Abstract
Frontier malaria is a biological, ecological, and sociodemographic phenomenon operating over time at three spatial scales (micro/individual, community, and state and national). We explicate these linkages by integrating data from remote sensing surveys, ground-level surveys and ethnographic appraisal, focusing on the Machadinho settlement project in Rondônia, Brazil. Spatially explicit analyses reveal that the early stages of frontier settlement are dominated by environmental risks, consequential to ecosystem transformations that promote larval habitats of Anopheles darlingi. With the advance of forest clearance and the establishment of agriculture, ranching, and urban development, malaria transmission is substantially reduced, and risks of new infection are largely driven by human behavioral factors. Malaria mitigation strategies for frontier settlements require a combination of preventive and curative methods and close collaboration between the health and agricultural sectors. Of fundamental importance is matching the agricultural potential of specific plots to the economic and technical capacities of new migrants. Equally important is providing an effective agricultural extension service.
Keywords: Brazilian Amazon, frontier malaria
Economically and politically driven human migration in the Amazon basin of Brazil over the past century has been accompanied by substantial ecosystem transformation and the promotion of malaria transmission (1–3). Research programs in parasitology, entomology, and epidemiology of vector-borne diseases were established in Brazil in the 1890s (4–6) followed, almost immediately, by translation into malaria mitigation strategies (3, 7). Major eradication and control campaigns in Amazonia, initiated in the 1950s and persisting until 1970 (1), succeeded in reducing the number of malaria cases in the region to ≈30,000 (in 1970) (roughly 60% of all cases reported in the country).
The modern era of Amazon frontier expansion began during the military government (1964–1985) with the introduction of large scale colonization projects focused on agriculture, mineral extraction, and wide-ranging human settlement (8–13). The human population of the Amazon grew from 7.2 million in 1970 to 11 million in 1980 and then to 18.7 million by 1996, accompanied by a dramatic increase in malaria cases (14, 15). As of 1999, there were ≈600,000 malaria cases in Brazil, 99.7% of which were concentrated in the legal Amazon. The spatial distribution of these cases was very irregular, and a lack of spatially targeted mitigation strategies resulted in inefficient allocation of resources. In 1986, 60% of all malaria cases in the Amazon were concentrated in 58% of the municipalities, but 70% of the budget for malaria control was spent in municipalities with only 3% of the cases (16).
Characterizing malaria risk in the rapidly transforming Amazon ecosystems requires considering biological and ecological phenomena acting at multiple spatial scales, juxtaposed with behavioral and economic conditions. In this regard, we adapt and add precision to the ideas of Sawyer (2, 17) and define frontier malaria as a phenomenon operating at three spatial scales. First, at a micro/individual level, vector densities are high [as a consequence of ecosystem transformations that promote Anopheles darlingi larval habitats (18–20) such as partial shade near the forest fringe and along river edges, clear standing water of high pH]; human exposure is intense [reflecting limited knowledge of transmission among settlers; A. darlingi has a bimodal biting pattern (21), at dawn and dusk, just when settlers are going to and returning from their fields]; Plasmodium falciparum is the primary parasite, augmented by limited abundance of Plasmodium vivax; morbidity is high, and mortality is low (reflecting an unusual evolution of virulence of P. falciparum in the Amazon); and immunity is low among new settlers (they come mostly from malaria-free areas). Housing quality is poor, thereby rendering indoor residual spraying ineffective. Curative health services are sparsely available, thereby limiting antimalarial drug distribution.
Second, at a community level, frontier malaria is characterized by weak institutions, minimal community cohesion, political marginality of the settlers, and high rates of both in- and out-migration. This combination of conditions severely limits organized attempts at ecosystem management to minimize malaria risk and development of health clinics. Human mobility ensures proliferation of parasites. Third, at a state and national level, frontier malaria is characterized by unplanned development of new settlement areas, stimulated by agricultural failures at previous settlement localities and by a desire of people to avoid further malaria episodes. This process, however, only serves to promote further transmission.
