Abstract
This study used positron-emission tomography to establish the patterns of brain activity involved in the isolated and concurrent experiences of thirst and pain. Ten subjects were scanned while experiencing pain evoked with noxious pressure, while experiencing thirst after the infusion of hypertonic saline, and while experiencing pain when thirsty. After the onset of thirst, noxious pressure evoked more intense sensations of pain. Noxious pressure did not change subjective ratings of thirst. Thirst caused activation in the anterior cingulate (Brodmann area 32) and the insula. Enhanced pain responses were associated with increased activity in cortical regions that are known to correlate with pain intensity, and also with unique activity in the pregenual anterior cingulate and ventral orbitofrontal cortex. These findings suggest a role for limbic and prefrontal cortices in the modulation of pain during the experience of thirst.
Keywords: interoception, neuroimaging
Sensations determined by interoceptors guide behaviors that maintain homeostasis. Experiences such as thirst, hunger, pain, and temperature sensation reflect imbalances of the internal state and have important implications for survival (1, 2). Interoceptive sensations can occur in tandem and create conflicts that are not resolved by a single behavior. Prevailing circumstances determine which internal imbalance requires the most urgent attention. Interoception would be more consistent with survival if competing sensations were modified to reflect relative priorities. To investigate competing interoceptive sensations, we measured brain activity during painful pressure and thirst, both in isolation and occurring concurrently.
Changes in sensations determined by interoceptors in response to changing environmental circumstances can be described as context-dependent responses (3). Both appetitive and aversive drives shape the state of an organism, and these drives must be reconciled to make the most adaptive behavioral choice. The juxtaposition of pain and thirst is particularly suited to the investigation of human brain activity associated with context-dependent changes in responses to competing drives. Both pain and thirst can be manipulated in a time frame compatible with imaging protocols, the former being particularly amenable to rapid onset and offset. There is evidence that the respective sensations are modified by contextual factors, although pain and thirst have yet to be examined contemporaneously. Finally, the concurrence of conditions likely to stimulate osmoreceptors and nociceptors would conceivably be associated with conflicting implications for behavioral responses. Drinking can be deferred without detrimental effect, but ultimately thirst must be sated to avoid desiccation. The acute onset of pain has more immediate implications for tissue integrity than the early experience of thirst. For example, during mild to moderate thirst, withdrawal from noxious stimuli would be more likely if thirst is decreased and/or pain is increased. Furthermore, the opposite changes in the two sensations, namely a decrease in pain and an increase in thirst, would accentuate the drive to drink.
Context-dependent responses involve integration of the physiological states that give rise to individual sensations, together with central mechanisms that modulate responses to different interoceptive stimuli. Experiments in animals have supported several brain structures as integration centers for interoceptive inputs, including the hypothalamus, thalamic intralaminar nuclei, and periaqueductal gray (4). Functional brain imaging studies in humans have focused attention on the pregenual anterior cingulate cortex as a key integrative region for interoception. The neural substrates of state-dependent changes in interoception have yet to be established but could include opioidergic systems. In studies in both humans and rats, the volume drunk after fluid deprivation is reduced by administration of an opioid antagonist (5, 6). However, the effect of naloxone on drinking after fluid deprivation may be a consequence of a change in satiation processes rather than a decrease in thirst intensity (5, 7), although it is notable that the “rush” induced by heroin is frequently associated with experiences of thirst (8). The capacity of opioids to achieve pain relief has been recognized by clinicians for centuries, but the pronociceptive effects of some opioid compounds has been a more recent discovery (9). The final pathway for opioid-mediated pain inhibition and facilitation is via projections from the rostral ventral medulla to neurons in the dorsal horn that are also the projection sites for nociceptive primary afferents (3).
