homologous recombination successfully allowed normal splicing of the MYLK gene, as illustrated by the normal expression of MLCK protein and the absence of telokin in ileum SM, which normally expresses high concentrations of telokin (27 μM) (23). As expected, if telokin increases MLCP activity, the level of RLC20 phosphorylation should negatively correlate with telokin muscle content. Indeed, resting RLC20 phosphorylation in the phasic guinea pig portal vein and tonic femoral artery is 10% and 20%, respectively, (20) correlating with the respective high and low telokin expression levels.
The 0.3 pCa units leftward shift in the force calcium curve in telokin KO ileal SM results in a 2-fold greater force for a given [Ca2+] over the physiologically relevant steep portion of the force curve. This shift is unlikely due to adaptive changes in expression of contractile proteins that occurred secondary to KO of telokin because the effect could be nearly completely rescued by the addition of 15 μM S13D telokin. We were unable to discern whether nonphospho- or phospho-telokin was responsible for the difference in the force calcium curves as both phospho- and nonphospho-telokin induce relaxation of telokin-depleted muscles (3), with the S13D phospho-telokin mimic being more potent (10). Indeed, a portion of telokin is in the phosphorylated state in resting ileum SM, and nonphospho-telokin added to permeabilized muscles is labeled with 32P ATP upon stimulation with cGMP (2).
Support for the hypothesis (3) that telokin’s action is through increased activity of MLCP rather than inhibition of MLCK comes from the following observations. First, we previously showed that telokin had no effect on MLCK-induced thiophosphorylation of RLCs (2) because thiophosphorylated RLCs are not a substrate for MLCP. Second, the presence of telokin did not affect Ca2+-induced force development when MYPT1 was inhibited by microcystin (10). Third, 8-Br-cGMP and recombinant telokin increase the rate and decrease the amplitude of force development upon photolysis of caged ATP (23). Taken together, results support the hypothesis (3) that in vivo, telokin-induced Ca2+ desensitization and associated decreases in RLC20 phosphorylation are mediated by acceleration of MLCP activity rather than inhibition of MLCK. NO-induced Ca2+ desensitization in resistance arteries (24) is also thought to reflect activation of MLCP. In the present study, we now show directly that MLCP activity is significantly greater in whole homogenates from WT ileal SM than from telokin KO homogenates, whereas neither MYPT1 nor MLCK content differed. These findings are further strengthened by the in vivo observations that the rates of relaxation, under conditions where MLCP rates are rate limiting, are significantly slowed in the telokin KO ileal SM but can be rescued by the addition of phospho-mimic S13D recombinant telokin. We have not compared the expected MLCP activity in WT vs. KO muscles based on the pCa-RLC20 phosphorylation measurements with measured MLCP activities because the magnitude of the difference in MLCP activity in the KO vs. WT muscle homogenates is likely an underestimate, reflecting the assay conditions of low salt and possible disruption of the physical relationship of the complex of molecules, including telokin partners associated with the RLC20 and possibly the need for native myosin filaments. A similar rationale may account for the inability of recombinant telokin to enhance the in vitro phosphatase activity of telokin KO SM homogenates (data not shown) while effectively rescuing in vivo relaxation rates of telokin KO muscles.
A well recognized but poorly understood characteristic of tonic arterial SMs is the greater sensitivity of the contractile proteins to Ca2+ compared to phasic SMs, such as the portal vein, resistance vessels, bladder, and gut. Although the content of both MLCK and MLCP are somewhat less in tonic SMs the ratio of MLCK:MLCP content are thought to be similar (25). Although the lower concentrations could account for the differences in the kinetics of force development in these slow and fast SMs, it is unlikely to account for the differences in the Ca2+-tension relationship. Phasic muscles have a high content of telokin (27 ± 4.6 μM in ileum), comparable to the myosin head concentration, whereas the tonic femoral has 4.5-fold less telokin (6 ± 1.7 μM) (23). Based on our present findings that the pCa tension curves did not differ in telokin KO and WT aortas and that the telokin KO ileum SM had a similar Ca2+ sensitivity as the WT aorta, we propose that telokin and its ability to increase MLCP activity accounts for the significant difference in the Ca2+-force relationship in phasic vs. tonic SM. The result also points to a general mechanism of regulation of smooth muscles in which the contractile properties of different smooth muscles are likely to be dictated by the expression levels of distinct subsets of signaling proteins.
