Abstract
Although urotensin II (UII) and somatostatin 1 (SS1) exhibit some structural similarities, their precursors do not show any appreciable sequence identity and, thus, it is widely accepted that the UII and SS1 genes do not derive from a common ancestral gene. The recent characterization of novel isoforms of these two peptides, namely urotensin II-related peptide (URP) and somatostatin 2 (SS2)/cortistatin (CST), provides new opportunity to revisit the phylogenetic relationships of UII and SS1 using a comparative genomics approach. In the present study, by radiation hybrid mapping and in silico sequence analysis, we have determined the chromosomal localization of the genes encoding UII- and somatostatin-related peptides in several vertebrate species, including human, chicken, and zebrafish. In most of the species investigated, the UII and URP genes are closely linked to the SS2/CST and SS1 genes, respectively. We also found that the UII-SS2/CST locus and the URP/SS1 locus are paralogous. Taken together, these data indicate that the UII and URP genes, on the one hand, and the SS1 and SS2/CST genes, on the other hand, arose through a segmental duplication of two ancestral genes that were already physically linked to each other. Our results also suggest that these two genes arose themselves through a tandem duplication of a single ancestral gene. It thus appears that the genes encoding UII- and somatostatin-related peptides belong to the same superfamily.
Keywords: duplication, multigenic family, neuropeptides, radiation hybrid mapping
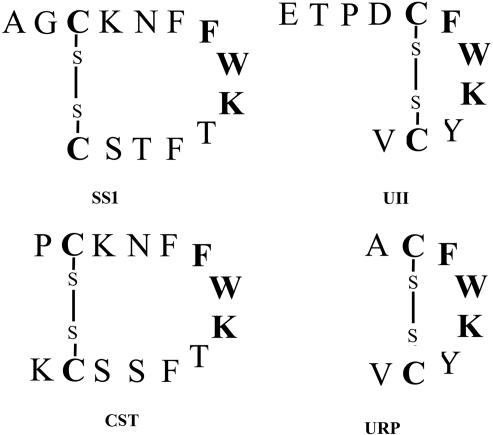
Urotensin II (UII) is a cyclic peptide initially isolated from the caudal neurosecretory system of teleosts, on the basis of its spasmogenic activity (1). Subsequently, UII has been characterized in the brain and spinal cord of various classes of vertebrates including amphibians and mammals (2–5). Recently, the occurrence of a second gene encoding the precursor of a UII paralog, named UII-related peptide (URP), has been reported in mouse, rat, and human (6). Both UII and URP exhibit limited structural identity to somatostatin 1 (SS1) (Fig. 1), and it has been found that UII and SS1 share some functional similarities (see ref. 7 for review), so that UII was originally described as a somatostatin-like peptide (1). However, characterization of the cDNA encoding the UII precursor has shown that prepro-UII and prepro-SS1 do not exhibit appreciable sequence identity (4, 5, 8), and hence, it was concluded that the UII and SS1 precursors were probably not derived from a common ancestral gene. The data currently available support the existence, in all vertebrate classes, of at least two somatostatin genes encoding two distinct somatostatin isoforms (Fig. 1), namely SS1 and somatostatin 2 (SS2), also termed cortistatin (CST) in mammals (3, 9–12). It is generally accepted that the SS2/CST gene arose through duplication of the SS1 gene, some 450 million years ago (11, 12). Teleost fish even possess a third gene encoding another somatostatin isoform named somatostatin II (SSII) (3, 9, 11).
Fig. 1.
Comparison of the amino acid sequences and predicted secondary structures of human UII, URP, SS1, and CST. Common residues are indicated in bold characters.
UII- and somatostatin-related peptides exert their effects through specific G protein-coupled receptors. Up to now, only one UII receptor, called UTR, has been characterized (13–16), and both UII and URP bind to this receptor with high affinity (17–19). In mammals, five somatostatin receptor subtypes (SST1–5) have been identified (20). The occurrence of a specific CST receptor, referred to as MgrX2, has also been reported in human (21).
The recent characterization of URP (6) and the existence of the somatostatin paralogs SS2/CST (22–31) provide the opportunity to revisit the possible relationships between the UII and somatostatin gene families. Using a comparative genomics approach, we show that the UII and somatostatin genes belong to the same gene superfamily.
Results
Struture of Predictive Peptides and Phylogenetical Relationships.
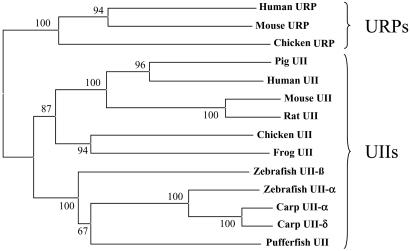
Two distinct cDNAs encoding UII-like sequences were characterized in both zebrafish and chicken (Fig. 2). All sequences are available in the GenBank database. In zebrafish, the two predictive mature peptides have been named UII-α and UII-β on the basis of their structural and phylogenetical relationships with other teleost UII variants. Zebrafish UII-α exhibits the same amino acid sequence as carp UII-α and displays only one substitution when compared with carp UII-δ, whereas zebrafish UII-β exhibits the same sequence as carp UII-β2 and displays only one substitution with carp UII-β1. In chicken, the two putative mature peptides correspond to UII and URP. Chicken UII possesses the same sequence as frog UII, whereas chicken URP exhibits only one substitution when compared with its mammalian counterpart. Phylogenetical analysis revealed that zebrafish UII-α and UII-β precursors are more closely related to the tetrapod UII precursors than to the tetrapod URP precursors (Fig. 3).
