Abstract
Engagement of Toll-like receptors (TLRs) on macrophages leads to activation of the mitogen-activated protein kinases (MAPKs), which contribute to innate immune responses. MAPK activity is regulated negatively by MAPK phosphatases (MKPs). MKP-1, the founding member of this family of dual-specificity phosphatases, has been implicated in regulating lipopolysaccharide (LPS) responses, but its role in TLR-mediated immune responses in vivo has not been defined. Here, we show that mice deficient in MKP-1 were highly susceptible to endotoxic shock in vivo, associated with enhanced production of proinflammatory cytokines TNF-α and IL-6 and an anti-inflammatory cytokine, IL-10. We further examined the regulation and function of MKP-1 in macrophages, a major cell type involved in endotoxic shock. MKP-1 was transiently induced by TLR stimulation through pathways mediated by both myeloid differentiation factor 88 (MyD88) and TIR domain-containing adaptor inducing IFN-β (TRIF). MKP-1 deficiency led to sustained activation of p38 MAPK and c-Jun N-terminal kinase (JNK) in LPS-treated macrophages. In response to TLR signals, MKP-1-deficient macrophages produced 5- to 10-fold higher IL-10, which could be blocked by a p38 MAPK inhibitor. Thus, p38 MAPK plays a critical role in mediating IL-10 synthesis in TLR signaling. TNF-α was found to be more abundant in MKP-1-deficient macrophages within 2 hours of TLR stimulation, but its production was rapidly down-regulated by IL-10. Our studies demonstrate that MKP-1 attenuates the activities of p38 MAPK and JNK to regulate both pro- and anti-inflammatory cytokines in TLR signaling. These results highlight the complex mechanisms by which the MAPKs regulate innate immunity.
Keywords: IL-10, innate immunity, phosphatase, Toll-like receptor signaling, TNF-α
The innate immune system is the first line of defense against invading pathogens through an evolutionarily conserved system of pattern recognition (1). Innate immune cells, including macrophages and dendritic cells, express a series of receptors known as Toll-like receptors (TLRs), which bind to highly conserved sequences expressed by microorganisms (2, 3). TLR4, the first cloned mammalian TLR, recognizes lipopolysaccharide (LPS) or endotoxin, a major component of Gram-negative bacterial outer membranes. TLR2, TLR3, TLR5, and TLR9 are activated by peptidoglycan (PGN), double-stranded RNA, flagellin, and bacterial CpG DNA, respectively (2). The engagement of TLR by these ligands results in a potent inflammatory response characterized by the release of proinflammatory cytokines, including TNF-α, IL-1β, IL-6, IL-12, and IL-18. Activation of the innate immune system is important for subsequent activation of lymphocytes and other cell types and clearance of infectious organisms. However, exuberant production of proinflammatory cytokines leads to severe immunopathology such as endotoxic shock (4). To prevent deleterious TLR activation, a number of signaling mechanisms are evoked. These mechanisms include the down-regulation of surface TLR expression, transcriptional induction of negative regulators such as IL-1 receptor-associated kinase (IRAK-M), suppressor of cytokine signaling 1 (SOCS1), and SH2-containing inositol phosphatase (SHIP), and production of anti-inflammatory cytokines, mainly IL-10 and TGF-β (5). Compared with the release of proinflammatory mediators, which occurs rapidly after TLR stimulation, production of these negative regulators is considerably slower, thus assuring proper regulation of the pro- and anti-inflammatory balance at the appropriate time (5).
TLR engagement results in activation of the mitogen-activated protein kinases (MAPKs), which, together with the NF-κB pathway, transduce extracellular signals to cellular responses (6). Activation of the MAPKs is mediated by a core kinase module comprised of MAP3K, MAP2K, and MAPK through sequential protein phosphorylation (6). Negative regulation of MAPK activity is effected primarily by MAPK phosphatases (MKPs), a group of 11 dual-specificity phosphatases that dephosphorylate the MAPKs on their regulatory threonine and tyrosine residues (7). MKP-1, the founding member of this family, was initially cloned as an early response gene induced by growth factors (8). MKP-1 localizes to the nucleus through its N terminus (9) and preferentially dephosphorylates activated p38 MAPK and c-Jun N-terminal kinase (JNK) relative to extracellular signal-regulated kinase (ERK) in vitro (10). Consistently, by using MKP-1-deficient cells, we and others have shown recently that MKP-1 deficiency results in enhanced p38 MAPK and JNK activation in response to serum and stress (11–13). Additionally, MKP-1-deficient fibroblasts exhibit enhanced sensitivity to apoptosis, suggesting an important role for MKP-1 in cell survival signaling (11). MKP-1 also has been shown to regulate TNF-α and IL-6 production after LPS treatment (12–15). However, the role of MKP-1 in TLR-mediated immune responses in vivo has not been defined. Here, by using MKP-1-deficient mice, we show that MKP-1 plays a nonredundant role in negatively regulating endotoxic shock responses. Moreover, lack of MKP-1 leads to dysregulated production of both proinflammatory and anti-inflammatory cytokines after TLR activation. These results provide insights into the complex mechanisms involved in the regulation of innate immunity by the MAPKs.
Results
MKP-1−/− Mice Are Hyperresponsive to Endotoxic Shock.
