Abstract
Successful reproduction requires maintenance of the reproductive axis within fine operating limits through negative feedback actions of sex steroids. Despite the importance of this homeostatic process, our understanding of the neural loci, pathways, and neurochemicals responsible remain incomplete. Here, we reveal a neuropeptidergic pathway that directly links gonadal steroid actions to regulation of the reproductive system. An RFamide (Arg-Phe-NH2) peptide that inhibits gonadotropin release from quail pituitary was recently identified and named gonadotropin-inhibitory hormone (GnIH). Birds are known to have specialized adaptations associated with gonadotropin-releasing hormone (GnRH) regulation to optimize reproduction (e.g., encephalic photoreceptors), and the existence of a hypothalamic peptide inhibiting gonadotropins may or may not be another such specialization. To determine whether GnIH serves as a signaling pathway for sex steroid regulation of the reproductive axis, we used immunohistochemistry and in situ hybridization to characterize the distribution and functional role of this peptide in hamsters, rats, and mice. GnIH-immunoreactive (GnIH-ir) cell bodies are clustered in the mediobasal hypothalamus with pronounced projections and terminals throughout the CNS. In vivo GnIH administration rapidly inhibits luteinizing hormone secretion. Additionally, GnIH-ir neurons form close appositions with GnRH cells, suggesting a direct means of GnRH modulation. Finally, GnIH-ir cells express estrogen receptor-α and exhibit robust immediate early gene expression after gonadal hormone stimulation. Taken together, the distribution of GnIH efferents to neural sites regulating reproductive behavior and neuroendocrine secretions, expression of steroid receptors in GnIH-ir nuclei, and GnIH inhibition of luteinizing hormone secretion indicate the discovery of a system regulating the mammalian reproductive axis.
Keywords: luteinizing, mating, reproduction
The final common pathway in the neural regulation of reproduction is the gonadotropin-releasing hormone (GnRH) neuronal system. Neurons that synthesize and secrete GnRH occupy a midventral continuum from the diagonal band of Broca to the mediobasal hypothalamus (1). GnRH neurons regulating gonadotropin secretion project to the median eminence to control synthesis and secretion of the pituitary gonadotropins, luteinizing hormone (LH), and follicle-stimulating hormone (FSH) (2–4).
In both males and females, gonadal steroids act through negative feedback to maintain the reproductive axis within the favorable operating limits necessary for fertility and successful mating. Negative feedback control of the GnRH system could be accomplished by sex hormones acting on either cognate receptors expressed in GnRH neurons or on gonadal-steroid-responsive systems upstream of GnRH. Although early evidence before this millennium suggested only the latter (5–11), more recent evidence suggests that both of these mechanisms act in concert to regulate precisely the reproductive axis in females (12–16), whereas the mechanisms regulating negative feedback in males remain obscure (5, 6).
Given the importance of reproductive axis regulation in normal fertility, it is essential to gain an understanding of the precise neural mechanisms responsible for its regulation. An RFamide (Arg-Phe-NH2 in the C terminus) peptide that inhibits gonadotropin secretion in vitro and in vivo, and named gonadotropin-inhibitory hormone (GnIH), has recently been identified in avian brain (17–21). In birds, regulation of reproduction is controlled, in part, by specialized adaptations such as deep hypothalamic photoreceptors that contact and potentially regulate GnRH (22, 23). To date, a hypothalamic neuropeptide negatively regulating gonadotropins at the level of the pituitary has not been identified in mammals. The goal of the present studies was to explore the possibility that GnIH occurs in mammals and to characterize the distribution and potential function of GnIH, its relationship to the reproductive system, and its possible role as a key neuropeptide involved in sex steroid negative feedback regulation in mammals.
Results
Rodent Brain Exhibits Robust GnIH-ir Peptide Expression.
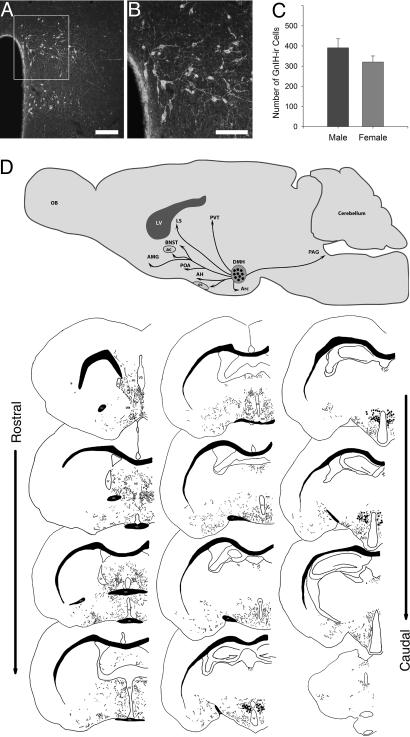
We first sought to determine the precise distribution of GnIH-ir cell bodies and fibers in hamsters, rats, and mice to explore GnIH connections. By characterizing GnIH-ir distribution, we aimed to gain further insight into the role that GnIH may play in mammalian reproduction and other functions. In all three rodent species, GnIH-ir cell bodies were concentrated in the dorsomedial nucleus of the hypothalamus (DMH) (Fig. 1A, B, and D and Fig. 2 E–H), with no other brain regions showing evidence of cell body staining. GnIH cells were mostly bi- and tripolar with a cell area of (622.52 ± 17.81 μm2). In hamsters, cell numbers (Fig. 1C) and fiber distribution did not differ between the sexes.