Frontier malaria was also conjectured (22) to follow a distinctive time path. At the opening of a settlement area, malaria rates rise rapidly, and the first two levels of spatial characterization are fully operative. After 6–8 years, the unstable human migration (both in and out) and the highly variable ecological transformations (driven by variation in land clearance practices and local ecology) is replaced by a more organized process of urbanization and development of community cohesion. Frontier malaria is replaced gradually by more stable low levels of transmission and lower malaria rates. The process of urbanization itself (especially the introduction of impervious surfaces and drains) is an important intervention because it creates environments that are inhospitable to A. darlingi larvae and that are increasingly remote from forest fringes, thereby substantially reducing human exposure.
The purpose of this article is to present an empirical analysis of frontier malaria, explicating the subtle linkages across different spatial and temporal scales. A central feature is the integration of data from remote sensing surveys, ground-level surveys, and ethnographic appraisal. The coalescence of all three lines of evidence is essential for characterizing malaria risk on the Amazon frontier.
Results
We focus on the Machadinho settlement project in the northeast corner of the state of Rondônia, Brazil (Fig. 1).
Fig. 1.
Location and physical structure of the Machadinho settlement project, Rondônia state (RO), Brazil. The traditional “fish-bone” pattern of settlements was replaced by an irregular land division that accounts for the local hydrology and topology, resulting in plots with frontage to roads and rear to a natural source of water. States portrayed in the map: AC, Acre; AM, Amazonas; PA, Pará; RO, Rondônia; MT, Mato Grosso.
Risk Profiles.
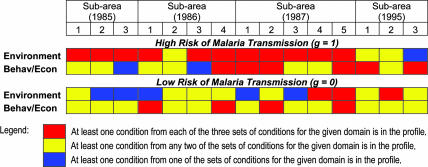
Risk profiles are specified by conditions in two broad domains: environmental and behavioral/economic. Within the environmental domain are three sets of conditions reflecting exposure, or the lack thereof, to A. darlingi larval habitats and/or adult mosquito biting preferences. These are: (i) housing characteristics (e.g., quality of roof and walls, effectiveness of house sealing), (ii) proximity to forest fringe or standing water [e.g., distance of house to forest, distance to small stream(s)], and (iii) land clearance (e.g., crop area planted, pasture area, cleared area). Within the behavioral/economic domain, there are also three sets of conditions that are indicators of the capacity of individuals to protect themselves or reflective of behaviors that may put them at more or less risk for acquiring malaria. These are: (i) level of education (of household head or spouse), (ii) migration history (e.g., place of origin and time of arrival at Machadinho, within settlement circular migration to urban area) and use of protective measures (e.g., insecticides), and (iii) economic circumstances (e.g., ownership of a chain saw, number and character of household goods owned).
We summarize the profile structures for Machadinho in Table 1. For each subarea as identified in Fig. 2, we score each of the major domains, environmental and behavioral/economic, as having a high, intermediate, or low contribution to a profile, because there are, respectively, three (high), two (intermediate), or one of the above-mentioned sets of conditions within the domain.
Table 1.
Structure of risk profiles
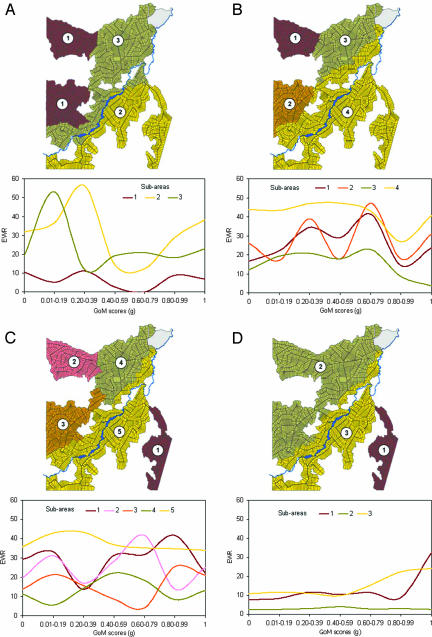
Fig. 2.
Subareas of malaria risk and EWR as a function of GoM scores (g). (A) 1985. (B) 1986. (C) 1987. (D) 1995. The number and delineation of subareas were chosen to reflect distinct spatial patterns of EWR, revealed by cluster analysis and spatially estimated EWR (for technical details, see the supporting information, which is published on the PNAS web site).