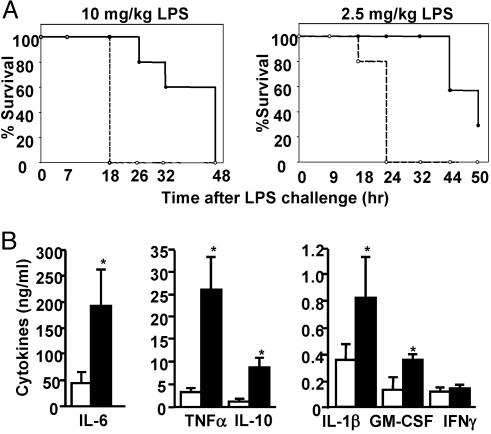
The reaction to bacterial LPS is a well characterized innate immune response and leads to endotoxic shock (4). To investigate the role of MKP-1 in innate immunity, we examined endotoxic shock induced in MKP-1−/− mice. Age- and sex-matched wild-type (WT) and MKP-1−/− mice were challenged with two different doses of LPS, and survival of mice was monitored up to 50 h (Fig. 1A). When LPS was administered at 10 mg/kg, the WT mice died between 24 and 48 h, whereas all of the MKP-1−/− mice died within 18 h. At the lower dose of LPS (2.5 mg/kg), 70% of the WT mice died at the end of the experiment. Again, MKP-1−/− mice died more rapidly, and all of the mice died within 24 h. Therefore, MKP-1−/− mice are more susceptible to LPS-induced lethality.
Fig. 1.
MKP-1−/− mice are hyperresponsive to endotoxic shock in vivo, associated with enhanced production of TNF-α, IL-6, and IL-10. (A) MKP-1+/+ (solid line) and MKP-1−/− (dotted line) mice were injected i.p. with LPS (10 mg/kg, Left, n ≥ 4; 2.5 mg/kg, Right, n ≥5), and lethality was monitored for up to 50 h. Both treatments resulted in significant differences between the two groups of mice (P < 0.005 and P < 0.001 for 10 and 2.5 mg/kg LPS challenges, respectively). (B) Serum from MKP-1+/+ (open bars) and MKP-1−/− (filled bars) mice was collected 3 h after 10 mg/kg LPS challenge, and cytokines were measured by Bio-plex assays or ELISA (IL-6). Data show mean ± SD for four or five mice from each group (∗, P < 0.05 determined using Student’s t test).
Endotoxic shock is mediated by an overproduction of proinflammatory cytokines, including TNF-α, IL-6, and IL-1β (4). To examine whether MKP-1 controls cytokine release in response to LPS stimulation in vivo, we measured levels of various cytokines in the serum from mice after 3 h of LPS challenge (Fig. 1B). Compared with WT mice, MKP-1−/− mice showed 8- and 4-fold higher levels of TNF-α and IL-6, respectively. Although less pronounced, we also found significant increases in the levels of IL-1β and granulocyte/macrophage colony-stimulating factor (GM-CSF) (Fig. 1B). Notably, IL-10, an anti-inflammatory cytokine, was elevated in MKP-1−/− mice by 8-fold. These results demonstrate that MKP-1 plays an important role in negatively regulating LPS-induced cytokine production in vivo.
TLR Stimulation Induces MKP-1 Expression Through Myeloid Differentiation Factor 88 (MyD88) and TIR Domain-Containing Adaptor Inducing IFN-β (TRIF)-Dependent Pathways.
To understand the cellular mechanisms by which MKP-1 controls endotoxic shock, we first examined whether development of immune cells was affected in MKP-1−/− mice. Flow-cytometry analysis of lymphoid and myeloid markers such as CD4, CD8, CD11b, and CD11c in the thymus, spleen, and lymph nodes of MKP-1−/− mice did not reveal major abnormalities in the development of the immune system (see Fig. 7, which is published as supporting information on the PNAS web site). In addition, expression of B220, Gr1, F4/80, CD62L, CD44, and CD25 were comparable between WT and MKP-1−/− mice (data not shown).
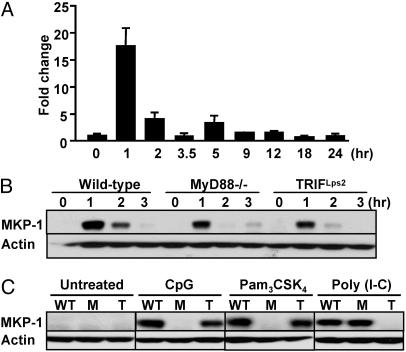
Because macrophages are robust producers of cytokines in response to endotoxic shock (4), we analyzed MKP-1 RNA and protein expression in response to LPS stimulation using bone marrow-derived macrophages (BMDM) from WT mice (Fig. 2A). Quantitative real-time PCR analysis showed that there was ≈15-fold induction of MKP-1 RNA expression after 1 h of exposure to LPS. MKP-1 RNA was down-regulated rapidly and returned to basal levels at 3–4 h after LPS treatment. MKP-1 protein was regulated similarly, showing a pattern of strong induction and rapid down-regulation (Fig. 2B).
Fig. 2.