Fig. 2.
Alignment of the amino acid sequences of chicken and zebrafish UII-related peptide precursors characterized in the present study. The sequences of mature peptides are indicated in boldface. Dashes indicate gaps. All of these sequences have been deposited in the GenBank database [accession nos. NM_212848 (prepro-urotensin II-α, zebrafish); NM_205591 (prepro-urotensin II-β, zebrafish); NM_206990 (prepro-urotensin II, chicken); and NM_206989 (prepro-urotensin II-related peptide, chicken)].
Fig. 3.
Phylogenetic tree of the UII gene family. An alignment of UII-related peptide precursor sequences currently known in vertebrates (data not shown) was used to calculate a neighbor-joining distance unrooted tree (33), using the phylo_win program (32). The values above the branches are the results (in percentages) of the bootstrap analysis.
Chromosomal Localization of Genes.
To localize the two UII genes to zebrafish linkage groups (LGs), radiation hybrid (RH) mapping using the LN54 panel was performed. The UII-α gene mapped to LG 23 at a distance of 7.58 centiRays∥ from the marker z3852 with a logarithm of odds score of 13.9, in the vicinity of the SS2/CST gene (12). The UII-β gene was assigned to LG 11 at 3.46 centiRays from z11067, with a logarithm of odds score of 14.1.
The chromosomal localization of the chicken URP and SS1 genes was investigated by using the ChickRH6 panel. The two genes mapped in the vicinity of several common markers: PSMD1, BDH, MCW0135, and FLJ20701. The distance between the URP gene and the SS1 gene was 52 centiRays, with a logarithm of odds score of 4.4. Because RH maps were available for only a limited number of chromosomes (32–37), the chromosomal assignment of the two genes could not be determined. They were finally localized on chromosome 9, owing to the data from the first draft sequence assembly of the whole chicken genome, available in ensembl (www.ensembl.org/index.html). In addition, based on the same data, the chicken UII and SS2/CST genes also appeared to be closely linked and could be assigned to chromosome 21.
Occurrence of Duplicate Gene Pairs on Human 1p36 and 3q28 Regions.
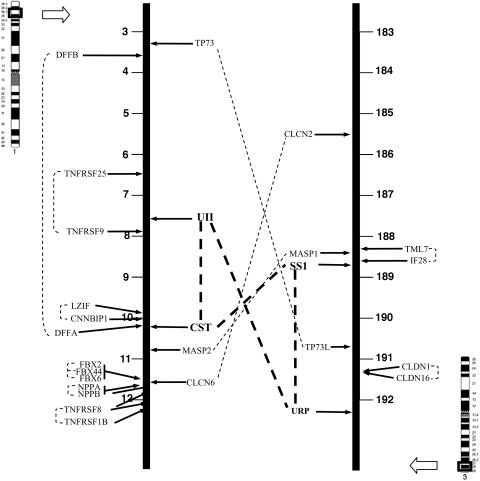
The duplicate gene pairs found on 1p36 and 3q28 are shown in Table 1. The chromosomal localization of these genes in human is depicted in Fig. 4. Several duplicate gene pairs displaying one copy on 1p36 and the other on 3q28 have been identified: TP73 and TP73L, MASP1 and MASP2, and CLCN2 and CLCN6. Most of the duplicate gene pairs that have been found on the same chromosome are arranged in tandem; i.e., LZIP and CNNBIP1; FBX2, FBX6, and FBX44; NPPA and NPPB; and TNFRSF1B and TNFRSF8 on 1p36; IF28 and TM7L, and CLDN1 and CLDN16 on 3q28. The genes from other pairs are relatively distant from each other; i.e., DFFA and DFFB, and TNFRSF25 and TNFRSF9 on 1p36. As shown in Table 1, the physical linkage of the orthologs of all these genes is perfectly conserved in tetrapods. Actually, most of the genes localized on 1p36 in human are even clustered in fish.
Table 1.