cGMP-induced relaxation of Ca2+-induced contractions was reduced by 50% in the telokin-deficient ileum under conditions where [Ca2+] was held constant, consistent with the increased potency of telokin phosphorylated by PKG (2, 3, 10). The remaining 50% relaxant activity of cGMP is not surprising in view of the multiple targets for active PKG (reviewed in ref. 1). cGMP induces Ca2+ desensitization of the femoral artery (26) even though telokin content is low and, in this case, is likely signaling to other cGMP targets. The ability of 8-Br-cGMP to dramatically reverse GTP-γS or agonist-induced activation of RhoA/Rho-kinase mediated Ca2+ sensitization of force and (8) demonstrates the antagonistic roles of RhoA/Rho-kinase and cyclic nucleotide-signaling pathways. However, PKG-induced Ca2+ desensitization can also be shown in preparations where the RhoA/ROK mechanism is inactive (24), and PKG can also increase MLCP activity through interaction between the leucine zipper motifs of PKG1α and MYPT1 (6). Because GTP-γS-induced Ca2+ sensitization did not change with deletion of telokin, it is unlikely that telokin is inhibiting the RhoA/Rho-kinase pathway. Interestingly, in vitro phosphorylation of MYPT1 at Ser-695 by PKG/PKA excludes phosphorylation of the adjacent Thr-696 site that is critical for inhibition of MLCP so, in this case, cGMP blocks the inhibition of MLCP (27). Which of the above mechanisms account for the remaining cGMP-mediated relaxation in telokin-deficient ileal SM at constant [Ca2+] is not defined, but it is clear that the absence of telokin strongly abrogates, by 50%, cGMP-induced relaxation.
The molecular basis for telokin’s ability to lead to an increase in MLCP activity remains to be determined. Telokin can localize to the S2 region of the myosin head (28) and could either enhance or inhibit the association of other molecules that target this region such as MYPT1, MYPT kinase (29), integrin-linked kinase (30), myotonic dystrophy-related kinase (31), PKG (6), ROCK, MLCK, CPI-17 (32), or M-RIP (33), resulting in modulation of MLCP activity, phosphorylation.
The physiological importance of PKG is demonstrated by the widespread importance of nitrovasodilators and natriuretic peptides in elevating cGMP, resulting in vasodilation and relaxation of SMs and physiological or pathophysiological states. In intact muscles, Ca2+ desensitization by PKG generally operates in parallel with mechanisms that reduce [Ca2+] and bring about relaxation. Although this relationship is not always the case, because NO-induced dilations in resistant arteries are not associated with decreases in cytoplasmic [Ca2+] (24). We conclude that telokin, a target of PKG, through activation of MLCP, plays a significant role in the complex panorama of chemical signal transduction that regulates SM contractility.
Methods
Telokin Promoter Deletion.