Important features of the settlement process, discernible from Table 1, are that: (i) in the initial years of settlement, environmental transformations and conditions dominated the high-risk profiles; (ii) by 1995, risky conditions essentially reflected personal behavior and economic circumstances because the substantial land-clearance process left much of Machadinho relatively inhospitable to A. darlingi larval development; (iii) low-risk conditions were initially dominated by behavioral and economic measures, particularly reflecting ownership of capital equipment (chain saws) and agricultural expertise, which leads to rapid land clearance and construction of a protective house, placing some settlers at considerable distance from the forest fringe and areas of partial shade; (iv) by 1995, there was substantial urban development in Machadinho city, cattle ranching activity, and housing that is protective. These positive environmental conditions, from the perspective of reducing exposure to A. darlingi, dominate low-risk profiles, especially in subarea 2, in 1995.
Malaria Rates.
Table 2 shows the minimum, median, and maximum of the exposure-weighted malaria illness rates (EWRs) reported for the subareas identified in Fig. 2 as a function of time.
Table 2.
Minimum, median, and maximum malaria rates observed in Machadinho subareas and overall malaria rate reported in each survey year
| Year | Malaria rates (per 100) |
|||
|---|---|---|---|---|
| Minimum | Median | Maximum | Global | |
| 1984 | 0 | 0 | 0 | 0 |
| 1985 | 7.4 | 24.8 | 35.9 | 22.7 |
| 1986 | 17.0 | 27.1 | 43.8 | 32.1 |
| 1987 | 14.9 | 24.3 | 38.5 | 23.6 |
| 1995 | 2.9 | 11.3 | 11.8 | 6.6 |
This pattern is consistent with the conjecture of Sawyer and Sawyer (22) about frontier malaria in that malaria rates rise rapidly at the opening of a settlement area, remain at a relatively high level for a few years, and then decay and maintain low levels after ≈10 years. The considerable range of rates across subareas in the early years of settlement [e.g., 7.40 (subarea 1) to 35.93 (subarea 2) in 1985; 17.0 (subarea 3) to 43.8 (subarea 4) in 1986] only conveys a rough picture of the variation in malaria rates in each year. For a more comprehensive picture, we require estimates of such rates as a function of proximity of plots to environmental and behavior/economic risk profiles (e.g., in 1987 rates for plots at the border of the largest protected forest reserve were as high as 80).
To this end, malaria rates as a function of proximity to high-risk profiles are shown in Fig. 2 A–D for all subareas in all survey years. In general, we would anticipate that the rates would increase with increasing values of g [i.e., the proximity to the high-risk or grade of membership (GoM) profile (23–27)]. For many, but certainly not for all, subareas this is the trend. However, reconciliation of seeming anomalies between malaria rates and the survey-based proximity (or GoM) scores require the amendment of ethnographic and satellite-based evidence. In this regard, we find that for 1985, subarea 1 has generally low rates, but the plots with low-risk profiles have rates as high, or higher, than the high-risk plots. This finding is accounted for by ethnographic evidence that reveals, in the southern part of subarea 1, that there are better soils with the potential for good farming, but many of these migrants only cleared and worked their plots and were not yet fully settled in Machadinho. They, nevertheless, provided a reservoir of parasites that were the basis of transmission to more stable households (i.e., those that satisfied inclusion conditions for the survey) with lower risk environmental conditions. In subareas 2 and 3 in 1985, there were people on lower risk plots (in the survey) whose higher malaria exposure was a consequence of the proximity of more mobile settlers (not in the survey), including rubber tappers acquiring plots in subarea 3.
In 1986, subarea 4 has persistently high malaria rates even for plots with a g value close to zero. From satellite imagery, we know that plots close to the low-risk plots in the survey are undergoing rapid ecological transformation, with creation of new zones of partial shade and redefinition of the forest fringe (ideal A. darlingi larval habitats) by new migrants whose plots do not meet inclusion criteria for the survey. We also know from ethnographic field notes that some of these plots are close to the forest reserve where exposure to a reservoir of parasites in infected rubber tappers facilitates transmission.