Duplicated gene pairs found in the vicinity of the UII- and somatostatin-related peptide encoding genes in human and their orthologs in mouse, rat, chicken, zebrafish, and Tetraodon
| Genes | Human | Mouse | Rat | Chicken | Zebrafish | Tetraodon |
|---|---|---|---|---|---|---|
| CST/SS2 | 1 (10.2) | 4 (147.2) | 5 (166.2) | 21 (4.0) | 23 (29.3) | 9 (2.2) |
| UII | 1 (7.8) | 4 (149.1) | 5 (168.2) | 21 (0.23) | α : 23; β : 11 | 9 (8.5) |
| CLCN6 | 1 (11.8) | 4 (145.9) | 5 (165.1) | 21 (5.3) | 8 (36.5) | Sc 7638 |
| Masp 2 | 1 (10.8) | 4 (146.7) | 5 (165.7) | 21 (3.9) | 23 (16.4) | N.D. |
| TP73 | 1 (3.3) | 4 (151.9) | 5 (170.9) | 21 (0.8) | 8 (36.6) | 9 (1.4) |
| DFFA | 1 (10.2) | 4 (147.2) | 5 (166.2) | 21 (3.6) | N.D. | 9 (2.2) |
| DFFB | 1 (3.6) | 4 (152.1) | 5 (170.8) | 21 (0.8) | 12 (1.7) | Sc 14659 |
| TNFRSF9 | 1 (7.9) | 4 (148.8) | N.D. | 21 (0.2) | N.D. | 9 (8.5) |
| TNFRSF8 | 1 (12.1) | 4 (143.6) | 5 (163.7) | 21 (5.2) | N.D. | N.D. |
| TNFRSF1B | 1 (12.2) | 4 (143.5) | 5 (163.7) | 21 (5.2) | N.D. | N.D. |
| TNFRSF25 | 1 (6.5) | 4 (150.0) | 5 (169.4) | Cg 30477.1 | N.D. | N.D. |
| FBX2 | 1 (11.6) | 4 (146.0) | 5 (165.2) | 21 (5.4) | 23 (17.8) | 9 (8.3) |
| FBX6 | 1 (11.7) | 4 (146.0) | 5 (165.2) | 21 (5.4) | 23 (17.8) | 9 (8.3) |
| FBXO44 | 1 (11.6) | 4 (146.0) | 5 (165.2) | 21 (5.4) | 23 (17.9) | 9 (8.3) |
| NPPA | 1 (11.6) | 4 (146.1) | 5 (165.1) | 21 (5.3) | 8 (34.6) | N.D. |
| NPPB | 1 (11.6) | 4 (146.1) | 5 (165.1) | N.D. | N.D. | N.D. |
| SS1 | 3 (188.7) | 16 (23.5) | 11 (79.2) | 9 (14.2) | 15 (31.8) | Sc 14677 |
| URP | 3 (192.3) | 16 (27.1) | N.D. | 9 (13.1) | N.D. | 3 (2.8) |
| CLCN2 | 3 (185.5) | 16 (20.5) | 11 (82.5) | Cg 23425 | 11 (4.9) | 16 (7.0) |
| Masp1 | 3 (188.3) | 16 (23.1) | 11 (79.6) | 9 (14.2) | 15 (31.6) | Sc 14677 |
| IF28 | 3 (188.6) | 16 (23.5) | 11 (79.2) | N.D. | N.D. | N.D. |
| TML7 | 3 (188.4) | 16 (23.3) | 11 (79.7) | N.D. | N.D. | N.D. |
| CLDN1 | 3 (191.3) | 16 (26.1) | 11 (76.5) | 9 (13.3) | N.D. | N.D. |
| CLDN16 | 3 (191.4) | 16 (26.2) | N.D. | 9 (13.3) | N.D. | N.D. |
| TP73L | 3 (190.7) | 16 (25.4) | 11 (77.0) | 9 (13.4) | 18 (32.4) | 1 (0.9) |
The first number is the chromosomal localization. The second number (between parentheses) is the exact position, in megabases. N.D., not determined. All data originate from ensembl and the present study.
Fig. 4.
Map showing chromosomal position of UII- and somatostatin-related peptide encoding genes in human. Other putative duplicated gene pairs localized in their vicinity are indicated. The duplicates of each pair are connected by broken line. All of the positions are expressed in megabases. CLCN2/6, chloride channel 2/6; MASP1/2, mannose-binding lectin-associated serine protease 1/2; TP73/73L, tumor protein p73/p73-like; DFFA/B, DNA fragmentation factor A/B; TNFRSF1B/8/9/25, tumor necrosis factor receptor superfamily member 1B/8/9/25; FBX2/6/44, F-box protein 2/6/44; NPPA/NPPB, A/B-type natriuretic peptide precursor; TML7, transmembrane protein 7; IF28, 28-kDa IFN-responsive protein; CLDN1/16, claudin 1/16.
Discussion
The present study has shown that, in human as in rodents and chicken, (i) the UII gene is closely linked to the gene encoding SS2/CST, and (ii) the gene encoding the novel UII-related peptide, URP, is located in the same chromosomal region as the SS1 gene (Fig. 4 and Table 1). This study also indicates that, in zebrafish, the SS2/CST gene is located in the vicinity of one of the two UII genes; i.e., the gene encoding the UII-α variant. It is likely that these two zebrafish UII genes originate from the whole-genome duplication that occurred in the ancestral fish lineage (38). In zebrafish, it was not possible to detect any URP-like gene in databases, suggesting that the URP gene has been lost in this species. In support of this view, an URP-like encoding sequence occurs in the genome of Tetraodon and Takifugu (available in ensembl). However, the primary structure of this putative peptide appears relatively atypical when compared with that of tetrapod URP, and its phylogenetic significance remains to be determined.
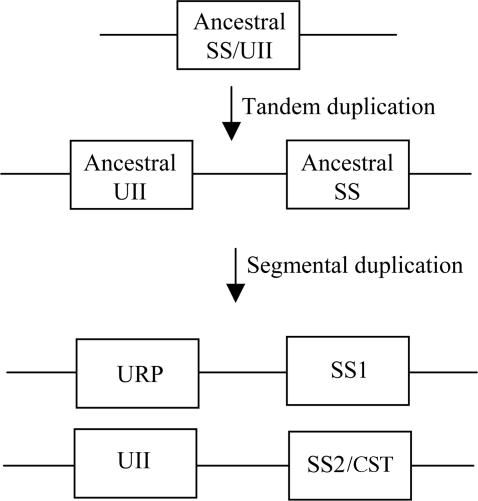
It is unlikely that the relative positions of the SS1 and SS2/CST genes and the UII and URP genes are only due to coincidence. Our study rather suggests that these four genes arose through segmental duplication from two common ancestral genes that were physically linked (Fig. 5). It is generally accepted that a large number of duplicated genes in vertebrate genomes have arisen through two rounds of genome duplication (2R hypothesis) that took place before the origin of gnathostomes (39). Consistent with this theory, we have found three additional duplicated gene pairs, namely the MASP1 and MASP2 genes, the CLCN2 and CLCN6 genes, and the TP73 and TP73L genes, which may have been duplicated along with UII- and somatostatin-related peptides-encoding genes. The occurrence of these genes indicates that, in human, the 1p36 and 3q28 regions are likely paralogous, in agreement with a recent report (40). Interestingly, there are >40 genes located on 1p36 between the SS2/CST gene and the UII gene, whereas there are only 16 genes on 3q28 between the SS1 gene and the URP gene, even though the distances between each gene pair are similar (≈3 Mb). This finding suggests that the 3q28 chromosomal segment may have lost numerous genes during evolution, after the putative segmental duplication, in agreement with the notion that the more likely outcome of a duplication event is the lost of the redundant gene copies (41).