MLCK isoforms (130 kDa and 220 kDa) and telokin are products of a single gene, the MYLK gene (Fig. 1). Telokin, a nonenzymatic protein, is an independently regulated gene embedded within the larger MYLK gene. Telokin expression is mediated by an independent promoter located in an intron upstream of exon 29 of the MYLK gene (Fig. 1B) and between the two exons encoding for the calmodulin-binding domain of MLCK. A 370-bp fragment of telokin is sufficient for maximal cell-specific promoter activity in vitro and to mimic endogenous telokin expression in vivo (34). Within this promoter, an AT-rich region and adjacent CArG box are critical for promoter activity (19). To knockdown expression of telokin in vivo and minimize possible effects on the normal splicing of the MYLK gene, a 30-nt fragment that includes the AT-rich region and CArG box were deleted from the endogenous telokin gene. The knockout vector for homologous recombination used Lexicon’s proprietary Lambda KOS system (35) (Lexicon Genetics, The Woodlands, TX) (Fig. 1B). The targeting vector contains 6 kb of 5′ homology and 3 kb of 3′ homology. The construct was designed to not significantly alter exon-intron-spacing (after cre-recombinase-induced excision of the neomycin phosphotransferase selectable marker gene). Chimeras were generated from targeted ES cell lines. The floxed PGKneo gene was removed by breeding of the mutant allele through male mice expressing Cre recombinase in their germ line. Heterozygotes were crossed to generate PGKneo-excised homozygotes deficient in telokin.
Generation of Telokin KO Congenic Lines.
A speed congenic approach (36) was used to eliminate the effect of variable genetic backgrounds in the telokin KO mice. The ES cells used for gene targeting were derived from 129/SvEvBrd. Chimeric founder males were crossed to C57BL/6 albino mice, followed by inbreeding to confirm the effects of promoter deletion. A male homozygous for the induced mutation was then genotyped to identify an informative microsatellite map for outcross to the C57BL/6 (B6) by using previously mapped loci (37). Offspring from five successive generations of backcrossing to the recipient B6 strain were selected for B6 homozygosity at 81 informative microsatellite loci representing a genomewide survey, excluding chromosome Y (which was introduced by a male B6 donor at generation 4). PCR amplification from genomic DNA was performed by using fluorochrome-labeled primers purchased from Research Genetics, (Huntsville, AL) by using a modification of methods in ref. 38. Products from 10–16 loci per animal were pooled after amplification and analyzed individually for allele sizes by using an ABI377 automated sequencer equipped with genescan 2.1 and geneotyper 2.0 software (PerkinElmer Applied Biosystems, Foster City, CA). Once no residual 129-derived microsatellite alleles could be detected, mice were inbred for propagation of the congenic strain. Subsequent heterozygote animals were continued to be backcrossed to the B6 strain as a source for the physiological and biochemical analysis.
Genotyping by Southern Blotting and PCR Analyses.
Southern blotting and PCR analyses were performed to genotype the targeted ES clones and weaned pups. For Southern blot analysis, genomic DNA was digested with BamHI and probed with 5′ and 3′ external probes as detailed in Supporting Methods, which is published as supporting information on the PNAS web site.
Tissue Preparation, Force, and RLC20 Phosphorylation Measurements.
Mice were anesthetized and killed with halothane according to protocols approved by the Animal Care and Use Committee at the University of Virginia.
Force measurements on intact, α-toxin, β-escin, or Triton X-100 permeabilized muscles were carried out as described previously and detailed in Supporting Methods.
The pCa-force relationships were obtained in permeabilized SMs by cumulative increasing the [Ca2+] from pCa 6.9 to pCa 4.5, with each force normalized to the maximal force developed by the same strip at pCa 4.5. Normalized pCa-force values obtained for each examined strip were fitted to the Hill equation [F = Fmax × (pCa)n/(pCa)n + EC50, where F and Fmax are steady force at given pCa and maximal force, respectively] to determine the pCa EC50 values.
Ca2+ sensitization and 8-Br-cGMP-induced relaxations were determined at intermediate [Ca2+] (pCa 6.3-pCa 6.5) where force was ≈50% of maximal force and characterized with the magnitudes of the response normalized to steady force developed before addition of agents. EC50 values were determined by fitting the dependences (magnitude vs. [agent]) to a hyperbola. The t1/2 of the time course of force decay induced by 8-Br-cGMP was used as an index of relaxation kinetics, rather than the rate constant, because the time course of force decay could not be fitted to a single exponential. In vivo index of phosphatase activity (t1/2 of force relaxation at 10°C) was measured after inhibition of MLCK as described in ref. 21.