The cortical representations of thirst and pain have much in common. Studies of thirst-related brain activity have identified a network including the hypothalamus, thalamus, insula, parahippocampus, somatosensory cortex, anterior cingulate, posterior cingulate, and cerebellum (1,10–12). A metaanalysis of pain imaging studies revealed a consistent network incorporating activity in the diencephalon and posterior parietal, insula, and limbic cortices and cerebellum (13). Craig (2) and Vogt (14) have suggested that the insula and anterior cingulate are key loci in the conscious experiences arising from interoceptive inputs. Comparisons of activation patterns have contrasted results from different laboratories and concluded that there are discrete representations for varied interoceptive sensations and emotional responses within the cingulate cortex (15, 16). Activations related to different interoceptive sensations have been reported in the insula, but no systematic comparison of the loci of activations in the insula has appeared in the literature. Nor have there been any reports of the functional topography of interoceptive sensations within the human cingulate and insula using multiple stimuli within a single group of subjects.
Our aim was to examine changes in regional cerebral blood flow (rCBF) associated with thirst and pain within the same subjects to identify regions in the anterior cingulate and insula that are activated by either or both of the two interoceptive sensations. Thirst was induced with an infusion of hypertonic saline, and pain was provoked with pressure to the thumbnail. We hypothesized that pain perception would be enhanced under conditions of contemporaneous thirst, and that ratings of thirst would be decreased during the experience of acute pain. Consistent with an integrative role for the pregenual anterior cingulate cortex, we hypothesized that the contemporaneous experience of thirst and pain would be associated with increases of rCBF in the pregenual area that exceeded changes associated with either of the sensations in isolation.
Results
Behavior and Physiology.
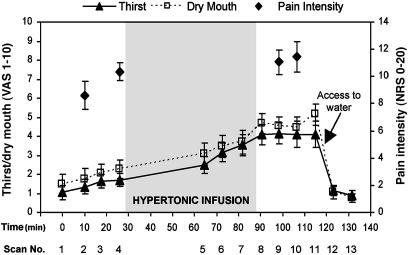
Pressures required to elicit faint pain and moderate pain were 2.3 (±0.8) and 7.8 (±2.8) kg/cm2, respectively. Thirst and dry-mouth ratings increased significantly after the hypertonic infusion [F(1,9) = 27.8, p < 0.001] but did not demonstrate any significant changes during pain versus no-pain scans [F(1,9) = 2.1, nonsignificant; Fig. 1]. Pain intensity ratings increased significantly after the hypertonic infusion [F(1,9) = 8.8, p < 0.016]. There were no significant order effects for pain ratings [F(1,9) = 2.1, p = 0.18], nor was there any interaction between the effects of order and hypertonic infusion [F(1,9) = 2.4, p = 0.16; Fig. 1].
Fig. 1.
Subjects were scanned on 13 occasions over ≈2 h. An infusion of hypertonic saline was commenced after the fourth scan and continued for 60 min. Noxious pressure was applied to the thumbnail during scans 2, 4, 9, and 10. Free access to water was given after scan 11. VAS, visual analogue scale; NRS, numerical rating scale.
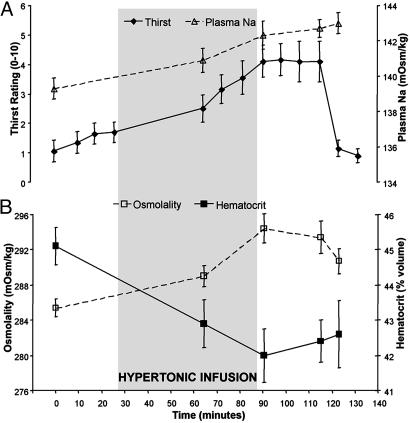
Plasma sodium and osmolality increased significantly between the commencement and termination of the infusion (p < 0.01), whereas the percentage of hematocrit decreased during the same period (p < 0.01; Fig. 2). The change in plasma Na levels between baseline and maximum thirst was 2.4% (±0.3). The percentage increase in plasma Na correlated with the increase in thirst ratings (r = 0.67, p < 0.05). Values for the osmolality of the blood generally reflected the increased levels of plasma sodium, although the final measure was significantly less than the maximum at the end of the infusion [t(9) = 3.7, p < 0.005].
Fig. 2.