TLR stimulation induces MKP-1 expression in macrophages. (A) BMDM from WT mice were treated with 10 ng/ml LPS for the indicated time points. RNA was isolated and subjected to quantitative RT-PCR analysis of MKP-1 levels. (B) BMDM from WT, MyD88−/−, and TRIFLps2 mice were treated with 10 ng/ml LPS, and whole-cell lysates were subjected to Western blot analysis using anti-MKP-1 and anti-Actin Abs. (C) BMDM from WT, MyD88−/− (M), and TRIFLps2 (T) mice were stimulated with 1 μM CpG, 50 μg/ml poly(I-C), or 200 ng/ml Pam3CSK4 for 1 h, and MKP-1 protein expression was examined as above.
LPS challenge leads to the activation of two pathways, which use MyD88 and TRIF to transduce signals that regulate discrete cellular responses (2, 3). To determine the upstream signals responsible for the induction of MKP-1, we examined the expression of MKP-1 in mice lacking MyD88 (16) and in mice with a null mutation of TRIF (TRIFLps2 mice) (17). After LPS treatment, the early induction of MKP-1 expression at 1 h was reduced in MyD88−/− and TRIFLps2 mice, as compared with WT mice. By 2 h after LPS stimulation, MKP-1 expression was more substantially inhibited in both MyD88−/− and TRIFLps2 mice. Therefore, both MyD88 and TRIF are required for optimal LPS-induced MKP-1 expression (Fig. 2B). We further examined whether MKP-1 could be induced by other TLR stimuli. WT macrophages were treated with various TLR agonists, including CpG, poly(I-C), and Pam3CSK4, which activate TLR9, TLR3, and TLR2, respectively. MKP-1 was induced by all of these stimuli (Fig. 2C). Among these TLRs, TLR9 and TLR2 use a signaling pathway dependent on MyD88, but not TRIF, whereas TLR3 signals only through TRIF (2, 3). In response to CpG (TLR9) and Pam3CSK4 (TLR2), MKP-1 induction was completely ablated in MyD88−/− mice but was normal in TRIFLps2 mice. Conversely, loss of TRIF, but not of MyD88 function, eliminated MKP-1 expression induced by poly(I-C) (TLR3; Fig. 2C). Together, these results demonstrate that MKP-1 is induced through MyD88 and TRIF-dependent pathways in response to various TLR ligands.
MKP-1 Negatively Regulates LPS-Induced p38 MAPK and JNK Activation and AP-1 DNA-Binding Activity.
TLR stimulation activates all three major subgroups of MAPKs: JNK, p38 MAPK, and ERK (2, 3), as well as MKP-1 (Fig. 2). Therefore, to determine whether MKP-1 plays an essential role in MAPK inactivation after TLR stimulation, we examined the activation of the MAPKs in response to TLR signaling in MKP-1−/− macrophages. WT and MKP-1−/− macrophages were treated with LPS for various times, and activation of the MAPKs was examined by using antibodies (Abs) that recognize the activated forms of these kinases (Fig. 3A). In response to LPS, p38 MAPK, JNK, and ERK activities were rapidly induced in both WT and MKP-1−/− cells. The initial phase of MAPK activation immediately after LPS stimulation (20 min) was comparable between WT and MKP-1−/− cells (Fig. 3A). Activities of p38 MAPK and JNK subsided in WT cells between 1 and 2 h after stimulation, whereas MKP-1−/− macrophages showed sustained p38 MAPK and JNK activation. In contrast to p38 MAPK and JNK, the activity of ERK was minimally affected in MKP-1−/− cells (Fig. 3A). Thus, MKP-1 deficiency results in sustained p38 MAPK and JNK activation, demonstrating a critical role for MKP-1 for the inactivation of p38 MAPK and JNK after TLR stimulation. Because AP-1 transcription factors are known targets of activated MAPKs, we examined whether MKP-1 deficiency affects AP-1 activity. As revealed by a gel shift assay using oligonucleotide sequences that contain DNA-binding sites for AP-1, there was a substantial increase of AP-1 activity in MKP-1−/− macrophages after LPS stimulation at 30 and 90 min (Fig. 3B). These findings demonstrate that MKP-1 negatively regulates LPS-induced p38 MAPK and JNK activation and AP-1 binding activity in macrophages.
Fig. 3.
MKP-1−/− macrophages exhibit higher p38 MAPK and JNK activation and increased AP-1 DNA-binding activity. (A) BMDM from MKP-1+/+ and MKP-1−/− mice were stimulated with 10 ng/ml LPS for the indicated time points, and activities of p38 MAPK, JNK, and ERK were examined by Western blot analysis using phosphospecific Abs. The total protein levels of p38 MAPK, JNK, and ERK also were measured. (B) BMDM from MKP-1+/+ and MKP-1−/− mice were stimulated with 10 ng/ml LPS, and AP-1 DNA-binding activity was measured by gel shift assay using AP-1 consensus oligonucleotide.
MKP-1 Differentially Controls TNF-α and IL-10 Expression in Macrophages.