Fig. 5.
Proposed evolutionary history of the somatostatin/UII gene family. According to this scenario, UII- and somatostatin-related peptide genes would result from two rounds of gene duplication; i.e., a tandem duplication followed by a segmental duplication. The latter duplication event may coincide with one of the two whole-genome duplications that took place during vertebrate evolution.
Although the SS1 and URP genes, on the one hand, and the SS2/CST and UII genes, on the other hand, are not strictly arranged in tandem, their physical linkage suggests that the genes of each locus may have arisen through a local duplication (Fig. 5). Two non-mutually exclusive hypotheses can be proposed to explain the large distances separating these genes (between 1.1 and 3.7 Mb depending on both the species and the genes): (i) the two copies of the ancestral gene were initially arranged in tandem and have been gradually separated by the insertion of additional genes, or (ii) the duplication event did not only affect the putative ancestral SS1/UII gene but also several surrounding genes, so that they became suddenly distant from each other. To test this latter hypothesis, we have searched for other putative duplicated genes in the vicinity of the human 1p36 and 3q28 regions. Several gene pairs were detected near the UII- and somatostatin-related genes, namely FBX2, FBX44 and FBX6, NPPA and NPPB, and TNFRSF8 and TNFRSF1B on 1p36; and TML7 and IF28, and CLDN1 and CLDN16 on 3q28. However, the fact that all these pairs are arranged in tandem suggests that they arose independently from each other. In contrast, two non-tandemly arranged gene pairs were observed in the 1p36 region; i.e., DFFA and DFFB, and TNFRSF9 and TNFRSF25. The occurrence of these latter genes supports the hypothesis that they arose simultaneously along with the UII and SS2/CST genes.
The major difference between the somatostatin genes and the UII genes concerns their exon–intron organization. Indeed, in almost all of the species investigated so far, the SS1 and SS2/CST genes possess only one intron, whereas the UII and URP genes possess three and four introns, respectively. However, although the structure of genes belonging to a same family is often conserved, various mechanisms, such as retrotransposition or reverse splicing (42), can modify the number of introns. One such mechanism may have affected the proto-SS1 gene or the proto-UII gene after duplication. As a matter of fact, according to ensembl resources, the chicken SS1 gene possesses three introns compared with one intron in the other species.
Several observations support the notion that the somatostatin-related genes and the UII-related genes belong to the same superfamily. (i) All four peptides possess a disulfide bridge and share, in their cyclic region, the motif Phe-Trp-Lys that is essential for their biological activity (Fig. 1) (17–19, 43, 44). (ii) The general organization of their precursors is identical, the sequence of the four peptides being located at the C-terminal extremity of the protein. The fact that prepro-UII and prepro-SS1 do not exhibit appreciable sequence identity, which has been regarded as a token that the two genes are not derived from a common precursor (3, 8), should be analyzed with a degree of caution. As a matter of fact, the percentage of identity between prepro-SS1 and prepro-SS2/CST (14%), two proteins that are clearly related (see above), is almost identical to the percentage of identity between prepro-SS1 and prepro-UII (13%). (iii) The UII receptor UTR shares high sequence identity with the somatostatin receptors (45), and the UTR gene is physically linked with the gene encoding one of the somatostatin receptors, SST3, at 17q23, suggesting that these two receptors arose by local duplication. These observations provide strong evidence for co-evolution of the somatostatin/UII family of peptides and their cognate receptors SSTs/UTR, consistent with the notion that related G protein-coupled receptors, such as NPY receptors, VIP/PACAP receptors, and opiate receptors, are usually activated by paralogous peptides (46–48).
In conclusion, the present study has shown that somatostatin- and UII-related peptide genes belong to the same superfamily. The physical linkage of the SS1 and URP genes on the one hand and the SS2/CST and UII genes on the other hand indicates that local duplication of an ancestral gene occurred before segmental duplication of the chromosome region (Fig. 5). The local duplication must have happened early during vertebrate evolution because both somatostatin and UII are present in lampreys (49–51), whereas the segmental duplication may correspond to one of the whole-genome duplications (39).
Molecular Cloning.