Blood samples were drawn from subjects after scans 1, 5, 8, 11, and 12. Plasma Na and osmolality increased during the infusion of hypertonic saline and were relatively stable after scan 8. Decreases in the proportion of hematocrit reflected increased plasma volume associated with the fluid load. Thirst ratings increased throughout the infusion, diminishing dramatically after free access to water.
Functional Brain Imaging.
Pain activations were evident in the contralateral (right) primary somatosensory cortex (S1), contralateral secondary somatosensory cortex (S2), midline supplementary motor area, bilateral insula, contralateral mid cingulate cortex, and ipsilateral cerebellum (Table 1). The pain activation with the peak voxel in S1 extended into the inferior parietal lobule and parietal operculum (Fig. 3E). Maximum thirst activations included the pregenual anterior cingulate cortex [Brodmann area (BA) 32], mid cingulate cortex (BA 24), insula, bilateral S1, and vermis of the cerebellum (Table 1). Maximum thirst activations were also apparent in the cuneus, lingual gyrus, inferior frontal gyrus, superior temporal gyrus, and supplementary motor area. The maximum thirst activation with a peak in the supplementary motor area extended posteriorly through the primary motor and S1 cortices.
Table 1.
Activations associated with pain provoked with noxious pressure produced by hypertonic saline, middle column, and the interaction between pain and maximum thirst
| Region | Pain |
Maximum thirst |
Pain and thirst |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BA | x | y | z | Z score | BA | x | y | z | Z score | BA | x | y | z | Z score | |
| S1/M1 | 4 | 34 | −18 | 60 | 7.53 | 5 | 10 | −40 | 66 | 5.69 | 3 | 46 | −22 | 52 | 5.53 |
| 2 | 46 | −24 | 48 | 7.36 | 4 | −48 | −14 | 32 | 5.54 | 3 | 50 | −18 | 40 | 4.70 | |
| 3 | 50 | −20 | 42 | 7.03 | 3 | −32 | −34 | 48 | 4.85 | 3 | −58 | −20 | 38 | 4.31 | |
| Inferior parietal lobule | 40 | 40 | −32 | 56 | 4.42 | ||||||||||
| SMA | 6 | 0 | 6 | 52 | 4.02 | 6 | 0 | −30 | 74 | 6.38 | 6 | 6 | −12 | 70 | 4.00 |
| 6 | 0 | −16 | 74 | 3.86 | |||||||||||
| Premotor | 6 | 58 | 2 | 22 | 3.88 | ||||||||||
| Anterior cingulate | 24 | 4 | 2 | 44 | 4.28 | 32 | 12 | 24 | 26 | 6.11 | 24 | 0 | −2 | 36 | 4.44 |
| 32 | 4 | 12 | 34 | 4.13 | 32 | 10 | 38 | 2 | 4.48 | 24 | 4 | −2 | 48 | 4.39 | |
| 32 | 6 | 44 | −2 | 3.97 | 24 | 4 | 32 | 18 | 3.86 | ||||||
| Insula/operculum | 13 | 40 | 0 | 12 | 5.33 | 13 | 38 | −28 | 6 | 5.25 | 13 | −40 | −2 | −2 | 3.88 |
| 13 | 42 | 10 | 4 | 4.77 | 13 | 28 | −28 | 24 | 4.44 | ||||||
| 13 | 32 | 14 | 14 | 4.43 | 13 | 32 | −32 | 18 | 3.64 | ||||||
| 13 | −38 | 8 | 8 | 3.91 | 13 | 34 | −10 | 12 | 4.96 | ||||||
| Orbitofrontal | 10 | 38 | 48 | −2 | 4.08 | ||||||||||
| 47 | 50 | 34 | −6 | 3.94 | |||||||||||
| Inferior frontal gyrus | 47 | −52 | 36 | −6 | 5.13 | ||||||||||
| 47 | −50 | 28 | −10 | 4.97 | |||||||||||
| Superior temp. gyrus | 38 | −50 | 24 | −18 | 4.81 | ||||||||||
| Cuneus | 23 | −18 | −70 | 10 | 5.49 | ||||||||||
| 18 | −4 | −74 | 8 | 4.39 | |||||||||||
| Lingual gyrus | 18 | 8 | −50 | 2 | 4.55 | ||||||||||
| Cerebellum | −18 | −48 | −22 | 4.28 | 0 | −56 | −4 | 4.46 | |||||||
S1/M1, primary somatosensory and motor cortices; SMA, supplementary motor cortex.