In response to LPS, MKP-1-deficient mice were found to exhibit enhanced cytokine production (Fig. 1). To investigate this finding further, we assessed whether MKP-1 regulates LPS-induced cytokine production in macrophages. TNF-α and IL-10 were chosen for detailed analyses, because these two cytokines were affected to the greatest degree by MKP-1 deficiency in vivo. BMDM from WT and MKP-1−/− mice were treated with LPS. TNF-α and IL-10 in the culture supernatants were measured by ELISA at different time points after LPS treatment (Fig. 4A). We found that MKP-1−/− macrophages produced higher levels of IL-10 at all time points examined, and these levels were 5- to 10-fold higher than those from WT cells after 5 h of LPS challenge. TNF-α levels also were enhanced in MKP-1−/− macrophages at earlier time points, but later on at 12–24 h after LPS stimulation, no consistent differences were observed (Fig. 4A).
Fig. 4.
MKP-1−/− macrophages show altered levels of IL-10 and TNF-α in response to LPS. (A) BMDM from MKP-1+/+ (open bars) and MKP-1−/− (filled bars) mice were stimulated with 10 ng/ml LPS for the indicated time points, and culture supernatants were harvested for ELISA analysis of IL-10 and TNF-α levels. (B) RNA was isolated and subjected to quantitative RT-PCR analysis of IL-10 and TNF-α levels. (C) BMDM from MKP-1+/+ and MKP-1−/− mice were incubated with (+) or without (−) an IL-10 neutralizing Ab (20 μg/ml) for 1 h, and activated with 10 ng/ml LPS for the indicated time points. Expression of TNF-α mRNA was measured by quantitative RT-PCR analysis. Shown is a representative result from three independent experiments. (D) BMDM from MKP-1+/+ (open bars) and MKP-1−/− (filled bars) mice were stimulated with 10 ng/ml LPS, and expression of Bcl-3 was examined by quantitative RT-PCR analysis.
To understand the mechanism underlying the changes in cytokine production, cytokine mRNA levels were determined in macrophages from WT and MKP-1−/− mice after exposure to LPS (Fig. 4B). Compared with ELISA, which measures the accumulative amount of proteins secreted, RNA analysis shows additional kinetic information concerning cytokine expression. In accordance with the ELISA data, IL-10 mRNA was enhanced in MKP-1−/− macrophages, with the greatest difference (>10-fold) observed after 5–12 h of LPS treatment (Fig. 4B). TNF-α RNA showed a distinct pattern of regulation. At early time points after LPS treatment (<2 h) examined, TNF-α RNA up-regulation was more pronounced in MKP-1−/− macrophages than in WT cells, consistent with the cytokine release results measured by ELISA. At later time points (>5 h), however, MKP-1−/− macrophages showed reduced mRNA levels of TNF-α (Fig. 4B). Such temporal regulation of TNF-α expression mediated by MKP-1 was likely responsible for the unaltered levels of TNF-α protein accumulated in the supernatants from MKP-1−/− macrophages as compared with those from WT cells at later time points.
Given the known function of IL-10 in suppressing TNF-α production (18, 19), we hypothesized that the reduced TNF-α expression in MKP-1−/− macrophages at later time points resulted from their responses to IL-10 overproduction by these cells in an autocrine/paracrine fashion. To address this issue, we treated WT and MKP-1−/− macrophages with a neutralizing IL-10 Ab for 1 h before LPS challenge (Fig. 4C). IL-10 neutralization enhanced TNF-α mRNA levels in both WT and MKP-1−/− cells. Importantly, TNF-α mRNA levels at 5 h were largely equivalent between WT and MKP-1−/− macrophages after IL-10 neutralization, suggesting that IL-10 overproduction in the absence of MKP-1 resulted in the down-regulation of TNF-α.
Among many inducible targets of IL-10, Bcl-3 is the only gene that has been genetically defined to mediate the function of IL-10 in suppressing TNF-α biosynthesis (20). We therefore measured whether Bcl-3 was differentially expressed between WT and MKP-1−/− cells (Fig. 4D). Bcl-3 RNA was induced upon LPS challenge in WT macrophages. MKP-1−/− cells showed a higher degree of Bcl-3 induction after 3 h of LPS stimulation (Fig. 4D), coinciding with the timing of enhanced IL-10 production observed in these cells.
Together, these results reveal an important function of MKP-1 in regulating both proinflammatory and anti-inflammatory cytokines in LPS-stimulated macrophages. Moreover, dysregulated IL-10 expression in MKP-1−/− cells affected levels of proinflammatory cytokines at later stages of macrophage activation, suggesting the functional significance of MKP-1-mediated regulation of IL-10.
p38 MAPK Activity Is Crucial for IL-10 Production After TLR Stimulation.