Fig. 1.
GnIH cells bodies are tightly clustered in the DMH and project throughout much of the brain of Syrian hamsters. (A) Medium-power photomicrograph depicting GnIH cell bodies clustered in the dorsal and ventral regions of the DMH. (Scale bar: 200 μm.) The box in the top image outlines the cells bodies shown at high power (B). (Scale bar: 100 μm.) The number of GnIH-immunoreactive cells was counted in both male and female hamsters (C). There were no differences between males and females in their cell counts or fiber distribution. A schematic diagram in the sagittal plane depicts the location of GnIH cell bodies and their projections (D Upper). Beneath the sagittal schematic is a tracing of the rostral-caudal extent of GnIH fiber projections and cell bodies (D Lower). Note that GnIH cell bodies are clustered in the dorsomedial region of the hypothalamus with diffuse projections throughout most of the brain, with a concentration of terminals in midline brain regions. ac, anterior commisure; AH, anterior hypothalamus; AMG, amygdala; Arc, arcuate nucleus; BNST, bed nucleus of the stria terminalis; LS, lateral septum; oc, optic chiasm; PAG, periaqueductal gray; POA, preoptic area; PVT, paraventricular nucleus of the thalamus.
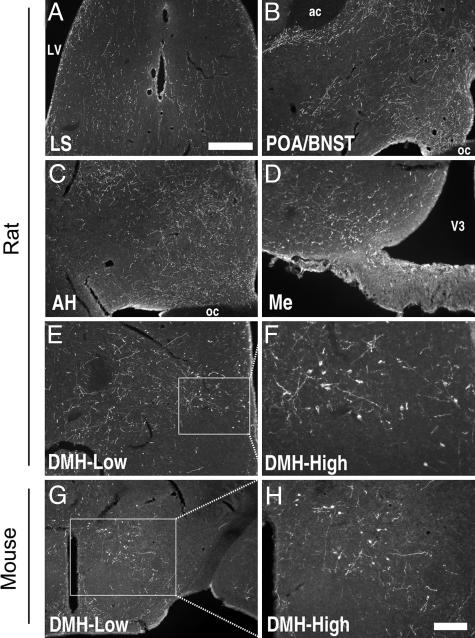
Fig. 2.
GnIH cell and fiber distribution in rats and mice. GnIH fibers are distributed throughout the rostrocaudal extent of the brain, with fiber terminals concentrated in midline brain regions in rat (A–F) and mice (G and H). In mice, the cell bodies tend to spread more lateral in the DMH in comparison with hamsters (G and H). The rat distribution of cell bodies is similar to that of hamster (E and F). ac, anterior commisure; AH, anterior hypothalamus; BNST, bed nucleus of the stria terminalis; LS, lateral septum; LV, lateral ventricle; POA, preoptic area. (Scale bars: 400 μm at low power, 100 μm at high power.)
As in birds, GnIH-ir fibers and terminal fields were omnipresent in midline brain regions that concentrate GnRH neurons and fibers (Fig. 1D); the medial septum, diagonal band of Broca, preoptic area, and anterior hypothalamus were all major targets for GnIH. This finding suggests that GnIH has the potential to modulate GnRH secretion through multiple upstream pathways. Staining for GnIH-ir was eliminated when the antiserum was preadsorbed with control GnIH peptides (see Materials and Methods). Importantly, fiber projections and terminal fields were robustly expressed throughout many brain regions that do not contain GnRH cells or fibers (see below). GnIH-ir fibers in hamsters (Fig. 1), rats (Fig. 2), and mice (Fig. 2) projected rostrally to midline, limbic regions (septal and preoptic areas, the amygdala, bed nucleus of the stria terminalis). Fibers projecting ventrally and rostrally targeted the anterior hypothalamus and arcuate nucleus. Fiber terminals were identified as far as the hindbrain and in the periaqueductal gray. GnIH fibers are sparse in the median eminence (Me), with most GnIH-ir fibers detected in the inner layer close to the third ventricle (Fig. 2D).
Deduced Amino Acid Sequence for the Syrian Hamster GnIH Precursor Polypeptide.
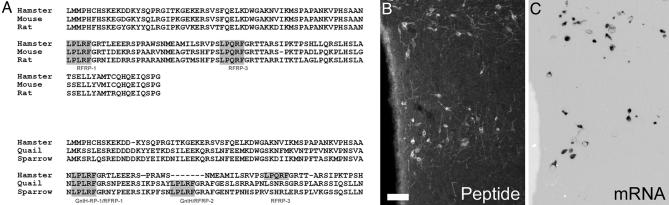
The deduced amino acid sequence of GnIH (Fig. 3) contains two LPXRF (X = L or Q) sequences that were both flanked by glycine residues as C-terminal amidation signal. Comparison of this partial preproprotein between those of mouse and rat RFamide-related peptides, white-crowned sparrow, and quail GnIHs were 81%, 77%, 43%, and 42%, homologous, respectively (Fig. 3).
Fig. 3.