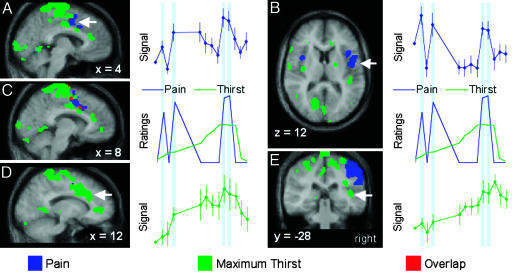
Fig. 3.
Activations for pain (blue regions), maximum thirst (green regions), and both conditions (red regions) in the anterior cingulate and insula cortices, shown on an average of the normalized magnetic resonance images for the 10 subjects. The vertical blue shaded regions in the time courses indicate scans during the pain stimuli. (A) Pain-related increases in the mid cingulate (arrow) cortex coincided with increasing intensity of pressure pain. (B) Pain-related signal change in the insula (arrow) did not show an increase at maximum thirst. (C) Pain and thirst activations were observed in medial and lateral mid cingulate cortex, respectively, with overlap at x = 8. (D) Regional CBF in the mid cingulate cortex (arrow) increased steadily in coincidence with the escalation of thirst ratings and decreased after drinking. (E) The rCBF in the posterior insula (arrow) also increased as thirst became more pronounced, but the post-drink reduction in rCBF in the insula was not as marked.
Pain and maximum thirst activations in the mid cingulate cortex were adjacent, with the pain activation medial to the maximum thirst activation. Despite some overlap (Fig. 3C) and proximity of peaks of activity (Table 1), the rCBF for pain and maximum thirst activations in the mid cingulate cortex had different time courses during the experimental protocol (Fig. 3 A and D). The rCBF for the pain activation in the mid cingulate cortex demonstrated increases consistent with the increased reports of pain intensity, whereas the rCBF from the adjacent maximum thirst activation clearly rose with the escalation of thirst and decreased substantially after drinking.
The activations for maximum thirst and pain in the insula cortex were discrete; there were no voxels showing activation in both contrasts (Fig. 3 B and E). Maximum thirst activations were primarily confined to the posterior portion of the right insula. Pain activations in the insula were in the mid and anterior regions, and although bilateral, they predominated in the hemisphere contralateral to stimulation. The rCBF in the maximum thirst activation in the right insula decreased marginally after drinking (Fig. 3E), whereas the rCBF in the major pain activation in the insula did not covary with reported levels of pain intensity.
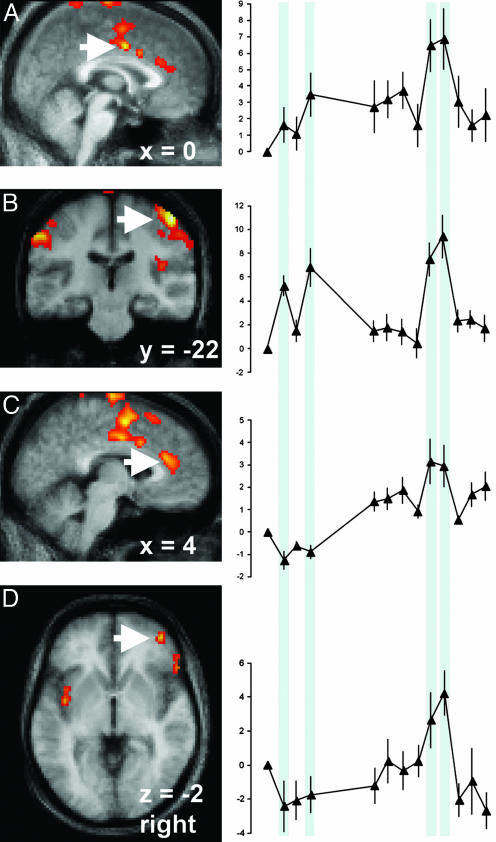
The interaction between thirst and pain was characterized by significant postinfusion increases of rCBF in regions associated with pain (Table 1). Pain intensity-dependent increases in rCBF were observed for activations in the mid cingulate cortex (Fig. 4A) and right S1 (Fig. 4B). The interaction also revealed significant increases in rCBF in the pregenual anterior cingulate cortex (Fig. 4C) and right ventral orbitofrontal cortex (Fig. 4D) that occurred during thirst and pain but were not activated during pain scans before the hypertonic infusion.