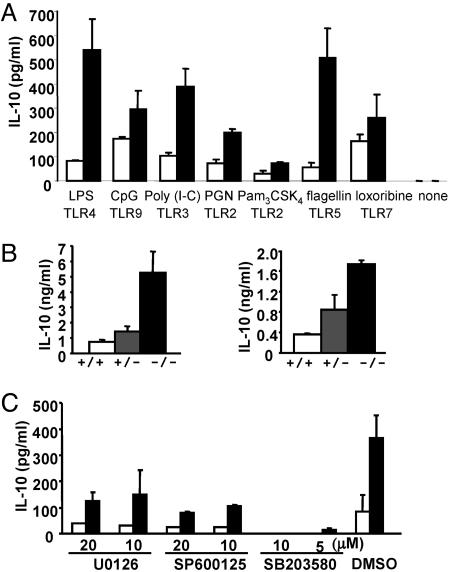
Next, we focused our analyses on the regulation of IL-10 by MKP-1. We first asked whether MKP-1 has a general role in regulating IL-10 production in responses to various TLR agonists. BMDM were treated with various TLR ligands, and the production of IL-10 was measured by ELISA (Fig. 5A). In response to all of the TLR ligands examined, MKP-1−/− macrophages produced higher levels of IL-10 as compared with WT cells. The greatest effects were observed after activation of TLR4 and TLR5, which resulted in >5-fold higher levels of IL-10 in MKP-1−/− macrophages relative to WT cells. We next examined whether MKP-1 showed gene dosage effects on the expression of IL-10. When MKP-1+/− mice were challenged with LPS in vivo, IL-10 levels in the serum from these mice were 2-fold higher than those from WT mice (Fig. 5B). Moreover, BMDM from MKP-1+/− mice produced higher levels of IL-10 after LPS challenge than WT controls (Fig. 5B). These results further support the notion that MKP-1 plays an essential function in the regulation of IL-10.
Fig. 5.
Enhanced IL-10 production in MKP-1−/− macrophages can be blocked by a p38 MAPK inhibitor. (A) BMDM from MKP-1+/+ (open bars) and MKP-1−/− (filled bars) mice were stimulated with 10 ng/ml LPS, 1 μM CpG, 50 μg/ml poly(I-C), 10 μg/ml peptidoglycan (PGN), 200 ng/ml Pam3CSK4, 100 ng/ml flagellin, or 200 μM loxoribine, or left untreated for 12 h. IL-10 production was measured by ELISA. (B Left) Serum from MKP-1+/+ (white bars), MKP-1+/− (gray bars), and MKP-1−/− (black bars) mice (n ≥ 3 for each group) was collected 3 h after LPS challenge, and IL-10 levels were measured by ELISA. (Right) BMDM from MKP-1+/+, MKP-1+/−, and MKP-1−/− mice were stimulated with 10 ng/ml LPS for 12 h, and IL-10 levels were measured by ELISA. (C) BMDM from MKP-1+/+ (open bars) and MKP-1−/− (filled bars) mice were treated with U0126 (ERK inhibitor), SP600125 (JNK inhibitor), SB203580 (p38 MAPK inhibitor), or vehicle alone (DMSO) at the indicated concentrations for 30 min and activated with 10 ng/ml LPS for 12 h. IL-10 levels were measured by ELISA.
Our data show that MKP-1-deficient macrophages exhibit enhanced MAPK activities (Fig. 3A). Therefore, we determined whether the increased MAPK activities were responsible for the enhanced levels of IL-10 observed in MKP-1−/− macrophages. WT and MKP-1−/− macrophages were treated with U0126 (an ERK inhibitor), SP600125 (a JNK inhibitor), or SB203580 (a p38 MAPK inhibitor) for 30 min before they were challenged with LPS (Fig. 5C). Whereas all of the drug inhibitors reduced IL-10 in WT and MKP-1−/− cells, only the p38 MAPK inhibitor completely abrogated the enhanced IL-10 production in MKP-1−/− cells. Similar results were obtained in flagellin-stimulated macrophages (data not shown). Therefore, the activity of p38 MAPK, and to a lesser extent ERK and JNK, play important roles in IL-10 production after TLR stimulation.
Discussion
Two major kinase-mediated signaling pathways are activated after TLR engagement: the MAPK and IκB kinase (IKK) complexes, which transduce various upstream signals to the activation of AP-1 and NF-κB transcription factors, respectively. Whereas the IKK–NF-κB pathway has been extensively studied in TLR signaling, the function and regulation of the MAPKs are less well understood because of the lack of appropriate genetic models. Recently, we have developed and characterized mice lacking MKP-5, which inactivates JNK preferentially, and identified a positive role for the JNK pathway in the regulation of innate immunity (21). In this work, by analyzing immune phenotypes in mice deficient in MKP-1, which regulates both p38 MAPK and JNK activities, we demonstrate that functions of MAPKs in innate immune responses are substantially more complex than previously appreciated. Specifically, we found that mice lacking MKP-1 showed enhanced sensitivity to endotoxic shock associated with hyperproduction of TNF-α and IL-6 in vivo, which is consistent with the role of the MAPKs as positive mediators of proinflammatory cytokines and TLR signaling. However, on closer examination of MKP-1−/− mice, we discovered that MKP-1 also negatively regulates IL-10, a critical anti-inflammatory cytokine in innate immunity. We propose a model in which the MAPKs play reciprocating roles in the temporal regulation of both pro- and anti-inflammatory cytokines (Fig. 6). In the initial phase of macrophage activation, the rapid synthesis of proinflammatory cytokines is partly mediated by MAPKs (6). The second phase of macrophage activation involves the delayed and more gradual production of immunosuppressive IL-10 (18), which is also dependent on the activities of MAPKs, and in particular, p38 MAPK. Our model is further supported by the dynamic expression patterns of MKP-1 after TLR stimulation. The initial induction of MKP-1 serves to restrain excessive production of proinflammatory cytokines, whereas the substantial down-regulation of MKP-1 after 2 h of stimulation allows the remaining active MAPKs to be sufficient for the synthesis of IL-10. By negatively regulating p38 MAPK and JNK activities, MKP-1 controls both pro- and anti-inflammatory mediators of TLR signaling. In a model of acute inflammation, endotoxic shock, it appears that the predominant role of MKP-1 is to suppress proinflammatory cytokines including TNF-α, IL-6, and IL-1β, whose overproduction in MKP-1-deficient mice is a likely cause for the susceptibility of these mice to LPS-induced toxicity. Although administration of IL-10 is effective to rescue mice from endotoxic shock (22, 23), the increased levels of IL-10 in MKP-1−/− mice may not be produced early enough, or in sufficient amounts, to suppress the detrimental effects of proinflammatory cytokines in this model. It will be of interest to examine the role of MKP-1 in chronic disease models that do not involve high levels of TNF-α production.