Total RNAs from zebrafish caudal spinal cord and chicken spinal cord were extracted by the acid guanidinium thiocyanate-phenol-chloroform procedure, using Tri reagent (Sigma). Poly(A+)RNAs were purified from total RNAs with the PolyA Tract mRNA Isolation System III,IV (Promega). 5′-RACE-ready and 3′-RACE-ready cDNAs were both constructed from 1 μg of poly(A+) RNA by using the SMART RACE cDNA amplification kit (Clontech). The 5′ end of two zebrafish UII cDNAs and one chicken UII cDNA was amplified by PCR using a degenerate primer (Uro N; Table 2) designed from the conserved cyclic region of UII (D/ECWKYCV). The 5′-sequence information obtained from the PCR products were then used to design novel specific primers (UII ZB alpha ext 5′, UII ZB Prox, and UII Pou Prox 5′, respectively; Table 2) to perform 3′ RACE reactions. Partial sequences of chicken URP were first identified in the GenBank EST division (accession nos. BU232439 and BU222609). Two novel specific primers (Uro Neo Pou For and Uro Neo Pou Rev; Table 2) were designed to obtain the full-length URP cDNA by RACE, as indicated above.
Table 2.
Sequences of the oligonucleotides used for PCR amplifications
| Primer | Sequence 5′ → 3′ |
|---|---|
| Uro N | ACR CAR TAY TTC CAR AAR CAN TC |
| UII ZB alpha ext 5′ | GTT TGT GTT CTG CTC TGT GCT C |
| UII ZB Prox | CTC CTG CTC TTT GCT GCT CCT TAC |
| UII Pou Prox 5′ | GAA TTA CGG GCG TTG ACA AGA C |
| Uro Neo Pou For | TAA GAA GTT TGC ATG GAG GAC AC |
| Uro Neo Pou Rev | CAG ATG CAG TAT TTC CAA AAG |
| D. rerio Uro II alpha F11 | TAT CCG ACC TCA CTG ATG TCC TGC |
| D. rerio Uro II alpha B11 | TTC TGC TCC CCA AAA GAC CAC TGG |
| D. rerio Uro II beta F6 | AGT TTC TCG GAG CAG GCG TAT C |
| D. rerio Uro II beta B1 | TTT AGG TCT GTC TCG GGC ATT G |
| Chicken URP Forward | ATG TTC CCC AGC TTC ACT TG |
| Chicken URP Reverse | TTG CAC CCA GAC TAC CTT CC |
| Chicken SS1 Forward | GCC AAG CCA GAC AGA AAA TG |
| Chicken SS1 Reverse | CAG GAT GTG AAA GTT TTC CAG AAG |
Phylogenetic Analysis.
Amino acid sequences of all of the UII and URP precursors currently known in vertebrates were aligned by using clustal x and manually optimized by using the seaview program (52). Tree construction and bootstrap analysis were carried out with the philo_win program (52). Distances were calculated according to ref. 53.
Linkage Analysis by Radiation Hybrid Mapping.
To locate the two UII genes to zebrafish linkage groups, RH mapping was performed by using the mouse/zebrafish RH DNA panel LN54 (54, 55), as reported in ref. 12. DNA (100 ng) from each of the 93 zebrafish X mouse RH was amplified by using a pair of gene-specific primers that amplified part of the 3′-UTR sequence of the two genes (Uro II alpha F11 and B11, and Uro II beta F6 and B1, respectively; Table 2). The reaction mixtures contained 1× PCR buffer, 1.5 mM MgCl2, 0.25 μM each forward and reverse primer, 0.2 mM each dNTP, and 1 unit of TaqDNA polymerase. The PCR templates for the controls were 100 ng of DNA from the two parental cell lines. After an initial denaturation at 94°C for 4 min, the PCR was subjected to 32 cycles of amplification for 30 sec at the appropriate annealing temperature for a given primer set (Table 2), 30 sec at 72°C, 30 sec at 94°C, and a final extension at 72°C for 7 min. The entire reaction (20 μl) was electrophoresed on a 1.5% (wt/vol) agarose gel.
The hamster/chicken RH DNA panel ChickRH6 (56) was used to map the chicken URP and SS1 genes. Chicken DNA was used as a positive control and hamster DNA and Tris-EDTA buffer as a negative controls. PCR amplifications were carried out for each gene in 15 μl containing 25 ng of hybrid DNA, 2 mM MgCl2, 0.3 units of TaqDNA polymerase (Life Technologies), 200 μM each dNTP, 0.2 μM each primer (Table 2), and 1× loading buffer, composed of 350 mM sucrose and 0.2 mM cresol. Amplifications were performed as follows: a first 10-min denaturation at 94°C was followed by 35 cycles each of denaturation at 94°C for 30 sec, annealing at 56°C (for URP) or 60°C (for SS1) for 30 sec, and elongation at 72°C for 30 sec. PCR products were analyzed on 2% agarose gels, electrophoresed in 1× TBE buffer, and visualized by ethidium bromide staining.
The two RH panels were scored based on the absence (0) or presence (1) of the expected DNA fragments, or an ambiguous result (2) to generate the radiation hybrid vectors. The precise chromosomal assignment of both the zebrafish and chicken genes was checked by using ensembl resources.
Search for Duplicate Gene Pairs on Human 1p36 and 3q28 Regions.
To search duplicate gene pairs in the vicinity of the human UII- and somatostatin-related peptide-encoding genes, all of the protein sequences located in the 1p36 and 3q28 regions were collected. Specifically, the sequences were selected from two segments of ≈10 Mb each around the UII and somatostatin genes. Two distinct database were thus created, containing 78 and 29 sequences, respectively. Each database was first used for a blastp search over the other, and then, all sequences from each database were individually used for a blastp search over the whole corresponding database. A computational program was designed to perform the latter search automatically. Only the sequences exhibiting the best scores (E-value ≤ 10−4) were retained, except for each query sequence. All putative duplicate gene pair sequences were aligned by using clustal x, to assert their relationships. The orthologs of all these sequences were searched in several other species such as mouse, rat, chicken, zebrafish, and Tetraodon, and their chromosomal localization was determined by using ensembl resources.