Fig. 4.
Activations associated with thirst when experiencing pain and with increased pain when thirsty demonstrated two distinct time courses. The vertical blue shaded regions in the time courses indicate the scans when noxious mechanical stimuli were applied. Activations having increased pain-related signal intensity during thirst were observed in the mid cingulate cortex (A, arrow) and contralateral S1 (B, arrow). By comparison, activations involving pain-related signal increases only during pain scans at maximum thirst were observed in the pregenual anterior cingulate cortex (C, arrow) and the ventral orbitofrontal cortex (D, arrow).
Discussion
Pain provoked by a fixed stimulus was more intense after hypertonic infusion, whereas thirst ratings did not vary when noxious pressure was applied. Activations in the mid cingulate cortex for maximum thirst and pain activations were in close proximity with some overlapping voxels. Conversely, activations for maximum thirst and pain occupied separate areas within the insula. Pain activations in the mid cingulate cortex and S1 increased during thirst when the standard pressure stimulus evoked more intense pain. Contemporaneous thirst and pain also produced activations in the pregenual anterior cingulate cortex and ventral orbitofrontal cortex that were not apparent during pain in the absence of thirst. These findings support the contention that interoceptive inputs may be integrated at a cortical level.
Pain-related activations in the pregenual anterior cingulate and ventral orbitofrontal cortex were apparent at maximum thirst but did not appear during pain at baseline. Many studies have demonstrated activation in the pregenual anterior cingulate cortex during conditions that modify pain responses such as anxiety, placebo, distraction, and capsaicin-induced hyperalgesia (17–20). Thus, pregenual anterior cingulate cortex activity occurs when pain sensitivity changes, irrespective of whether sensitivity is enhanced or diminished. A human imaging study assessing the functional connectivity of the pregenual anterior cingulate cortex during pain and distraction has identified correlated behavior in the periaqueductal gray (21). Consequently, these converging lines of evidence implicate the pregenual anterior cingulate cortex in top-down control of midbrain regions, where these regions have established roles in the descending modulation of dorsal horn responses to noxious peripheral stimuli.
The orbitofrontal cortex, like the pregenual cingulate cortex, is rarely reported as a region showing pain-related activations under normal physiological conditions (13). Unlike the pregenual anterior cingulate cortex, which shows pain-related activations when pain sensitivity is enhanced or diminished, and consistent with the study reported here, pain-related activations in the lateral ventral orbitofrontal cortex only occur when pain sensitivity in humans is enhanced (20). Stimulation of the ventral orbitofrontal cortex in rats leads to inhibition of rostral ventromedial medulla off cells, facilitation of on cells, and decreased latency of the tail-flick response (22). These pronociceptive effects are presumably mediated via projections from the ventral orbitofrontal cortex to the periaqueductal gray, because the orbitofrontal cortex does not have direction connections to the rostral ventromedial medulla. Similar mechanisms could explain the observations made in the study reported herein.
Anterior cingulate activations associated with pain and maximum thirst, respectively, were present in the middle portion of the gyrus corresponding with BA 24. This activity was increased with thirst ratings, decreased after drinking, and also increased with increasing pain intensity. A small proportion of voxels showed activity in response to both maximum thirst and pain, but generally the thirst and pain activations did not overlap significantly. Pain-related activity was more medial; thirst-related activity was lateral, being located deep in the cingulate sulcus. Previously reported loci of activations for maximum thirst are consistent with the activation site reported in this study (1).