The amino acid sequence of the preproprotein that encodes the Syrian hamster GnIH homolog exhibits high homology with other mammalian LPXRF-amide peptides and avian GnIH. (A) Alignment of preproproteins that encode RF-amide-related peptides (RFRP-1, -2, and -3) and GnIH-related peptides (GnIH, -RP-1, and -RP-2) in mammals (hamster, rat, and mouse) and birds (quail and white-crowned sparrow). The predicted amino acid sequence for the Syrian hamster GnIH preproprotein homolog exhibits high homology to previously identified RFRPs in mouse and rat. The hamster amino acid sequence is highly homologous to the avian GnIH-RP-1. (B and C) Comparison of immunocytochemical staining (B) with RNA labeling (C) at similar levels of the DMH. Note that both labels exhibit a dorsal and ventral population in the same general pattern of expression. Mouse and rat, ref. 38; white-crowned sparrow, ref. 17; quail, ref. 20. (Scale bar: 100 μm.)
GnIH mRNA Is Expressed in the DMH.
In situ hybridization using an antisense probe generated from the partial Syrian hamster GnIH clone revealed cell body labeling in the DMH, the neural locus where cell bodies stained immunohistochemically for GnIH are seen (Fig. 3). Extended processes and cell nuclei were devoid of reaction product. Importantly, the distribution of cells was identical to that seen with immunohistochemistry, with cell bodies spreading laterally from the third ventricle, just dorsal to the ventromedial hypothalamus, and lateral to the dorsal tip of the third ventricle. Staining was not seen in tissue processed using the GnIH sense probe (data not shown).
GnIH-ir Cells Project to GnRH Neurons.
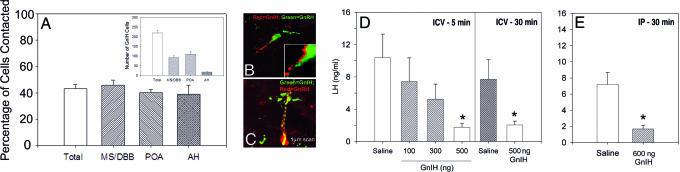
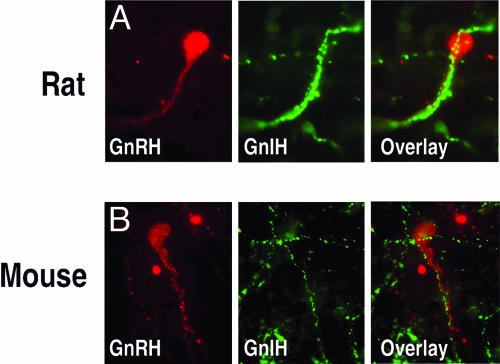
Because the GnIH system projects to brain areas rich in GnRH cells, we used double-label immunohistochemistry to determine whether GnIH fibers directly contact GnRH cells. Contacts from GnIH-ir fibers were examined using both conventional and confocal microscopy. A large percentage of GnRH cells (>40%) receive projections from the GnIH system in female Syrian hamsters, suggesting the potential for direct inhibition (Fig. 4A–C). In rats and mice, similar contacts were identified but not quantified (Fig. 5). Although we only quantified axo-somatic contacts, a number of axo-dendritic contacts were observed. Although most GnRH cells are concentrated rostrally in septal regions and the preoptic area with few cells in the AH, an equal percentage of cells was contacted within each subregion (Fig. 4A). Whereas confocal and light microscopic analyses cannot be used to identify synaptic connections, the existence of pronounced close contacts suggests important functional implications.
Fig. 4.
GnIH cells target a large proportion of GnRH somata. The GnRH system is distributed from the septum to the caudal aspect of the hypothalamus, and GnRH cells in all brain regions are similarly contacted (Inset depicts the distribution of GnRH-immunoreactive cells numbers across brain regions) (A). GnRH cells were investigated by using wide-field (B) and confocal (C) microscopy. Both B and C depict fibers for GnIH “tracking” the GnRH fiber and cell body; presumptive boutons are evident. GnIH administration either i.c.v. (D) or i.p. (E) leads to marked and rapid reductions in LH.
Fig. 5.
GnIH fibers contact GnRH cells in rats and mice. As in hamsters, GnIH fibers target GnRH cells in rats (A) and mice (B). Note how GnIH cells “track” the fiber following it to the cell body, with several presumptive boutons along the path. For visibility, images are shown as GnRH (red) alone and GnIH fibers (green) alone, followed by their respective overlays. Images were taken at ×1,000 at the light level.
GnIH Inhibits LH Secretion.
Given the similarity in the distribution of GnIH-ir cells and fibers in both sexes and among rodent species studied, we focused on exploring the functional role of GnIH using the hamster model. We investigated female Syrian hamsters because the precise regulation of hormones of the reproductive axis is critical for female reproductive function (ovulation, mating, pregnancy, and lactation). Furthermore, the timing of reproductive events is most precisely temporally regulated (estrus occurs at the same time every 4 days) in hamsters relative to rats and mice, making female hamsters an excellent model system to establish a role for GnIH. Ovariectomized Syrian hamsters were injected with GnIH either intracerebroventricularly (i.c.v.) (0, 100, 300, or 500 ng) or peripherally (0 or 600 ng). When injected i.c.v., GnIH rapidly reduced LH concentrations with suppression sustained 30 min after the most effective dose (Fig. 4 D and E). Similar results were obtained with peripheral injections. Together, these findings provide compelling evidence for a role of GnIH as a mammalian gonadotropin inhibitory factor.
GnIH-ir Cells Express Sex Steroid Receptors and Respond to Gonadal Steroids with Increased Immediate Early Gene Expression.