Acknowledgments
We thank Dr. Philippe Vernier (Institut Alfred Fessard, Gif-sur-Yvette, France), Dr. Bruno Quérat (Muséum National d’Histoire Naturelle, Paris), Dr. Hugues Roest-Crollius (Ecole Normale Supérieure, Paris), and Dr. Michele Trabucchi (Howard Hughes Medical Institute, La Jolla, CA) for helpful discussions; Benoît Bély, Arnaud Lefebvre, Dr. Thierry Lecroq, and Dr. Joël Alexandre (Atelier de Biologie, Informatique, Statistiques et Sociolinquistique, University of Rouen, Mont-Saint-Aignan, France) for their contribution in computational analysis; and Marie Mille, Roxane Piblinger, and Romain Vauchelles for their help in molecular cloning experiments. This work was supported by Institut National de la Santé et de la Recherche Médicale Unité 413 and the Conseil Régional de Haute-Normandie.
Glossary
Abbreviations:
- CST
cortistatin
- RH
radiation hybrid
- SS1
somatostatin 1
- SS2
somatostatin 2
- UII
urotensin II
- URP
urotensin II-related peptide.
Footnotes
Conflict of interest statement: No conflicts declared.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database [accession nos. NM_212848 (prepro-urotensin II-α, zebrafish); NM_205591 (prepro-urotensin II-β, zebrafish); NM_206990 (prepro-urotensin II, chicken); and NM_206989 (prepro-urotensin II-related peptide, chicken)].
A measure of the frequency of chromosome breakage between DNA markers in radiation-reduced somatic cell hybrids. One centiRay is equivalent to a 1% probability that a chromosome break (double-stranded break for diploid hybrid panels) has occurred between two markers in a given hybrid cell line.
References
- 1.Pearson D., Shively J. E., Clark B. R., Geschwind I. I., Barkley M., Nishioka R. S., Bern H. A. Proc. Natl. Acad. Sci. USA. 1980;77:5021–5024. doi: 10.1073/pnas.77.8.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conlon J. M., O’Harte F., Smith D. D., Tonon M. C., Vaudry H. Biochem. Biophys. Res. Commun. 1992;188:578–583. doi: 10.1016/0006-291x(92)91095-8. [DOI] [PubMed] [Google Scholar]
- 3.Conlon J. M., Tostivint H., Vaudry H. Regul. Pept. 1997;69:95–103. doi: 10.1016/s0167-0115(97)02135-6. [DOI] [PubMed] [Google Scholar]
- 4.Coulouarn Y., Lihrmann I., Jegou S., Anouar Y., Tostivint H., Beauvillain J. C., Conlon J. M., Bern H. A., Vaudry H. Proc. Natl. Acad. Sci. USA. 1998;95:15803–15808. doi: 10.1073/pnas.95.26.15803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coulouarn Y., Jégou S., Tostivint H., Vaudry H., Lihrmann I. FEBS Lett. 1999;457:28–32. doi: 10.1016/s0014-5793(99)01003-0. [DOI] [PubMed] [Google Scholar]
- 6.Sugo T., Murakami Y., Shimomura Y., Harada M., Abe M., Ishibashi Y., Kitada C., Miyajima N., Suzuki N., Mori M., Fujino M. Biochem. Biophys. Res. Commun. 2003;310:860–868. doi: 10.1016/j.bbrc.2003.09.102. [DOI] [PubMed] [Google Scholar]
- 7.Conlon J. M., Yano K., Waugh D., Hazon N. J. Exp. Zool. 1996;275:226–238. [PubMed] [Google Scholar]
- 8.Ohsako S., Ishida I., Ichikawa T., Deguchi T. J. Neurosci. 1986;6:2730–2735. doi: 10.1523/JNEUROSCI.06-09-02730.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin X., Otto C. J., Cardenas R., Peter R. E. Can. J. Physiol. Pharmacol. 2000;78:1053–1066. [PubMed] [Google Scholar]
- 10.Spier A. D., de Lecea L. Brain Res. Rev. 2000;33:228–241. doi: 10.1016/s0165-0173(00)00031-x. [DOI] [PubMed] [Google Scholar]
- 11.Tostivint H., Trabucchi M., Vallarino M., Conlon J. M., Lihrmann I., Vaudry H. In: Somatostatin. Srikant C. B., editor. Dordrecht, The Netherlands: Kluwer; 2004. pp. 47–64. [Google Scholar]
- 12.Tostivint H., Joly L., Lihrmann I., Ekker M., Vaudry H. J. Mol. Endocrinol. 2004;33:R1–R8. doi: 10.1677/jme.1.01602. [DOI] [PubMed] [Google Scholar]
- 13.Ames R. S., Sarau H. M., Chambers J. K., Willette R. N., Aiyar N. V., Romanic A. M., Louden C. S., Foley J. J., Sauermelch C. F., Coatney R. W., et al. Nature. 1999;401:282–286. doi: 10.1038/45809. [DOI] [PubMed] [Google Scholar]
- 14.Nothacker H. P., Wang Z., McNeill A. M., Saito Y., Merten S., O’Dowd B., Duckles S. P., Civelli O. Nat. Cell Biol. 1999;1:383–385. doi: 10.1038/14081. [DOI] [PubMed] [Google Scholar]