Recombinant mutant telokin was prepared, purified, and buffer exchanged as described in refs. 3 and 10 and used at a concentration of 10–15 μM to rescue the force responses.
The ratio of phospho-RLC20:total RLC20 was determined by immunoblotting with a rabbit polyclonal phospho-specific antibody (Cell Signaling Technology, Beverly, MA) and an antibody to detect total RLC20 kindly provided by Kris Kamm (University of Texas Southwestern Medical Center, Dallas).
MLCP Assay.
Turkey gizzard myosin was prepared and phosphorylated at room temperature in buffer as detailed in Supporting Methods. MLCP activity of the homogenates from WT and KO tissue was determined by measuring the rate of dephosphorylation of the prephosphorylated myosin. Tissues were weighed and homogenized at 0°C in extraction buffer (50 mM Tris·HCl, pH 7.0/0.1 mM EGTA), plus 1% protease inhibitor mixture at a ratio of 1 mg of tissue to 20 μl of buffer. Total protein content was adjusted to 0.25 mg/ml. At 20°C, the homogenate (1 μl of total protein) was mixed with phosphorylated myosin (120 μl), and the reaction was terminated at 2,5,15, and 30 min by pipetting 30-μl aliquots of the mixture into the ice-cold acetone (1:5 ratio vol/vol). UREA-Glycerol PAGE was used to separate phospho-, and unphospho-RLC20 (39) and determine the percent of phospho-RLC20 to total RLC20 at different time points. The data were fitted to a decaying exponential with rate constant k and the initial rate determined as the product of k and the initial RLC20 phosphorylation level. MLCP activity was expressed as an initial rate of MLCP activity decrease per microgram of homogenate protein. Samples from KO and WT were run on the same gel and compared by paired t test. MYPT content in WT and KO homogenate were determined by Western blotting and densitometry over the linear range of protein loads with MYPT monoclonal antibody (B.D. Transduction Laboratories, Franklin Lakes, NJ). MYPT content was normalized to RLC20 and GAPDH content.
Electronmicroscopy.
Preparatory procedures were detailed in Supporting Methods.
Supplementary Material
Acknowledgments
We thank Ms. Jama Coartney for preparation of the illustrations and Mr. Howard Phipps for his preparation of the manuscript. This work was supported by National Institutes of Health Program Project Grants 5 P01 HL48807-11A1 and 5 P01 HL19242-29.
Glossary
Abbreviations:
- KO
knockout
- MLCK
myosin light-chain kinase
- MLCP
myosin light-chain phosphatase
- SM
smooth muscle.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Somlyo A. P., Somlyo A. V. Physiological Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 2.Wu X., Somlyo A. V., Somlyo A. P. Biochem. Biophys. Res. Commun. 1996;220:658–663. doi: 10.1006/bbrc.1996.0460. [DOI] [PubMed] [Google Scholar]
- 3.Wu X., Haystead T. A., Nakamoto R. K., Somlyo A. V., Somlyo A. P. J. Biol. Chem. 1998;273:11362–11369. doi: 10.1074/jbc.273.18.11362. [DOI] [PubMed] [Google Scholar]