Anatomically distinct regions of activation associated with thirst and pain were observed in the insula. This finding in a single group of subjects provides strong evidence for functional topography of interoceptive experiences within the insula. Consistent with our previous report, the experience of maximum thirst was represented in the posterior right insula (1). Pain-related activity in the insula was predominately contralateral to thumb pressure. The absence of an increase in pain activation in the insula after the hypertonic infusion may have been a function of the use of noxious pressure (23), because others have previously reported intensity-dependent activations in the insula in response to thermal stimuli (24–27).
Maximum thirst and pain activations in regions other than the insula and anterior cingulate cortex were largely consistent with previous imaging reports. This is an important finding because the induction and satiation of thirst, by its very nature, is exceedingly difficult to replicate in a single session. The test–retest validity of the thirst activations has been borne out by the pattern of activations from this study being largely consistent with those from our earlier studies. In addition to the cingulate and insula, the maximum thirst contrast identified activity in the frontal, temporal, and occipital cortices. We have previously reported frontal and temporal activity for moderate thirst, whereas activity in the cuneus was confined to the experience of mild thirst (1, 11). The extensive activity in the medial frontal gyrus and paracentral lobule is a novel finding for the maximum thirst contrast, although this area was beyond the field of view in the earlier report (1). The most substantial departure from the earlier study was the absence of a significant maximum thirst activation in the parahippocampus. In almost all respects, the pattern of pain activations corresponded with previous studies (13). An exception was the exclusively contralateral distribution of activity in the S2 region; many pain studies report bilateral activity. However, thermodes are more frequently the stimulus of choice for pain imaging studies, and the only other report of thumb pressure pain in healthy volunteers was notable for S2 activity confined to the contralateral hemisphere (28). Of note among the pain activations were signal changes in the S1 and anterior cingulate that were consistent with changes in pain report during the application of constant stimulus intensities. This observation would suggest a role for these regions in the perceptual processes of pain experience.
Pain responses to a fixed stimulus can varying dramatically in association with a wide range of environmental, cognitive, and tissue-related factors. Our findings confirm that sensitivity to noxious pressure is enhanced under circumstances of increased plasma osmolality and associated thirst. The interaction between pain and thirst has not previously been examined in humans although there is evidence that ingestion of large volumes of water can produce an increase in pain threshold with a concomitant decrease in the EEG response to a noxious laser stimulus (29). We hypothesized a reduction in thirst ratings during noxious pressure. The levels of thirst reported by the subjects at maximum thirst were relatively low, which may have precluded a further reduction during the experience of pain. Alternatively, moderate thirst may be resistant to extraneous influences, although there is some evidence from animal models that drinking behavior diminishes in response to pain provoked by repeated tail pinch (30). Under more extreme circumstances, such as intense thirst, persistent behavioral responses to pain could be maladaptive. Indeed, unlike schedule-induced drinking, deprivation-induced drinking in rats is not influenced by tail pinch (30).
Interaction of concurrent interoceptive experiences in humans has been examined by using tourniquet ischemia and hypercapnia (31). Increased respiratory drive is associated with increased levels of dyspnea during painful ischemia, whereas hypercapnia during tourniquet application leads to diminished reports of pain intensity. Hunger and pain also elicit different behavioral responses depending on the relative priority of the competing sensations. For example, rats operantly conditioned to seek food ingest fewer pellets than nonconditioned rats in the acute phase of a painful inflammation of the paw (32). However, in the later phases of the inflammatory state, conditioned rats spend more time acquiring food and exhibit less pain behavior than nonconditioned rats.
Conclusion
We have examined brain activity associated with thirst and pain experiences in a single group of subjects, to examine the commonality and differences of activation in the anterior cingulate cortex and insulae. Pain perception was enhanced under conditions of contemporaneous thirst, whereas thirst ratings were unaffected by pain. These sensations elicited activations in adjacent regions of the anterior cingulate cortex, but in different locations in the insulae, suggesting a functional topography for pain and thirst in the two regions. The concurrence of pain and thirst were notable for activations in the pregenual cingulate cortex and ventral orbitofrontal cortex that were not in evidence for either sensation in isolation, pointing toward an integrative role for the regions in the processes of context-dependent changes in sensation.