- 15.Liu Q., Pong S. S., Zeng Z., Zhang Q., Howard A. D., Williams D. L., Jr., Davidoff M., Wang R., Austin C. P., McDonald T. P., et al. Biochem. Biophys. Res. Commun. 1999;266:174–178. doi: 10.1006/bbrc.1999.1796. [DOI] [PubMed] [Google Scholar]
- 16.Mori M., Sugo T., Abe M., Shimomura Y., Kurihara M., Kitada C., Kikuchi K., Shintani Y., Kurokawa T., Onda H., et al. Biochem. Biophys. Res. Commun. 1999;265:123–129. doi: 10.1006/bbrc.1999.1640. [DOI] [PubMed] [Google Scholar]
- 17.Flohr S., Kurz M., Kostenis E., Brkovich A., Fournier A., Klabunde T. J. Med. Chem. 2002;45:1799–1805. doi: 10.1021/jm0111043. [DOI] [PubMed] [Google Scholar]
- 18.Labarrère P., Chatenet D., Leprince J., Marionneau C., Loirand G., Tonon M. C., Dubessy C., Scalbert E., Pfeiffer B., Renard P., et al. J. Enzyme Inhib. Med. Chem. 2003;18:77–88. doi: 10.1080/1475636031000093507. [DOI] [PubMed] [Google Scholar]
- 19.Chatenet D., Dubessy C., Leprince J., Boularan C., Carlier L., Segalas-Milazzo I., Guilhaudis L., Oulyadi H., Davoust D., Scalbert E., et al. Peptides. 2004;25:1819–1830. doi: 10.1016/j.peptides.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 20.Olias G., Viollet C., Kusserow H., Epelbaum J., Meyerhof W. J. Neurochem. 2004;89:1057–1091. doi: 10.1111/j.1471-4159.2004.02402.x. [DOI] [PubMed] [Google Scholar]
- 21.Robas N., Mead E., Fidock M. J. Biol. Chem. 2003;278:44400–44404. doi: 10.1074/jbc.M302456200. [DOI] [PubMed] [Google Scholar]
- 22.Vaudry H., Chartrel N., Conlon J. M. Biochem. Biophys. Res. Commun. 1992;188:477–482. doi: 10.1016/0006-291x(92)92409-q. [DOI] [PubMed] [Google Scholar]
- 23.Nishii M., Moverus B., Bukovskaya O. S., Takahashi A., Kawauchi H. Gen. Comp. Endocrinol. 1995;99:6–12. doi: 10.1006/gcen.1995.1078. [DOI] [PubMed] [Google Scholar]
- 24.Tostivint H., Lihrmann I., Bucharles C., Vieau D., Coulouarn Y., Fournier A., Conlon J. M., Vaudry H. Proc. Natl. Acad. Sci. USA. 1996;93:12605–12610. doi: 10.1073/pnas.93.22.12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Lecea L., Criado J. R., Prospero-Garcia O., Gautvik K. M., Schweitzer P., Danielson P. E., Dunlop C. L. M., Siggins G. R., Henriksen S. J., Sutcliffe J. G. Nature. 1996;381:242–245. doi: 10.1038/381242a0. [DOI] [PubMed] [Google Scholar]
- 26.de Lecea L., Ruiz-Lozano P., Danielson P. E., Peelle-Kirley J., Foye P. E., Frankel W. N., Sutcliffe J. G. Genomics. 1997;42:499–506. doi: 10.1006/geno.1997.4763. [DOI] [PubMed] [Google Scholar]
- 27.Fukusumi S., Kitada C., Takekawa S., Kizawa H., Sakamoto J., Miyamoto M., Hinuma S., Kitano K., Fujino M. Biochem. Biophys. Res. Commun. 1997;232:157–163. doi: 10.1006/bbrc.1997.6252. [DOI] [PubMed] [Google Scholar]
- 28.Lin X., Otto C. J., Peter R. E. Endocrinology. 1999;140:2089–2099. doi: 10.1210/endo.140.5.6706. [DOI] [PubMed] [Google Scholar]
- 29.Trabucchi M., Tostivint H., Lihrmann I., Jégou S., Vallarino M., Vaudry H. J. Comp. Neurol. 1999;410:643–652. doi: 10.1002/(sici)1096-9861(19990809)410:4<643::aid-cne10>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 30.Trabucchi M., Tostivint H., Lihrmann I., Sollars C., Vallarino M., Dores R. M., Vaudry H. J. Comp. Neurol. 2002;443:332–345. doi: 10.1002/cne.10126. [DOI] [PubMed] [Google Scholar]
- 31.Trabucchi M., Tostivint H., Lihrmann I., Bläser S., Vallarino M., Vaudry H. J. Comp. Neurol. 2003;461:441–451. doi: 10.1002/cne.10690. [DOI] [PubMed] [Google Scholar]
- 32.Jennen D. G., Crooijmans R. P., Morisson M., Grootemaat A. E., Van Der Poel J. J., Vignal A., Groenen M. A. Anim. Genet. 2004;35:63–65. doi: 10.1111/j.1365-2052.2003.01082.x. [DOI] [PubMed] [Google Scholar]
- 33.Morisson M., Jiguet-Jiglaire C., Leroux S., Faraut T., Bardes S., Feve K., Genet C., Pitel F., Milan D., Vignal A. Mamm. Genome. 2004;5:732–739. doi: 10.1007/s00335-004-3003-y. [DOI] [PubMed] [Google Scholar]