Finally, we point out that in one instance (subarea 1 in 1995), the survey-based profiles are irrelevant for discussing malaria risk. The high rates are largely a consequence of a parasite reservoir among illegal settlers in the area just to the right of subarea 1. This phenomenon is clearly visible from a satellite image (Fig. 3) and represents the result of illegal forest clearance in 1994 (documented in ethnographic field notes).
Fig. 3.
Illegal deforestation of ≈33.5 km2 in size that occurred at the southeastern portion of Machadinho.
Discussion
Malaria Mitigation.
On the basis of the observed rapid rise in the EWR in the early stages of settlement and their subsequent decay to low levels 11 years later (Table 2), this study contains the first verification of the conjectured (22) general pattern of infection for frontier malaria. If we take it as given that further frontier settlements will continue to be established, and this is indeed what has been taking place (28), then the question arises as to what feasible strategies might be used to prevent the malaria pattern we have seen from repeating at each new site. Four interventions would seem to be of importance:
Match the soil composition and agricultural potential of plots to the capacity of settlers for farming at the time plots are allocated.
Encourage, and even support, rapid initial clearance of land for agriculture and house construction that is protective.
Make available an effective agricultural extension service.
Establish health clinic facilities with the opening of the settlement area.
Items 1 and 3 pertain to facilitating effective farming, thereby ensuring substantial land clearance that will keep settlers away from the forest fringe and major zones of partial shade that comprise A. darlingi larval habitats. These steps will also maximize the chances for economic success, thereby reducing the probability that farmers already infected with malaria parasites would abandon a plot and move to another site. This kind of out-migration facilitated the spread of malaria to incoming settlers in the early years of the Machadinho project, as well as to persons at other colonization projects. It is ironic that soil sampling, formal scoring of the agricultural potential of all plots, and mapping of the entire Machadinho area with inclusion of this information was carried out before any of the plots were allocated to settlers (29). However, none of this information was used when allocation of plots was carried out. This omission created major mismatches between the agricultural knowledge and economic capacity, particularly the availability of capital equipment for forest cutting and clearance, of new settlers and the characteristics of plots allocated to them. The primary consequence of this omission was that many settlers lacked the capacity to clear large areas of forest and rapidly establish farming, thereby leaving them in close proximity to A. darlingi habitats for many months.
Item 2 is a preventive measure that minimizes the exposure time of new settlers to A. darlingi habitats upon their initial arrival at the settlement site. In this regard, it is important to recall that there have been no malaria outbreaks in corporate-sponsored openings of frontier areas for natural resource extraction or cattle ranching (2). This is a consequence of their carrying out rapid initial land clearance with a small number of personnel who take protective measures against mosquito exposure. In government-sponsored colonization projects in which knowledge of the mechanics of malaria transmission and the features of risky exposure are minimally understood by the settlers, malaria outbreaks are a consequence of both lack of knowledge about the disease per se and an inability, based on lack of capital equipment, to rapidly cut down and clear forested areas. Finally, item 4, the installation of health clinics, provides a basis for not only diagnosis and treatment of malaria when it may occur but also can serve as centers for health education, emphasizing preventive measures throughout the settlement area.
Monitoring and Surveillance.
Although the structured surveys used in this study provided the core evidence about both environmental and behavioral/economic risks over time, they represent an inefficient methodology for monitoring and surveillance purposes at colonization sites more generally. Satellite images can readily identify environmental risks at different levels of resolution. Although in this study we used Landsat images, higher resolution satellite imagery, such as Kometa (Sovinformsputnik, Moscow) with 2 m of spatial resolution, Ikonos (Space Imaging, Thornton, CO) with one meter of resolution, and Quickbird (Digital Globe, Longmont, CO) with the best spatial resolution commercially available to date (namely 0.61 m), would provide for greater accuracy in that distances to forest fringe, streams, and a diversity of sources of standing water could be assessed within a few meters as opposed to the estimates obtained from heads of households in the present surveys. Furthermore, information about activities on plots not included in the survey but relevant for characterizing risk would be readily ascertainable from satellite images, thereby reducing dependency on ethnographic studies for this kind of environmental risk assessment.