Methods
Subject Recruitment and Procedures Before Attendance for Imaging.
Procedures for recruitment, obtaining informed consent, and the execution of experimental procedures were approved by the Institutional Review Board of the University of Texas Health Sciences Center. Ten healthy male subjects with a mean age of 23.7 (± 2.8 years; range 21–30) were instructed to abstain from the consumption of alcohol on the day before their attendance at the Research Imaging Center. All subjects were tested in the morning after a light breakfast including one glass (150 ml) of water or fruit juice and excluding any other beverages.
Psychophysical Measures.
Pressure stimuli were delivered to the left thumbnail. Pain was rated with a combined numerical, word descriptor scale that is based on results from cross modality procedures (range 0–20) (33). Brief stimuli (5-s duration) were delivered in a double random staircase to establish the pressure required to elicit faint pain (pain threshold, 0.5 on pain scale) and moderate pain (11.5 on pain scale) (34). Subjects were instructed to provide ratings of thirst by using a 10-point visual analogue scale with the limits “no thirst” and “most intense thirst ever experienced,” scored, respectively, as 0 and 10. Dry mouth was rated with a 10-point visual analogue scale with the limits “mouth not dry” and “most intense mouth dryness ever experienced,” scored, respectively, as 0 and 10.
Image Acquisition.
Structural images were acquired with a 1.9-T Elscint/GE magnetic resonance scanner by using a T1-weighted, 3D, gradient recalled echo sequence (repetition time = 35 ms, echo time = 7 ms, flip angle = 60°, 1.0-mm2 sagittal acquisition, 1.3-mm slice thickness). Positron-emission tomography (PET) images were acquired with an ECAT HR+ PET scanner (CTI Molecular Imaging, Knoxville, TN) with 63 transverse slices of 2.5-mm thickness. For each scan, subjects received a 15-mCi (1 Ci = 37 GBq) bolus injection of H215O.
Experimental Protocol.
After completion of psychophysical procedures, subjects were prepared for PET imaging. Each subject was fitted with a thermoplastic face mask to reduce head movement. A cannula was inserted in the right antecubital vein for administration of H215O doses and the collection of blood samples. A second cannula was inserted in the left arm for infusion of hypertonic saline. Ratings of thirst and dry mouth were collected after all scans. The first and third scans were performed during rest, eyes closed. Pressure, at intensities likely to elicit moderate pain (values previously determined by staircase method), were applied to the left thumbnail during acquisition of the second and fourth scans. Pressure was released for 1 s every 5 s during the course of the pain scans because intermittent pressure is better tolerated than constant pressure and less likely to summate (28). After each pain scan, pressure was reapplied to the thumb and ratings of thirst, dry mouth, and pain were collected. Hypertonic infusions were commenced after the fourth scan (5% saline, infusion volume = 3.0 ± 0.4 ml/kg, duration = 61.8 ± 4.9 min). Scans 5–8 were performed during the course of the hypertonic infusion. Scans 9–11 were collected after the infusion at maximum thirst. Subjects were given free access to water, and two post-drink scans were acquired. Moderately painful pressure was applied and rated at maximum thirst (scans 9 and 10) as for baseline (scans 2 and 4). Five blood samples were collected. The samples were collected at baseline, start and end of infusion, maximum thirst, and post-drink (scans 1, 5, 8, 11, and 12, respectively).
Analysis
Psychometric and Physiological Measures.
Repeated-measures analysis of variance was used to test for the effects of time, pain, and hypertonic infusion on thirst and dry mouth during baseline and after infusion periods. Pain ratings were also assessed with repeated-measures analysis of variance to establish any effects of order and hypertonic solution. Sequential pairs of physiological measures (plasma Na, hematocrit, plasma osmolality) were tested for significant change with paired t tests.
Functional Brain Images.