Fig. 6.
Model of MKP-1-mediated temporal regulation of cytokine production in TLR signaling. Macrophage activation is divided into two phases: an initial phase characterized by rapid production of proinflammatory cytokines such as TNF-α and a second phase that involves the synthesis of IL-10. In the first phase, p38 MAPK and JNK are rapidly activated (≈20 min) and contribute to the expression of TNF-α. This early event is followed by the induction of MKP-1 (≈60 min) mediated by MyD88 and TRIF pathways, which serves as a negative feedback mechanism to down-regulate p38 MAPK and JNK activities and TNF-α production. In the second phase, activities of JNK and p38 MAPK are reduced because of MKP-1 but still higher than basal levels, whereas MKP-1 expression is suppressed to allow the remaining active p38 MAPK and JNK to promote IL-10 expression. IL-10, in turn, further limits TNF-α expression. For simplicity, only pathways relevant to MKP-1 are shown.
Although IL-10 is capable of stimulating proliferation of B cells, mast cells, and thymocytes, it is a potent anti-inflammatory regulator of innate immune responses (19). IL-10 has recently been shown to induce the expression of MKP-1 in macrophages (24), which may act to down-regulate IL-10 production in a negative feedback loop. IL-10 is produced by macrophages, certain types of T cells (TH2 and regulatory T cells), B cells, and cell types of nonhemopoietic lineage (19). Upstream signals leading to IL-10 production in activated macrophages are not fully understood, although IL-10 synthesis appears to be mediated by distinct adaptor molecules from those involved in the production of proinflammatory cytokines (25). Based on the studies using pharmacological inhibitors of the MAPKs, we found that all three major subgroups of MAPKs play a role in the regulation of IL-10. Whereas U0126 and SB203580 are reasonably specific inhibitors of ERK and p38, respectively, the specificity of SP600125 to JNK has been questioned (26), and the role of JNK in IL-10 regulation remains to be genetically defined. Importantly, IL-10 production was more substantially reduced by inhibition of p38 MAPK as compared with either JNK or ERK. Furthermore, only inhibition of p38 MAPK resulted in a complete abrogation of the increased IL-10 levels in MKP-1−/− macrophages, suggesting that p38 MAPK plays an important role in IL-10 production. Notably, IL-10 production in TH2 cells has been shown to require Jun/AP-1 proteins (27, 28). Consistent with this finding, we observed increased AP-1 binding activity in LPS-stimulated MKP-1−/− macrophages. Future work is required to identify the downstream targets of p38 MAPK that mediate IL-10 regulation in macrophages.
Previous work has focused on examining the role of MKP-1 in the regulation of TNF-α in macrophages. In one report, MKP-1−/− macrophages produced ≈100% and 30% higher TNF-α after 4 and 6 h of LPS stimulation, respectively (13). In another study, WT and MKP-1−/− macrophages produced similar levels of TNF-α after 24 h of exposure to LPS (12). Our data showed that MKP-1 deficiency resulted in an increased production of TNF-α only at early phases of LPS stimulation. At later time points, TNF-α production in MKP-1−/− macrophages was suppressed because of the increased IL-10 and Bcl-3 expression in these cells. This finding demonstrates that the kinetics of TNF-α production in response to LPS is biphasic, suggesting a more complex regulatory feature involving the synthesis of this cytokine.
In light of the important function of MKP-1 in the regulation of cytokine expression in a temporally specific manner, it is interesting to note that MKP-1 expression itself is regulated by TLR signaling. MKP-1 is induced rapidly after activation of various TLRs. Deficiency in either MyD88 or TRIF reduces, but does not abrogate completely, MKP-1 induction in LPS-treated macrophages. In contrast, expression of MKP-1 is entirely dependent on TRIF and MyD88 in response to TLR3 and TLR9 stimulation, respectively. Thus, both MyD88 and TRIF pathways are required for TLR-induced MKP-1 expression. Equally important is the termination of MKP-1 expression, because prolonged MKP-1 expression is anticipated to suppress the induction of IL-10. In growth factor-stimulated cells, MKP-1 protein is degraded by the ubiquitin-directed proteasome complex in a pathway controlled by the ERK activity (29). Whether a similar mechanism regulates MKP-1 expression in TLR signaling remains to be determined.
In summary, our studies provide important insights into the regulation of innate immune responses by the MAPK pathways. We have shown that MKP-1 suppresses endotoxic shock and proinflammatory cytokine production in vivo. Moreover, p38 MAPK and MKP-1 are critical positive and negative regulators of TLR-induced IL-10 production, respectively. We propose a dynamic interplay between the MAPKs and MKPs in the control of immune balance by temporally regulating both pro- and anti-inflammatory mediators of TLR signaling.