Behavioral patterns leading to exposure to A. darlingi, the specifics of agricultural practices, utilization of health services, and direct assessment of malaria episodes (via blood sampling and self-reports) requires ground-based assessments. Substantial advances in survey design and methodology (30–33) could readily be incorporated in Demographic Surveillance Systems (DSS) at colonization sites and in nearby towns, thereby providing high-quality appraisals of behavioral risks over time. Linkage of DSS longitudinal surveys to standard official data collection would also provide a basis for malaria monitoring over much wider geographical areas than would be feasible with community based in-depth surveys of any kind.
Although our focus is malaria, it is important that the monitoring and evaluation aspect of malaria mitigation be integrated in an overall evidence-based planning system for health at district, regional, and national levels (31). Such a systemic approach is particularly important in light of the fact that many health information systems are weak because monitoring and evaluation is not treated as a coherent element in a routine pathway to evidence-based decision making. We emphasize this point because there are many examples of interventions that should have been adopted much earlier than they were, policies that should have been changed sooner, and epidemics that deserved swifter responses. Data and information (the product of analyses) were frequently available, but proper packaging, communication, and follow through with relevant policy makers inhibited the needed action. In the context of frontier colonization projects, malaria mitigation strategies, of necessity, also involve cooperation between the agriculture and health sectors because environmental management is a critical preventive ingredient in the early stages of settlement. Finally, the health sector could benefit from the large amount of data being generated by the System for Surveillance of the Amazon (SIVAM), a large-scale sophisticated radar system currently oriented to national security and environmental protection objectives. Such intersectoral cooperation will be required if the malaria experience of Machadinho is to be avoided in the future.
Materials and Methods
Study Area.
Colonization in Rondônia, Brazil was promoted by the Northwest Regional Integrated Development Program, POLONOROESTE, a project initiated in 1984 and cosponsored by the Brazilian federal government and the World Bank (34). Before 1984, the project area was primarily jungle. The adjacent “protected reserves” contained a sparsely settled population of rubber tappers, not officially acknowledged by the government. The rubber tappers, long established in the area and largely asymptomatic to malaria infection, were an important reservoir of parasites. They, together with infected migrants from the nearby malaria-infested areas of Jaru and Ariquemes, played a central role in stimulating a malaria epidemic among all groups of migrants to Machadinho. Within 1 year of initial occupation of project-designated plots, the Annual Parasite Index (API) reached 3,400 positive slides per thousand people (35).
Field Surveys.
A household survey was given to settlers living on 70% of what were regarded as “occupied plots” in 1985 and 100% of such plots in 1986, 1987, and 1995. An occupied plot is one in which settlers cleared some of their land and at least lived part-time in Machadinho. Based on this definition, 13%, 20%, 33%, and 51% of the plots in tract 1 (Fig. 1) were occupied in 1985, 1986, 1987, and 1995, respectively. In tract 2, these numbers were 18%, 38%, 49%, and 57% for the same period. The survey instruments included information on health and on demographic, economic, social, ecological, and agricultural characteristics of the people and their immediate environment on the plot (35, 36). A core set of questions remained the same throughout all 4 years of data collection, thereby facilitating longitudinal data archives with the plot as the unit of analysis.
Malaria episodes over the 12-month period before each survey were ascertained by self-report. Prior validation studies in Southern Pará (37) show a sensitivity of 80.9% and a specificity of 66.7% for detecting P. falciparum infections in nonimmune populations similar in sociodemographic characteristics to those living at Machadinho. The relatively high misclassification in which people report malaria episodes but are negative for a P. falciparum-specific antibody assay is probably a result of P. vivax infections that are also present in these populations. Malaria self-reports are a useful measure in settings in which people are nonimmune, are sparsely scattered over a broad geographic area, and one or more household members is very mobile. Migrants to Machadinho are educated upon arrival about malaria symptomotology and, to a minimal extent (unfortunately), about the mechanism of transmission.
Remote Sensing.