To assess the role of GnIH in negative feedback of the reproductive system, we explored whether GnIH-ir cells express sex steroid receptors. In female hamsters, double-label immunofluorescence for GnIH and ERα indicates that a large subset (39.41 ± 2.06%) of GnIH-ir cells express ERα (Fig. 6E). Similar results were obtained in male hamsters when brains were labeled for AR expression in GnIH-ir cells (data not shown).
Fig. 6.
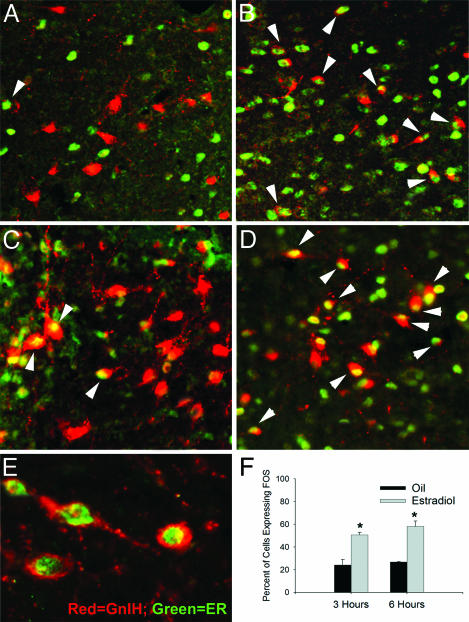
GnIH cells are activated by sex steroid exposure. We pursued the role of GnIH in modulation of estrogen negative feedback because of the importance of this process in regulation of ovulation and the coordination of receptivity. Ovariectomized hamsters were injected with either estradiol (B and D) or oil vehicle (A and C) and killed either 3 (A and B) or 6 (C and D) h after injection. The percentages of double-labeled GnIH and FOS neurons were counted (F). In oil-treated controls, few GnIH cells expressed FOS (A and C), whereas robust expression of FOS was evident in GnIH cells after estradiol treatment (B and D). Because estrogen administration led to FOS expression in GnIH cells, it was necessary to see whether estrogen was acting on GnIH neurons or systems upstream. Double-label immunofluorescence was used to colabel GnIH cells and ERα. ERα is expressed in a subset of GnIH cells in female hamsters, suggesting direct actions of estradiol on GnIH cell activation (E).
We reasoned that if estrogen acts on GnIH-ir cells to stimulate their secretion, then these cells should be “activated” (express FOS) in ovariectomized females after estrogen administration. To determine whether this FOS activation occurs in GnIH-ir cells in the DMH, hamsters were ovariectomized and injected subcutaneously (s.c.) with either estrogen (10 μg in 0.1 ml in sesame oil) or oil vehicle. GnIH-ir cells (number of GnIH-ir cells investigated: 3 h oil, 103.0 ± 4.16; 3 h estrogen, 112.3 ± 5.01; 6 h oil, 107.5 ± 5.50; 6 h estrogen, 115.2 ± 4.26) from four brain sections were assessed for FOS expression by observers unaware of the conditions to which the animals had been exposed. FOS expression was markedly elevated in the DMH of estradiol-treated females relative to oil-injected control animals both 3 and 6 h after estradiol treatment. GnIH-ir cells from oil-injected controls exhibited low levels of FOS expression compared to the robust FOS expression seen after estrogen injections (Fig. 6 A–D and F). Together with the expression of ERα in GnIH-ir cells, these findings suggest that estrogen feeds back to the brain to act (at least in part) on GnIH cells.
Discussion
Our data provide evidence for a previously uncharacterized mammalian RFamide-related peptide in the signaling pathway regulating negative feedback effects of gonadal steroids on the reproductive axis in mammals. The results herein characterize previously unidentified projections from GnIH-ir neurons to the GnRH system, provide evidence that GnIH inhibits activity of the reproductive axis, and suggest that this inhibitory pathway is sensitive to sex steroids.
It is noteworthy that GnIH-ir cells are clustered in the DMH. The DMH projects extensively to neuroendocrine and preautonomic hypothalamic regions, and to a lesser extent to extrahypothalamic sites (24). The DMH, along with five other preoptic nuclei, forms a complex interconnected network that plays a role in coordinating neuroendocrine, autonomic, and somatic responses to external stimuli, sensory feedback, and cognitive/motivational input (25). In male and female Syrian hamsters, lesions of the DMH prevent the seasonal onset of reproductive quiescence thought to be mediated through enhanced gonadal steroid negative feedback (26, 27). The importance of the DMH in regulating motivated behaviors and endocrine function, along with the present results indicating extensive projections of GnIH-ir cells to hypothalamic and extrahypothalamic brain regions, suggests that GnIH may be an important neurochemical mediator contributing to a broad spectrum of physiological and behavioral systems. The observation that GnIH-ir cells send pronounced projections to limbic brain regions (e.g., septum, striatum, amygdala, and hypothalamus) important for motivated behaviors (28) provides further support for this speculation and suggests opportunity for study of GnIH beyond reproductive events.