Materials and Methods
Reagents.
The following TLR ligands were used: LPS from Escherichia coli serotype O111:B4 (Sigma), peptidoglycan (PGN) from Staphylococcus aureus (Fluka), poly(I-C) (Amersham Pharmacia Biotech), phosphorothioate-modified CpG oligonucleotide DNA (TCCATGACGTTCCTGACGTT, synthesized by the Keck Facility at Yale University), synthetic lipoprotein Pam3CSK4, flagellin, and loxoribine (all from InvivoGen, San Diego). ELISA Abs for IL-6, TNF-α, and IL-10, the neutralizing Ab for IL-10 (JES5–2A5), flow-cytometry Abs, and recombinant cytokines were from BD Pharmingen. Abs against total and phosphospecific MAPKs were from Cell Signaling Technology (Beverly, MA). Anti-MKP-1 and anti-Actin Abs were from Santa Cruz Biotechnology. Inhibitors for MAPKs (SP600125, SB203580, and U0126) were from Calbiochem.
Mice and Endotoxic Shock.
MKP-1−/− mice were rederived from cryopreserved embryos obtained from Bristol-Myers Squibb (11, 30). Mice deficient in MyD88 were kindly provided by S. Akira (Osaka University, Suita, Osaka, Japan) (16). Mice with a frameshift mutation in the TRIF gene (TRIFLps2 mice) were kindly provided by B. Beutler (The Scripps Research Institute, La Jolla, CA) (17). Age- and sex-matched WT and MKP-1−/− mice were challenged by i.p. injection of 10 or 2.5 mg/kg of LPS, and survival of mice was monitored for up to 50 h. The incidence of mouse lethality was compared and analyzed by using the log rank test, performed by prism (Version 3.0a for Macintosh, GraphPad, San Diego). The animal experiments were conducted in accordance with the guidelines of Institutional Animal Care and Usage Committee of Yale University.
BMDM Culture.
Bone marrow cells were differentiated in DMEM supplemented with 20% heat-inactivated FCS and 30% L929 supernatants containing macrophage-stimulating factor (M-CSF). Bone marrow cells were cultured at an initial density of ≈106 cells/ml for 5–7 days, and fresh medium was added at day 3. Cells were harvested with cold 0.2% EDTA (Invitrogen) and plated at a density of 2–4 × 105 per ml in DMEM supplemented with 10% FBS. Macrophages were cultured for at least 12 h before stimulation.
Measurement of Cytokine Levels.
Cytokines including IL-6, TNF-α, and IL-10 were measured by ELISA, as described in ref. 31. Serum levels of cytokines also were measured by using the Beadlyte mouse multicytokine detection system (Upstate Biotechnology, Lake Placid, NY) on a Bio-plex Instrument (Bio-Rad) per manufacturer’s protocols.
Quantitative RT-PCR.
RNA was extracted by using TRIzol reagent, and cDNA was synthesized by Superscript II reverse-transcriptase per manufacturer’s protocols (Invitrogen). Quantitative PCR was performed on the Applied Biosystems 7500 real-time PCR system by using primer/probe sets purchased from Applied Biosystems or synthesized by Biosearch, as described in ref. 31. RNA expression levels of the target genes were normalized against those of hypoxanthine phosphoribosyltransferase. Mean values of at least two individual mice per group were shown.
Immunoblotting.
Protein extracts were separated on NuPAGE gels (Invitrogen), blotted, and probed with primary Abs with the following dilutions: anti-phospho and total MAPK Abs were used at 1:1,000, anti-MKP-1 at 1:500, and anti-actin at 1:4,000.
Gel Shift Assay.
Nuclear extracts were prepared and gel shift was performed by using 5 or 10 μg of protein per sample (quantified with the Bio-Rad protein assay) as described in ref. 28.
Supplementary Material
Acknowledgments
We thank M. Chen for technical assistance, F. Sutterwala and Y. Y. Wan for scientific discussions, Bristol-Myers Squibb for MKP-1−/− embryos, S. Akira for MyD88−/− mice, and B. Beutler for TRIFLps2 mice. This work was supported in part by National Institutes of Health Grants P01-AI36529 (to R.A.F.), P01-DK57751 (to A.M.B.), and T32-CA09085 (to J.J.W.). H.C. is supported by the Arthritis National Research Foundation and Charles H. Hood Foundation. R.A.F. is an investigator of the Howard Hughes Medical Institute.