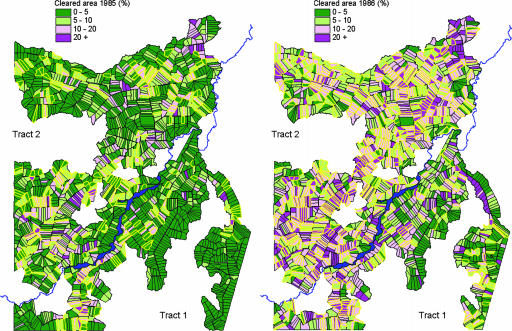
Satellite images provided additional information not collected by the field survey. We used Landsat thematic mapper images with a 30-m resolution for 1985, 1986, 1994, and 1995. The amount of cleared land was estimated for all plots in tracts 1 and 2. As Fig. 4 shows, many plots not included in the field survey experienced extensive environmental disturbance, facilitating the risk of transmission in their immediate neighborhood. Landsat thematic mapper images from July, 1994 and April, 1995 (Fig. 3) revealed a large area (≈33.5 km2) of rapid and illegal forest clearance, bringing temporary workers and new settlers into close proximity with new A. darlingi larval habitats.
Fig. 4.
Man-made environmental transformation in Machadinho in 1985 and 1986. The percentage of cleared area is given by Landsat thematic mapper images. Plots with bold yellow borders are those included in the field survey.
Ethnography.
The criterion for an occupied plot maximizes the chances of identifying settlers who can be followed longitudinally via a structured survey. An important limitation of this criterion is that the survey does not identify some key people (e.g., rubber tappers), who are central to the malaria transmission process. To expand the population coverage and ascertain subtle behavioral nuances that influence transmission, an ethnographic assessment of the Machadinho area was carried out over time by one of us (R.L.M.-M.) in parallel with the surveys. Field notes recorded the presence of, and population estimates for, rubber tappers in zones defined as forest reserves and that were declared by the government to be empty of people. Rubber tappers did not officially exist and were not taken into account in the government planning process for the opening of the Machadinho settlement project.
With the opening of Machadinho via a network of roads and designated plots (Fig. 4), rubber tappers bought plots close to the forest reserve, establishing second homes, and regularly moved back-and-forth between their primary home and the new site within the project boundaries. This provided substantial contact between infected rubber tappers and A. darlingi that were also proximal to nonimmune migrants. This transmission pattern is reflected in the high malaria rates among settlers on occupied plots proximal to forest reserves. Equally important for facilitating transmission in the early stages of Machadinho settlement were parasite-infected migrants from the nearby malaria-infested municipalities of Jaru and Ariquemes.
Analysis Strategy.
The primary outcome variable is the EWR, defined as:
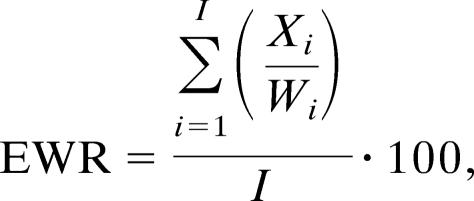 |
where I is the total number of persons in Machadinho during the past year, Xi is the number of reported months with malaria episodes for person i during the past year, and Wi is the number of months person i lived in Machadinho during the past year. Therefore, EWR ranges between 0 and 100, and its value varies substantially across geographic boundaries within Machadinho. By using a local indicator of spatial association, Gi (d) statistic (38–41), clusters of high and low malaria rates were identified for all survey years. Rates were interpolated for “unoccupied” plots by using kriging estimators (42–44). The interpolated rates combined with the cluster analysis facilitated the definition of distinctive subareas (in terms of malaria risk) within Machadinho (Fig. 2).
For each subarea in each of the years, 1985–87 and 1995, GoM analysis (23–27) was carried out by using the survey data to identify combinations of conditions that placed individuals at various gradations of risk for malaria. The plot was the basic unit of analysis. Two-profile GoM models were fitted in all instances. They were identified with conditions of low and high malaria risk, respectively. Thus, each plot has a degree-of-similarity score, g, indicating the proximity of its characteristics to the low- (g = 0) and high-risk (g = 1) profiles. A plot with g = 2/3, for example, would have more of the characteristics of a high-risk profile while still having 1/3 of its own features corresponding to low-risk conditions.
Supplementary Material
Acknowledgments
We thank the Office of Population Research and Center for Health and Wellbeing (Princeton University, Princeton), the Andrew W. Mellon Foundation [Program on Migration and Urbanization (Princeton University)], the Rockefeller Foundation, and International Development Research Centre Grant 94-0206-00 for financial support.