- 4.Nishimura J., van Breemen C. Biochem. Biophys. Res. Commun. 1989;163:929–935. doi: 10.1016/0006-291x(89)92311-5. [DOI] [PubMed] [Google Scholar]
- 5.Pfitzer G., Hofmann F., DiSalvo J., Ruegg J. C. Pflugers Arch. Eur. J. Physiol. 1984;401:277–280. doi: 10.1007/BF00582596. [DOI] [PubMed] [Google Scholar]
- 6.Surks H. K., Mochizuki N., Kasai Y., Georgescu S. P., Tang K. M., Ito M., Lincoln T. M., Mendelsohn M. E. Science. 1999;286:1583–1587. doi: 10.1126/science.286.5444.1583. [DOI] [PubMed] [Google Scholar]
- 7.Khatri J. J., Joyce K. M., Brozovich F. V., Fisher S. A. J. Biol. Chem. 2001;276:37250–37257. doi: 10.1074/jbc.M105275200. [DOI] [PubMed] [Google Scholar]
- 8.Sauzeau V., Le Jeune J., Cario-Toumaniantz C., Smolenski A., Lohmann S. M., Bertoglio J., Chardin P., Pacaud P., Loirand G. J. Biol. Chem. 2000;275:21722–21729. doi: 10.1074/jbc.M000753200. [DOI] [PubMed] [Google Scholar]
- 9.MacDonald J. A., Walker L. A., Nakamoto R. K., Gorenne I., Somlyo A. V., Somlyo A. P., Haystead T. A. FEBS Lett. 2000;479:83–88. doi: 10.1016/s0014-5793(00)01884-6. [DOI] [PubMed] [Google Scholar]
- 10.Walker L. A., MacDonald J. A., Liu X., Nakamoto R. K., Haystead T. A., Somlyo A. V., Somlyo A. P. J. Biol. Chem. 2001;276:24519–24524. doi: 10.1074/jbc.M103560200. [DOI] [PubMed] [Google Scholar]
- 11.Ito M., Dabrowska R., Guerriero V., Jr., Hartshorne D. J. J. Biol. Chem. 1989;264:13971–13974. [PubMed] [Google Scholar]
- 12.Gallagher P. J., Herring B. P. J. Biol. Chem. 1991;266:23945–23952. [PMC free article] [PubMed] [Google Scholar]
- 13.Herring B. P., Smith A. F. Am. J. Physiol. 1996;270:C1656–C1665. doi: 10.1152/ajpcell.1996.270.6.C1656. [DOI] [PubMed] [Google Scholar]
- 14.Shirinsky V. P., Vorotnikov A. V., Birukov K. G., Nanaev A. K., Collinge M., Lukas T. J., Sellers J. R., Watterson D. M. J. Biol. Chem. 1993;268:16578–16583. [PubMed] [Google Scholar]
- 15.Katayama E., Scott-Woo G., Ikebe M. J. Biol. Chem. 1995;270:3919–3925. doi: 10.1074/jbc.270.8.3919. [DOI] [PubMed] [Google Scholar]
- 16.Silver D. L., Vorotnikov A. V., Watterson D. M., Shirinsky V. P., Sellers J. R. J. Biol. Chem. 1997;272:25353–25359. doi: 10.1074/jbc.272.40.25353. [DOI] [PubMed] [Google Scholar]
- 17.Nieznanski K., Sobieszek A. Biochem. J. 1997;322:65–71. doi: 10.1042/bj3220065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamm K. E., Stull J. T. J. Biol. Chem. 2001;276:4527–4530. doi: 10.1074/jbc.R000028200. [DOI] [PubMed] [Google Scholar]
- 19.Herring B. P., Smith A. F. Am. J. Physiol. 1997;272:C1394–C1404. doi: 10.1152/ajpcell.1997.272.4.C1394. [DOI] [PubMed] [Google Scholar]
- 20.Kitazawa T., Gaylinn B. D., Denney G. H., Somlyo A. P. J. Biol. Chem. 1991;266:1708–1715. [PubMed] [Google Scholar]
- 21.Kitazawa T., Masuo M., Somlyo A. P. Proc. Natl. Acad. Sci. USA. 1991;88:9307–9310. doi: 10.1073/pnas.88.20.9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitsui T., Kitazawa T., Ikebe M. J. Biol. Chem. 1994;269:5842–5848. [PubMed] [Google Scholar]