Glossary
Abbreviations:
- TLR
Toll-like receptor
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MKP
MAPK phosphatase
- MyD88
myeloid differentiation factor 88
- TRIF
TIR domain-containing adaptor inducing IFN-β
- BMDM
bone marrow-derived macrophages
- JNK
c-Jun N-terminal kinase
- ERK
extracellular signal-regulated kinase.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Janeway C. A., Jr, Medzhitov R. Annu. Rev. Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 2.Takeda K., Kaisho T., Akira S. Annu. Rev. Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 3.Takeda K., Akira S. Int. Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 4.Lopez-Bojorquez L. N., Dehesa A. Z., Reyes-Teran G. Arch. Med. Res. 2004;35:465–479. doi: 10.1016/j.arcmed.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Liew F. Y., Xu D., Brint E. K., O’Neill L. A. J. Nat. Rev. Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 6.Dong C., Davis R. J., Flavell R. A. Annu. Rev. Immunol. 2002;20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- 7.Farooq A., Zhou M. M. Cell. Signalling. 2004;16:769–779. doi: 10.1016/j.cellsig.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Lau L. F., Nathans D. EMBO J. 1985;4:3145–3151. doi: 10.1002/j.1460-2075.1985.tb04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu J. J., Zhang L., Bennett A. M. Mol. Cell. Biol. 2005;25:4792–4803. doi: 10.1128/MCB.25.11.4792-4803.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franklin C. C., Kraft A. S. J. Biol. Chem. 1997;272:16917–16923. doi: 10.1074/jbc.272.27.16917. [DOI] [PubMed] [Google Scholar]
- 11.Wu J. J., Bennett A. M. J. Biol. Chem. 2005;280:16461–16466. doi: 10.1074/jbc.M501762200. [DOI] [PubMed] [Google Scholar]
- 12.Nimah M., Zhao B., Denenberg A. G., Bueno O., Molkentin J., Wong H. R., Shanley T. P. Shock. 2005;23:80–87. doi: 10.1097/01.shk.0000145206.28812.60. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Q., Shepherd E. G., Manson M. E., Nelin L. D., Sorokin A., Liu Y. J. Biol. Chem. 2005;280:8101–8108. doi: 10.1074/jbc.M411760200. [DOI] [PubMed] [Google Scholar]
- 14.Chen P., Li J., Barnes J., Kokkonen G. C., Lee J. C., Liu Y. J. Immunol. 2002;169:6408–6416. doi: 10.4049/jimmunol.169.11.6408. [DOI] [PubMed] [Google Scholar]
- 15.Shepherd E. G., Zhao Q., Welty S. E., Hansen T. N., Smith C. V., Liu Y. J. Biol. Chem. 2004;279:54023–54031. doi: 10.1074/jbc.M408444200. [DOI] [PubMed] [Google Scholar]
- 16.Adachi O., Kawai T., Takeda K., Matsumoto M., Tsutsui H., Sakagami M., Nakanishi K., Akira S. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 17.Hoebe K., Du X., Georgel P., Janssen E., Tabeta K., Kim S. O., Goode J., Lin P., Mann N., Mudd S., et al. Nature. 2003;424:743–748. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]
- 18.de Waal Malefyt R., Abrams J., Bennett B., Figdor C. G., de Vries J. E. J. Exp. Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore K. W., de Waal Malefyt R., Coffman R. L., O’Garra A. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 20.Kuwata H., Watanabe Y., Miyoshi H., Yamamoto M., Kaisho T., Takeda K., Akira S. Blood. 2003;102:4123–4129. doi: 10.1182/blood-2003-04-1228. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y., Blattman J. N., Kennedy N. J., Duong J., Nguyen T., Wang Y., Davis R. J., Greenberg P. D., Flavell R. A., Dong C. Nature. 2004;430:793–797. doi: 10.1038/nature02764. [DOI] [PubMed] [Google Scholar]
- 22.Howard M., Muchamuel T., Andrade S., Menon S. J. Exp. Med. 1993;177:1205–1208. doi: 10.1084/jem.177.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerard C., Bruyns C., Marchant A., Abramowicz D., Vandenabeele P., Delvaux A., Fiers W., Goldman M., Velu T. J. Exp. Med. 1993;177:547–550. doi: 10.1084/jem.177.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hammer M., Mages J., Dietrich H., Schmitz F., Striebel F., Murray P. J., Wagner H., Lang R. Eur. J. Immunol. 2005;35:2991–3001. doi: 10.1002/eji.200526192. [DOI] [PubMed] [Google Scholar]
- 25.Hacker H., Redecke V., Blagoev B., Kratchmarova I., Hsu L. C., Wang G. G., Kamps M. P., Raz E., Wagner H., Hacker G., et al. Nature. Vol. 439. 2005. pp. 204–207. (lett.) [DOI] [PubMed] [Google Scholar]
- 26.Bain J., McLauchlan H., Elliott M., Cohen P. Biochem. J. 2003;371:199–204. doi: 10.1042/BJ20021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Z. Y., Sato H., Kusam S., Sehra S., Toney L. M., Dent A. L. J. Immunol. 2005;174:2098–2105. doi: 10.4049/jimmunol.174.4.2098. [DOI] [PubMed] [Google Scholar]
- 28.Jones E. A., Flavell R. A. J. Immunol. 2005;175:7437–7446. doi: 10.4049/jimmunol.175.11.7437. [DOI] [PubMed] [Google Scholar]
- 29.Brondello J.-M., Pouysségur J., McKenzie F. R. Science. 1999;286:2514–2517. doi: 10.1126/science.286.5449.2514. [DOI] [PubMed] [Google Scholar]
- 30.Dorfman K., Carrasco D., Gruda M., Ryan C., Lira S. A., Bravo R. Oncogene. 1996;13:925–931. [PubMed] [Google Scholar]
- 31.Chi H., Lu B., Takekawa M., Davis R. J., Flavell R. A. EMBO J. 2004;23:1576–1586. doi: 10.1038/sj.emboj.7600173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.