Glossary
Abbreviations:
- GoM
grade of membership
- EWR
exposure-weighted malaria illness rate.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Deane L. M. Am. J. Trop. Med. Hyg. 1988;38:223–230. doi: 10.4269/ajtmh.1988.38.223. [DOI] [PubMed] [Google Scholar]
- 2.Sawyer D. R. Malaria and the Environment. Brasília: Instituto SPN; 1992. [Google Scholar]
- 3.Stepan N. L. In: Disease in the History of Modern Latin America: From Malaria to AIDS. Armus D., editor. Durham, NC: Duke Univ. Press; 2003. pp. 25–50. [Google Scholar]
- 4.Neiva A. Mem. Inst. Oswaldo Cruz. 1910;2:131–140. [Google Scholar]
- 5.Neiva A. Rev. Clube Eng. 1940;VI:60–75. [Google Scholar]
- 6.Stepan N. Beginnings of Brazilian Science: Oswaldo Cruz, Medical Research and Policy. New York: Science History Publications; 1976. pp. 1890–1920. [Google Scholar]
- 7.Cruz O. In: The Prevention of Malaria. Ross R., editor. New York: E. P. Dutton; 1910. pp. 390–399. [Google Scholar]
- 8.Benchimol S. In: Change in the Amazon Basin: The Frontier after a Decade of Colonization. Hemming J., editor. Vol. II. Manchester, U.K.: Manchester Univ. Press; 1985. pp. 37–50. [Google Scholar]
- 9.Moran E. F. In: Change in the Amazon Basin: The Frontier after a Decade of Colonization. Hemming J., editor. Vol. II. Manchester, U.K.: Manchester Univ. Press; 1985. pp. 91–102. [Google Scholar]
- 10.Fearnside P. M. Human Carrying Capacity of the Brazilian Rainforest. New York: Columbia Univ. Press; 1986. [Google Scholar]
- 11.Sawyer D. R. SE. Asian J. Trop. Med. Public Health. Vol. 17. 1986. pp. 342–345. [PubMed] [Google Scholar]
- 12.Browder J. O., Godfrey B. J. Rainforest Cities: Urbanization Development and Globalization of the Brazilian Amazon. Columbia Univ. Press: New York; 1997. [Google Scholar]
- 13.Schmink M., Wood C. H. Contested Frontiers in Amazonia. New York: Columbia Univ. Press; 1992. [Google Scholar]
- 14.Coimbra M. E. L. S. SUCAM Malaria Control. Brazil: Centro de Desenvolvimento e Planejamento Regional Belo Horizonte; 1985. [Google Scholar]
- 15.Marques A. C. Parasitol. Today. 1987:166–170. [Google Scholar]
- 16.Akhavan D., Musgrove P., Abrantes A., Gusmão R. A. Soc. Sci. Med. 1999;49:1385–1399. doi: 10.1016/s0277-9536(99)00214-2. [DOI] [PubMed] [Google Scholar]
- 17.Sawyer D. R. Frontier Malaria in the Amazon Region of Brazil: Types of Malaria Situations and Some Implications for Control. Brasília: Pan American Health Organization/WHO/The Special Programme for Research Training in Tropical Diseases; 1988. [Google Scholar]
- 18.Charlwood J. D. B. Entomol. Res. 1980;70:685–692. [Google Scholar]
- 19.Charlwood J. D. Mem. Inst. Oswaldo Cruz. 1996;91:391–398. doi: 10.1590/s0074-02761996000400001. [DOI] [PubMed] [Google Scholar]
- 20.Tadei W. P., Thatcher B. D., Santos J. M. M. d., Scarpassa V. M., Rodrigues I. B., Rafael M. S. Am. J. Trop. Med. Hyg. 1998;59:325–335. doi: 10.4269/ajtmh.1998.59.325. [DOI] [PubMed] [Google Scholar]
- 21.Klein T. A., Lima J. B. P. J. Am. Mosq. Control Assoc. 1990;6:700–707. [PubMed] [Google Scholar]
- 22.Sawyer D. R., Sawyer D. O. In: Advancing the Health in Developing Countries: The Role of Social Research. Chen L. C., editor. Westport, CT: Auburn House; 1992. pp. 105–122. [Google Scholar]