- 34.Pitel F., Abasht B., Morisson M., Crooijmans R. P., Vignoles F., Leroux S., Fève K., Bardes S., Milan D., Lagarrigue S., et al. BMC Genomics. 2004;5:66. doi: 10.1186/1471-2164-5-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rabie T. S., Crooijmans R. P., Morisson M., Andryszkiewicz J., van der Poel J. J., Vignal A., Groenen M. A. Mamm. Genome. 2004;15:560–569. doi: 10.1007/s00335-004-2362-8. [DOI] [PubMed] [Google Scholar]
- 36.Leroux S., Dottax M., Bardes S., Vignoles F., Fève K., Pitel F., Morisson M., Vignal A. BMC Genomics. 2005;6:12. doi: 10.1186/1471-2164-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morisson M., Leroux S., Jiguet-Jiglaire C., Assaf S., Pitel F., Lagarrigue S, Bardes S, Feve K., Faraut T., Milan D., Vignal A. Genet. Sel. Evol. 2005;37:229–251. doi: 10.1186/1297-9686-37-3-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amores A., Force A., Joly L., Yan Y. L., Amemiya C., Fritz A., Ho R., Langeland J., Prince V., Wang Y. L., et al. Science. 1998;282:1711–1714. doi: 10.1126/science.282.5394.1711. [DOI] [PubMed] [Google Scholar]
- 39.Lundin L. G., Larhammar D., Hallbook F. J. Struct. Funct. Genomics. 2003;3:53–63. [PubMed] [Google Scholar]
- 40.Popovici C., Leveugle M., Birnbaum D., Coulier F. Biochem. Biophys. Res. Commun. 2005;288:362–370. doi: 10.1006/bbrc.2001.5794. [DOI] [PubMed] [Google Scholar]
- 41.Lynch M., Conery J. S. Science. 2000;290:1151–1155. doi: 10.1126/science.290.5494.1151. [DOI] [PubMed] [Google Scholar]
- 42.Dibb N. J., Newman A. J. EMBO J. 1989;8:2015–2021. doi: 10.1002/j.1460-2075.1989.tb03609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Veber D. F., Holly F. W., Nutt R. F., Bergstrand S. J., Brady S. F., Hirschmann R., Glitzer M. S., Saperstein R. Nature. 1979;280:512–514. doi: 10.1038/280512a0. [DOI] [PubMed] [Google Scholar]
- 44.Criado J. R., Li H., Jiang X., Spina M., Huitron-Resendiz S., Liapakis G., Calbet M., Siehler S., Henriksen S. J., Koob G., et al. J. Neurosci. Res. 1999;56:611–619. doi: 10.1002/(SICI)1097-4547(19990615)56:6<611::AID-JNR7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 45.Marchese A., Heiber M., Nguyen T., Heig H. H., Saldivia V. R., Cheng R., Murphy P. M., Tsui L. C., Shi X., Gregor P., et al. Genomics. 1995;29:335–344. doi: 10.1006/geno.1995.9996. [DOI] [PubMed] [Google Scholar]
- 46.Cerda-Reverter J. M., Larhammar D. Biochem. Cell Biol. 2000;78:371–392. [PubMed] [Google Scholar]
- 47.Sherwood N. M., Krueckl S. L., McRory J. E. Endocr. Rev. 2000;21:619–670. doi: 10.1210/edrv.21.6.0414. [DOI] [PubMed] [Google Scholar]
- 48.Dores R. M., Lecaudé S., Bauer D., Danielson P. B. Mass Spectrom. Rev. 2002;21:220–243. doi: 10.1002/mas.10029. [DOI] [PubMed] [Google Scholar]
- 49.Andrews P. C., Pollock H. G., Elliott W. M., Youson J. H., Plisetskaya E. M. J. Biol. Chem. 1988;263:15809–15814. [PubMed] [Google Scholar]
- 50.Conlon J. M., Bondereva V., Rusakov Y., Plisetskaya E. M., Mynarcik D. C., Whittaker J. Gen. Comp. Endocrinol. 1995;100:96–105. doi: 10.1006/gcen.1995.1138. [DOI] [PubMed] [Google Scholar]
- 51.Conlon J. M., Nielsen P. F., Youson J. H., Potter I. C. Gen. Comp. Endocrinol. 1995;100:413–422. doi: 10.1006/gcen.1995.1172. [DOI] [PubMed] [Google Scholar]
- 52.Galtier N., Gouy M., Gautier C. Comput. Appl. Biosci. 1996;12:543–548. doi: 10.1093/bioinformatics/12.6.543. [DOI] [PubMed] [Google Scholar]
- 53.Saitou N., Nei M. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 54.Hukriede N. A., Joly L., Tsang M., Miles J., Tellis P., Epstein J. A., Barbazuk W. B., Li F. N., Paw B., Postlethwait J. H., et al. Proc. Natl. Acad. Sci. USA. 1999;96:9745–9750. doi: 10.1073/pnas.96.17.9745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hukriede N., Fisher D., Epstein J., Joly L., Tellis P., Zhou Y., Barbazuk B., Cox K., Fenton-Noriega L., Hersey C., et al. Genome Res. 2001;11:2127–2132. doi: 10.1101/gr.210601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morisson M., Lemiere A., Bosc S., Galan M., Plisson-Petit F., Pinton P., Delcros C., Feve K., Pitel F., Fillon V., et al. Genet. Sel. Evol. 2002;34:521–533. doi: 10.1186/1297-9686-34-4-521. [DOI] [PMC free article] [PubMed] [Google Scholar]