- 23.Choudhury N., Khromov A. S., Somlyo A. P., Somlyo A. V. J. Muscle Res. Cell Motil. 2004;25:657–665. doi: 10.1007/s10974-004-7807-x. [DOI] [PubMed] [Google Scholar]
- 24.Bolz S. S., Vogel L., Sollinger D., Derwand R., de Wit C., Loirand G., Pohl U. Circulation. 2003;107:3081–3087. doi: 10.1161/01.CIR.0000074202.19612.8C. [DOI] [PubMed] [Google Scholar]
- 25.Gong M. C., Cohen P., Kitazawa T., Ikebe M., Masuo M., Somlyo A. P., Somlyo A. V. J. Biol. Chem. 1992;267:14662–14668. [PubMed] [Google Scholar]
- 26.Lee M. R., Li L., Kitazawa T. J. Biol. Chem. 1997;272:5063–5068. doi: 10.1074/jbc.272.8.5063. [DOI] [PubMed] [Google Scholar]
- 27.Wooldridge A. A., MacDonald J. A., Erdodi F., Ma C., Borman M. A., Hartshorne D. J., Haystead T. A. J. Biol. Chem. 2004;279:34496–34504. doi: 10.1074/jbc.M405957200. [DOI] [PubMed] [Google Scholar]
- 28.Masato T., Numata T., Katoh T., Morita F., Yazawa M. J. Biochem. 1997;121:225–230. [PubMed] [Google Scholar]
- 29.MacDonald J. A., Borman M. A., Muranyi A., Somlyo A. V., Hartshorne D. J., Haystead T. A. Proc. Natl. Acad. Sci. USA. 2001;98:2419–2424. doi: 10.1073/pnas.041331498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muranyi A., MacDonald J. A., Deng J. T., Wilson D. P., Haystead T. A., Walsh M. P., Erdodi F., Kiss E., Wu Y., Hartshorne D. J. Biochem. J. 2002;366:211–216. doi: 10.1042/BJ20020401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muranyi A., Zhang R., Liu F., Hirano K., Ito M., Epstein H. F., Hartshorne D. J. FEBS Lett. 2001;493:80–84. doi: 10.1016/s0014-5793(01)02283-9. [DOI] [PubMed] [Google Scholar]
- 32.Eto M., Ohmori T., Suzuki M., Furuya K., Morita F. J. Biochem. (Tokyo) 1995;118:1104–1107. doi: 10.1093/oxfordjournals.jbchem.a124993. [DOI] [PubMed] [Google Scholar]
- 33.Surks H. K., Richards C. T., Mendelsohn M. E. J. Biol. Chem. 2003;278:51484–51493. doi: 10.1074/jbc.M305622200. [DOI] [PubMed] [Google Scholar]
- 34.Hoggatt A. M., Simon G. M., Herring B. P. Circ. Res. 2002;91:1151–1159. doi: 10.1161/01.res.0000047508.30800.4f. [DOI] [PubMed] [Google Scholar]
- 35.Wattler S., Kelly M., Nehls M. BioTechniques. 1999;26:1150–1156. doi: 10.2144/99266rr02. [DOI] [PubMed] [Google Scholar]
- 36.Wakeland E., Morel L., Achey K., Yui M., Longmate J. Immunol. Today. 1997;18:472–477. doi: 10.1016/s0167-5699(97)01126-2. [DOI] [PubMed] [Google Scholar]
- 37.Dietrich W. F., Miller J., Steen R., Merchant M. A., Damron-Boles D., Husain Z., Dredge R., Daly M. J., Ingalls K. A., O’Connor T. J. Nature. 1996;380:149–152. doi: 10.1038/380149a0. [DOI] [PubMed] [Google Scholar]
- 38.Dietrich W., Katz H., Lincoln S. E., Shin H. S., Friedman J., Dracopoli N. C., Lander E. S. Genetics. 1992;131:423–447. doi: 10.1093/genetics/131.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Facemyer K. C., Cremo C. R. Bioconjugate Chem. 1992;3:408–413. doi: 10.1021/bc00017a009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.