The reproductive axis integrates information from a wide range of systems via a number of direct and indirect neurochemical inputs (29–31). These neurochemical modulators allow the GnRH system to monitor the internal and external environments and adjust reproductive function according to current conditions. For example, during nutritional deficits, GnRH secretion is inhibited and reproduction is curtailed (32). Likewise, acute and chronic stress can have marked effects on GnRH secretion and reproductive function (33, 34). The distribution of GnIH-ir in the present studies, indicating substantial innervation of midline hypothalamic regions, suggests the possibility that GnIH neurons may act through intermediary systems to influence the hypothalamic–pituitary–gonadal axis. For example, RFamide peptides have been implicated in control of hormone release and feeding via interactions with the opiatergic systems (35–39). The presence of GnIH-ir terminals in the arcuate and PVH suggest that GnIH is in a position to monitor nutritional state and modify the reproductive axis accordingly. Additionally, projections of GnIH-ir fibers to the PVH provide the potential for GnIH to regulate corticotropin-releasing hormone to hormonally influence GnRH function.
In avian species, GnIH neurons are found only in the PVN with extensive fibers projecting rostrally to the ventral paleostriatum, lateral and medial septum, and preoptic area and caudally to the median eminence, optic tectum, and brainstem (18, 19), and alterations in GnIH have been implicated in seasonal changes in reproduction (17, 18, 40–42), with melatonin acting directly on GnIH-expressing cells (43). Neuroanatomical and functional studies suggest that GnIH, in turn, modulates gonadotropin secretion by acting directly on GnRH neurons (18) as well as the anterior pituitary (17, 21, 40). The possibility that GnIH cells express sex-steroid receptors has not been investigated in avian species. As seen in the present study, the general rodent pattern of cell and fiber distribution is similar to that of birds. Although innervation of the median eminence is sparse, these projections may be functional. In rats, GnIH inhibits LH and GnRH-stimulated release of LH, indicating a potential role for GnIH at the pituitary level.∥,** Together, these findings point to convergent roles for GnIH across species and underscore the importance of comparative investigations for this peptide.
RFamide-related peptides (RFRPs) homologous to GnIH as well as its receptor have been identified previously in rats and mice (38, 44, 45). As with the Syrian hamster fragment identified in the present study, the rat and mouse prepro-RFRP polypeptide encodes two RFRPs, called RFRP-1 and RFRP-3, with these precursor polypeptides sharing ≈80% homology among all three species (Fig. 3). The rat homolog is a potent stimulator of prolactin secretion, with reportedly no effects on other pituitary hormones, including LH (38). However, this study was conducted in gonad-intact male rats; as a result, LH suppression via sex steroid negative feedback was maximal at the time of testing. In the present study, using ovariectomized animals to release the hypothalamic–pituitary–gonadal axis from negative feedback, we now establish a role for this RFamide in regulating LH secretion.
In summary, RFamide peptides are emerging as important regulators of neuroendocrine function (38, 46, 47). Our data indicate an important role for the RFRP, GnIH, in regulating the mammalian reproductive axis. The present findings point to a previously unidentified neurochemical pathway by which sex steroids act on the brain to regulate the reproductive axis. The extensive fiber projections of GnIH throughout the brain point to opportunity for investigating the role of this peptide in an array of motivated behaviors.
Materials and Methods
Animals.
Thirty-five (>60 days) female and five male LVG hamsters (Mesocricetus auratus), five female Sprague–Dawley rats (Rattus norvegicus), and five female C57BL/6 mice (Mus musculus) were used in the present experiment. All animals were purchased from Charles River Breeding Laboratories. Animals were housed in translucent propylene cages (48 × 27 × 20 cm for hamsters and rats; 29 × 19 × 12.5 cm for mice), provided with ad libitum access to food and water for the duration of the study, and cared for in accordance with the Columbia University Institutional Animal Care and Use Committee and Animal Welfare.
Double-Label Immunofluorescence.
For simultaneous visualization of GnIH and GnRH, every fourth 30-μm section was double-labeled (five brains for each condition for each species and sex) by using a rabbit anti-white-crowned sparrow GnIH antibody (PAC 123a) and a guinea pig anti-GnRH antibody (antigenic determinants are amino acids 6–10; Advanced ChemTech, Louisville, KY). Specific methodological details are described in Supporting Methods, which is published as supporting information on the PNAS web site.
DNA Sequencing of the Partial Syrian Hamster GnIH cDNA Fragment.
Total RNA of the hypothalamus was isolated by the sepasol extraction method. Three forward (5′-CAGCCTACAGGAATCTCA-3′, 5′-GATGCCCCATTTTCACAGC-3′, and 5′-GTTGACTTTAGCCACTTC-3′) and three reverse (5′-CCATGAATGCTTGTCTCC-3′, 5′-TCCCTTCTTCATCGTCTG-3′, and 5′-TGCTGGCAAGTCATGGCGGT-3′) primers were synthesized (Sigma Genosys) based on the known sequence for the Siberian hamster (Phodopus sungorus) GnIH precursor cDNA (K.I., T.U., K.U., L.J.K., and K.T., unpublished work). First-strand cDNA was synthesized by RT-PCR (ImProm-II reverse transcription system, Promega) in accordance with the manufacturer’s instructions, using nine different primer combinations. After gel electrophoresis, the strongest band resulted from the combination of forward primer 5′-GATGCCCCATTTTCACAGC-3′ and reverse primer 5′-TGCTGGCAAGTCATGGCGT-3′. cDNA PCR product was purified with Amersham Pharmacia MicroSpin columns according to the manufacturer’s instructions. The purified PCR products were subcloned into a pGEM-T Easy vector in accordance with the manufacturer’s instructions (Promega). The DNA inserts of the positive clones were amplified by PCR with universal M13 primers. The nucleotide sequence was determined by cycle sequencing reactions, using fluorescent dideoxynucleotide terminators and loading onto an ABI 3730XL DNA analyzer (Applied Biosystems).