- 23.Woodbury M. A., Clive J., Garson A., Jr. Comput. Biomed. Res. 1978;11:277–298. doi: 10.1016/0010-4809(78)90012-5. [DOI] [PubMed] [Google Scholar]
- 24.Woodbury M. A., Manton K. G. Methods Inf. Med. 1982;21:210–220. [PubMed] [Google Scholar]
- 25.Singer B. H. In: Probability, Statistics, and Mathematics: Papers in Honor of Samuel Karlin. Anderson T. W., Athreya K. B., Iglehart D. L., editors. Boston: Academic; 1989. pp. 317–334. [Google Scholar]
- 26.Manton K. G., Woodbury M. A., Tolley H. D. Statistical Applications Using Fuzzy Sets. New York: Wiley; 1994. [Google Scholar]
- 27.Erosheva E. A., Fienberg S. E., Lafferty J. Proc. Natl. Acad. Sci. USA. Vol. 97. 2004. pp. 11885–11892. [Google Scholar]
- 28.Gonçalves M. J. F., Alecrim W. D. Rev. Salud Publica. 2004;6:156–166. doi: 10.1590/s0124-00642004000200003. [DOI] [PubMed] [Google Scholar]
- 29.Instituto Nacional de Colonização e Reforma Agrária (INCRA) Levantamento pedológico de reconhecimento com alta intensidade, aptidão agrícola e zoneamento agrícola das glebas 1 e 6, Projeto de Assentamento Machadinho. Brasília: INCRA & Ministério da Agricultura; 1984. [Google Scholar]
- 30.Byass P., Berhane Y., Emmelin A., Kebede D., Andersson T., Hogberg U., Wall S. Public Health. Vol. 116. 2002. pp. 145–150. [DOI] [PubMed] [Google Scholar]
- 31.De Savigny D., Binka F. N. Am. J. Trop. Med. Hyg. 2004;71(Suppl. 2):224–231. [PubMed] [Google Scholar]
- 32.D’souza S. Rural Demogr. 1981;8:29–51. [PubMed] [Google Scholar]
- 33.Ngom P., Binka F. N., Phillips J. F., Pence B., MacLeod B. Health Policy Plan. Vol. 16. 2001. pp. 337–344. [DOI] [PubMed] [Google Scholar]
- 34.Martine G. In: The Future of Amazonia: Destruction or Sustainable Development? Goodman D., Hall A. L., editors. New York: Palgrave Macmillan; 1990. pp. 23–48. [Google Scholar]
- 35.Sawyer D. R., Sawyer D. O. Malaria on the Amazon Frontier: Economic and Social Aspects of Transmission and Control. Belo Horizonte, Brazil: Centro de Desenvolvimento e Planejamento Regional; 1987. [Google Scholar]
- 36.Sawyer D. R. Research Design and Feasibility in the Machadinho Settlement Project. Belo Horizonte, Brazil: Centro de Desenvolvimento e Planejamento Regional; 1985. [Google Scholar]
- 37.Singer B. H., Sawyer D. O. Health Policy Plan. 1992;7:40–45. [Google Scholar]
- 38.Getis A., Ord J. K. Geogr. Anal. Vol. 24. 1992. pp. 189–206. [Google Scholar]
- 39.Anselin L. Geogr. Anal. Vol. 27. 1995. pp. 93–115. [Google Scholar]
- 40.Ord J. K, Getis A. Geogr. Anal. 1995;27:286–306. [Google Scholar]
- 41.Getis A., Ord K. J. In: Spatial Analysis: Modelling in a GIS Environment. Longley P., Batty M., editors. New York: Wiley; 1996. pp. 261–277. [Google Scholar]
- 42.Cressie N. Statistics for Spatial Data. New York: Wiley; 1993. [Google Scholar]
- 43.Isaaks E. H., Srivastava R. M. Applied Geostatistics. New York: Oxford Univ. Press; 1989. [Google Scholar]
- 44.Singer B. H., Castro M. C. Ann. NY Acad. Sci. 2001;954:184–222. doi: 10.1111/j.1749-6632.2001.tb02753.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.