In Situ Hybridization of GnIH mRNA.
The localization of GnIH mRNA expression was identified by in situ hybridization. Two female Syrian hamsters were deeply anesthetized with sodium pentobarbital before transcardial perfusion with PBS, followed by fixative solution (4% paraformaldehyde in PBS). Brains were collected in the middle of the light/dark cycle on the day of diestrus to maximize GnIH detection. Specific details of the procedure are described in Supporting Methods.
Effects of GnIH on LH Concentrations.
Ovariectomized animals were injected either i.c.v. (5 μl of 0, 100, 300, or 500 ng of GnIH in saline; n = 8 per dose) or peripherally (0.2 ml, i.p. with 0 or 600 ng GnIH; n = 10 per dose). All injections commenced 3 h after light onset (light/dark, 14/10; lights on at 1500 hours). Injections were infused over a 5-min period, and a blood sample was collected either 5 (i.c.v.) or 30 (i.c.v. and i.p.) min after the end of the injection. At least a 1-week recovery was allowed between each sample collection. Animals fitted with cannulae were injected with each i.c.v. dose in a counterbalanced design. For i.p. treatments, all animals injected were injected with either saline or GnIH in a counterbalanced design. All surgical details are described in Supporting Methods.
LH Radioimmunoassay.
LH was assayed in blood plasma collected from female hamsters by the National Hormone and Pituitary Program. Reference preparation RP-3 was used as standard, NIDDK rLH I-9 (AFP-10250C) was used for iodination, and NIDDK S-10 anti-LH was used. Bound LH was precipitated by using goat anti-rabbit IgG. Standard and hamster serum produce parallel inhibition curves in this assay system. All samples were run in a single assay.
Estrogen Injections.
Twelve ovariectomized hamsters were injected (s.c.) with either estradiol (10 μg in 0.1 ml in sesame oil) or oil vehicle 3 h after light onset (L/D, 14/10; lights on at 1500 hours). This amount and route of estradiol administration resulted in elevated estrogen concentrations within 1 h, peaking after 5–6 h (unpublished results). Animals were killed either 3 (n = 3 per group) or 6 (n = 3 per group) h after the injection, and their brains were double-labeled for GnIH and FOS (see above).
Conventional Light Microscopy.
GnRH-ir cell counts and GnIH contacts on GnRH-ir cell bodies were investigated by using a Nikon Eclipse E800 microscope. Sections were examined by using the standard wavelengths for Cy-2 (488 nm) and Cy-3 (568 nm). Every fourth section from the medial septum to the caudal aspect of the anterior hypothalamus was evaluated. Specifically, the medial septum/diagonal band (MS/DBB), medial and lateral preoptic areas, and anterior hypothalamus, were investigated. GnRH cells were counted, and putative axosomatic contacts of GnIH-ir fibers on GnRH soma were screened at ×200. Contacts were assessed at ×400 and ×1,000. A contact was scored only if a GnIH-ir bouton-like structure was observed in close proximity to a GnRH cell body (with both the bouton and cell body being in the same plane of focus), and with examination of the fine focal plane revealing the continuity of the GnIH-ir fiber. All contacts were digitally captured to further confirm GnIH contacts in 8-bit grayscale by using a cooled charge-coupled device camera (SPOT, Diagnostic Instruments). Each image was captured as a single image without moving the position of the stage or plane of focus between captures. Images were superimposed digitally by using spot software (Diagnostic Instruments).
Confocal Microscopy.
Brain sections used for light microscopy were also used for the confocal scans to confirm that the close contacts were on the same 0.5-to 1.0-μm plane. To this end, GnRH-ir cells with GnIH-ir contacts identified at the light level were evaluated. Cells were observed under a Zeiss Axiovert 100TV fluorescence microscope with a Zeiss LSM 410 laser scanning confocal attachment. The sections were excited with an Argon-Krypton laser by using the standard excitation wavelengths for Cy-2 and Cy-3. Stacked images were collected as 0.5-μm multitract optical sections (with sequential excitation by each laser to avoid “cross-talk” between the two wavelengths). Using lsm 3.95 software (Zeiss), red and green images of the sections were superimposed. Each cell was examined through its entirety in 0.5-μm steps, and axosomatic appositions were assessed.
Supplementary Material
Acknowledgments
We thank Sean Duffy, Hamed Bayat, and Sachi Jain for technical assistance, and Dr. Irv Zucker for valuable comments on an earlier version of this manuscript. This work was supported by National Institute of Neurological Disorders and Stroke Grant 37919 (to R.S.).
Glossary
Abbreviations:
- DMH
dorsomedial hypothalamus
- GnIH
gonadotropin-inhibitory hormone
- GnIH-ir
GnIH-immunoreactive
- GnRH
gonadotropin-releasing hormone
- i.c.v.
intracerebroventricular(ly)
- LH
luteinizing hormone
- RFamide
Arg-Phe-NH2
- RFRP
RFamide-related peptide.
Footnotes
Conflict of interest statement: No conflicts declared.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. DQ371799).
Anderson, G. M., Steyn, F. J., Tsutsui, K., Ukena, K., Bentley, G. E. & Herbison, A. E. (2005) Soc. Neurosci., Abstr. 759.6.
Johnson, M. A., Bentley, G. E., Ukena, K., Tsutsui, K. & Fraley, G. S. (2005) Soc. Neurosci., Abstr. 758.8.
References
- 1.Silverman A. J. Physiol. Reprod. 1994;1:1683–1709. [Google Scholar]
- 2.Silverman A. J., Witkin J. W., Silverman R. C., Gibson M. J. Synapse. 1990;6:154–160. doi: 10.1002/syn.890060206. [DOI] [PubMed] [Google Scholar]
- 3.Sawyer C. H. Neuroendocrinology. 1975;17:97–124. doi: 10.1159/000122347. [DOI] [PubMed] [Google Scholar]
- 4.Jennes L., Stumpf W. E. Cell Tissue Res. 1980;209:239–256. doi: 10.1007/BF00237629. [DOI] [PubMed] [Google Scholar]
- 5.Huang X., Harlan R. E. Brain Res. 1993;624:309–311. doi: 10.1016/0006-8993(93)90094-4. [DOI] [PubMed] [Google Scholar]
- 6.Herbison A. E., Skinner D. C., Robinson J. E., King I. S. Neuroendocrinology. 1996;63:120–131. doi: 10.1159/000126948. [DOI] [PubMed] [Google Scholar]
- 7.Shivers B. D., Harlan R. E., Morrell J. I., Pfaff D. W. Nature. 1983;304:345–347. doi: 10.1038/304345a0. [DOI] [PubMed] [Google Scholar]
- 8.Lehman M. N., Karsch F. J. Endocrinology. 1993;133:887–895. doi: 10.1210/endo.133.2.8102098. [DOI] [PubMed] [Google Scholar]
- 9.Herbison A. E., Theodosis D. T. Neuroscience. 1992;50:283–298. doi: 10.1016/0306-4522(92)90423-y. [DOI] [PubMed] [Google Scholar]
- 10.Herbison A. E. Endocr. Rev. 1998;19:302–330. doi: 10.1210/edrv.19.3.0332. [DOI] [PubMed] [Google Scholar]
- 11.Gore A. C., Roberts J. L. Front. Neuroendocrinol. 1997;18:209–245. doi: 10.1006/frne.1996.0149. [DOI] [PubMed] [Google Scholar]
- 12.Abraham I. M., Han S. K., Todman M. G., Korach K. S., Herbison A. E. J. Neurosci. 2003;23:5771–5777. doi: 10.1523/JNEUROSCI.23-13-05771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butler J. A., Sjoberg M., Coen C. W. J. Neuroendocrinol. 1999;11:331–335. doi: 10.1046/j.1365-2826.1999.00347.x. [DOI] [PubMed] [Google Scholar]
- 14.Hrabovszky E., Shughrue P. J., Merchenthaler I., Hajszan T., Carpenter C. D., Liposits Z., Petersen S. L. Endocrinology. 2000;141:3506–3509. doi: 10.1210/endo.141.9.7788. [DOI] [PubMed] [Google Scholar]
- 15.Skynner M. J., Sim J. A., Herbison A. E. Endocrinology. 1999;140:5195–5201. doi: 10.1210/endo.140.11.7146. [DOI] [PubMed] [Google Scholar]
- 16.Petersen S. L., Ottem E. N., Carpenter C. D. Biol. Reprod. 2003;69:1771–1778. doi: 10.1095/biolreprod.103.019745. [DOI] [PubMed] [Google Scholar]
- 17.Osugi T., Ukena K., Bentley G. E., O’Brien S., Moore I. T., Wingfield J. C., Tsutsui K. J. Endocrinol. 2004;182:33–42. doi: 10.1677/joe.0.1820033. [DOI] [PubMed] [Google Scholar]
- 18.Bentley G. E., Perfito N., Ukena K., Tsutsui K., Wingfield J. C. J. Neuroendocrinol. 2003;15:794–802. doi: 10.1046/j.1365-2826.2003.01062.x. [DOI] [PubMed] [Google Scholar]
- 19.Ukena K., Ubuka T., Tsutsui K. Cell Tissue Res. 2003;312:73–79. doi: 10.1007/s00441-003-0700-x. [DOI] [PubMed] [Google Scholar]
- 20.Satake H., Hisada M., Kawada T., Minakata H., Ukena K., Tsutsui K. Biochem. J. 2001;354:379–385. doi: 10.1042/0264-6021:3540379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsutsui K., Saigoh E., Ukena K., Teranishi H., Fujisawa Y., Kikuchi M., Ishii S., Sharp P. J. Biochem. Biophys. Res. Commun. 2000;275:661–667. doi: 10.1006/bbrc.2000.3350. [DOI] [PubMed] [Google Scholar]
- 22.Saldanha C. J., Silverman A. J., Silver R. J. Biol. Rhythms. 2001;16:39–49. doi: 10.1177/074873040101600105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silver R., Witkovsky P., Horvath P., Alones V., Barnstable C. J., Lehman M. N. Cell Tissue Res. 1988;253:189–198. doi: 10.1007/BF00221754. [DOI] [PubMed] [Google Scholar]
- 24.Thompson R. H., Canteras N. S., Swanson L. W. J. Comp. Neurol. 1996;376:143–173. doi: 10.1002/(SICI)1096-9861(19961202)376:1<143::AID-CNE9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 25.Thompson R. H., Swanson L. W. Brain Res. Brain Res. Rev. 2003;41:153–202. doi: 10.1016/s0165-0173(02)00232-1. [DOI] [PubMed] [Google Scholar]
- 26.Lewis D., Freeman D. A., Dark J., Wynne-Edwards K. E., Zucker I. J. Neuroendocrinol. 2002;14:294–299. doi: 10.1046/j.1365-2826.2002.00779.x. [DOI] [PubMed] [Google Scholar]
- 27.Maywood E. S., Bittman E. L., Hastings M. H. Biol. Reprod. 1996;54:470–477. doi: 10.1095/biolreprod54.2.470. [DOI] [PubMed] [Google Scholar]
- 28.Simerly R. B. Annu. Rev. Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- 29.Gore A. C. Endocrinology. 2004;145:4016–4017. doi: 10.1210/en.2004-0855. [DOI] [PubMed] [Google Scholar]
- 30.Smith M. S., Grove K. L. Front. Neuroendocrinol. 2002;23:225–256. doi: 10.1016/s0091-3022(02)00002-x. [DOI] [PubMed] [Google Scholar]
- 31.Clarke I. J., Pompolo S. Anim. Reprod. Sci. 2005;88:29–55. doi: 10.1016/j.anireprosci.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Wade G. N., Jones J. E. Am. J. Physiol. 2004;287:R1277–R1296. doi: 10.1152/ajpregu.00475.2004. [DOI] [PubMed] [Google Scholar]
- 33.Tilbrook A. J., Turner A. I., Clarke I. J. Stress. 2002;5:83–100. doi: 10.1080/10253890290027912. [DOI] [PubMed] [Google Scholar]
- 34.Belsham D. D., Lovejoy D. A. Vitam. Horm. 2005;71:59–94. doi: 10.1016/S0083-6729(05)71003-7. [DOI] [PubMed] [Google Scholar]
- 35.Adams L. A., Vician L., Clifton D. K., Steiner R. A. Endocrinology. 1991;128:1881–1886. doi: 10.1210/endo-128-4-1881. [DOI] [PubMed] [Google Scholar]
- 36.Chartrel N., Dujardin C., Anouar Y., Leprince J., Decker A., Clerens S., Do-Rego J. C., Vandesande F., Llorens-Cortes C., Costentin J., et al. Proc. Natl. Acad. Sci. USA. 2003;100:15247–15252. doi: 10.1073/pnas.2434676100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fukusumi S., Yoshida H., Fujii R., Maruyama M., Komatsu H., Habata Y., Shintani Y., Hinuma S., Fujino M. J. Biol. Chem. 2003;278:46387–46395. doi: 10.1074/jbc.M305270200. [DOI] [PubMed] [Google Scholar]
- 38.Hinuma S., Shintani Y., Fukusumi S., Iijima N., Matsumoto Y., Hosoya M., Fujii R., Watanabe T., Kikuchi K., Terao Y., et al. Nat. Cell Biol. 2000;2:703–708. doi: 10.1038/35036326. [DOI] [PubMed] [Google Scholar]
- 39.Ibata Y., Iijima N., Kataoka Y., Kakihara K., Tanaka M., Hosoya M., Hinuma S. Neurosci. Res. 2000;38:223–230. doi: 10.1016/s0168-0102(00)00182-6. [DOI] [PubMed] [Google Scholar]
- 40.Ciccone N. A., Dunn I. C., Boswell T., Tsutsui K., Ubuka T., Ukena K., Sharp P. J. J. Neuroendocrinol. 2004;16:999–1006. doi: 10.1111/j.1365-2826.2005.01260.x. [DOI] [PubMed] [Google Scholar]
- 41.Yin H., Ukena K., Ubuka T., Tsutsui K. J. Endocrinol. 2005;184:257–266. doi: 10.1677/joe.1.05926. [DOI] [PubMed] [Google Scholar]
- 42.Ciccone N. A., Tsutsui K., Sharp P. J., Dunn I. C. Br. Poultry Sci. 2004;45(Suppl. 1):S28–S29. doi: 10.1080/00071660410001698100. [DOI] [PubMed] [Google Scholar]
- 43.Ubuka T., Bentley G. E., Ukena K., Wingfield J. C., Tsutsui K. Proc. Natl. Acad. Sci. USA. 2005;102:3052–3057. doi: 10.1073/pnas.0403840102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ukena K., Iwakoshi E., Minakata H., Tsutsui K. FEBS Lett. 2002;512:255–258. doi: 10.1016/s0014-5793(02)02275-5. [DOI] [PubMed] [Google Scholar]
- 45.Ukena K., Tsutsui K. Neurosci. Lett. 2001;300:153–156. doi: 10.1016/s0304-3940(01)01583-x. [DOI] [PubMed] [Google Scholar]
- 46.Hoek R. M., Li K. W., van Minnen J., Lodder J. C., de Jong-Brink M., Smit A. B., van Kesteren R. E. J. Neurochem. 2005;92:1073–1080. doi: 10.1111/j.1471-4159.2004.02927.x. [DOI] [PubMed] [Google Scholar]
- 47.Yang H. Y., Tang J., Iadarola M., Panula P., Costa E. Prog. Clin. Biol. Res. 1985;192:313–322. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.