After global normalization [value-normalized to whole brain mean activity and then scaled to an arbitrary mean of 1,000 (35–37)] of each individual PET scan image, interscan, intrasubject movement was assessed and corrected by using mcflirt (38). PET and magnetic resonance images were coregistered and spatially normalized to the Talairach and Tournoux atlas (39) by using an affine, nine-parameter transformation (40, 41). Images were spatially smoothed by using spm2 (Wellcome Department of Cognitive Neurology, London). A statistical parametric map of the pain response was generated by contrasting noxious pressure (scans 2, 4, 9, and 10) with no pressure (scans 1, 3, 8, and 11). A maximum thirst statistical parametric map was generated by contrasting the baseline (scans 1–4) with maximum thirst (scans 8–11). Interactions between pain and maximum thirst were tested by contrasting concurrent pain and thirst (scans 9 and 10) with maximum thirst (scans 8 and 11) and pain (scans 2 and 4) in isolation. Significant clusters of activation were identified by using a cluster level threshold (pcorr < 0.05) and a single voxel inclusion threshold of Z > 2.33 (p < 0.01). The mean percentage change in the globally normalized signal for each of the 13 scans was calculated for selected activations from each of the contrasts to demonstrate patterns of change during the experimental protocol.
Acknowledgments
This work was supported by the Robert J. Jr. and Helen C. Kleberg Foundation, the G. Harold and Leila Y. Mathers Charitable Foundation, the Brown Foundation, the Search Foundation, and the National Health and Medical Research Council of Australia.
Glossary
Abbreviations:
- BA
Brodmann area
- PET
positron-emission tomography
- rCBF
regional cerebral blood flow
- S1
primary somatosensory cortex
- S2
secondary somatosensory cortex
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Denton D., Shade R., Zamarippa F., Egan G., Blair-West J., McKinley M., Lancaster J., Fox P. Proc. Natl. Acad. Sci. USA. 1999;96:5304–5309. doi: 10.1073/pnas.96.9.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Craig A. D. Curr. Opin. Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 3.Fields H. Nat. Rev. Neurosci. 2004;5:565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- 4.Sewards T. V., Sewards M. A. Brain Res. Bull. 2003;61:25–49. doi: 10.1016/s0361-9230(03)00069-8. [DOI] [PubMed] [Google Scholar]
- 5.Leventhal L., Bodnar R. J. Brain Res. 1996;741:300–308. doi: 10.1016/s0006-8993(96)00951-1. [DOI] [PubMed] [Google Scholar]
- 6.Silver A. J., Morley J. E. J. Am. Geriatr. Soc. 1992;40:556–560. doi: 10.1111/j.1532-5415.1992.tb02102.x. [DOI] [PubMed] [Google Scholar]
- 7.Seckl J. R., Johnson M. R., Lightman S. L. Clin. Endocrinol. (Oxford) 1989;30:513–518. doi: 10.1111/j.1365-2265.1989.tb01422.x. [DOI] [PubMed] [Google Scholar]
- 8.Seecof R., Tennant F. S., Jr. Am. J. Drug Alcohol Abuse. 1986;12:79–87. doi: 10.3109/00952998609083744. [DOI] [PubMed] [Google Scholar]
- 9.Pan Z. Z., Tershner S. A., Fields H. L. Nature. 1997;389:382–385. doi: 10.1038/38730. [DOI] [PubMed] [Google Scholar]
- 10.Denton D., Shade R., Zamarippa F., Egan G., Blair-West J., McKinley M., Fox P. Proc. Natl. Acad. Sci. USA. 1999;96:2532–2537. doi: 10.1073/pnas.96.5.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egan G., Silk T., Zamarripa F., Williams J., Federico P., Cunnington R., Carabott L., Blair-West J., Shade R., McKinley M., et al. Proc. Natl. Acad. Sci. USA. 2003;100:15241–15246. doi: 10.1073/pnas.2136650100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parsons L. M., Denton D., Egan G., McKinley M., Shade R., Lancaster J., Fox P. T. Proc. Natl. Acad. Sci. USA. 2000;97:2332–2336. doi: 10.1073/pnas.040555497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farrell M. J., Laird A. R., Egan G. F. Hum. Brain Mapp. 2005;25:129–139. doi: 10.1002/hbm.20125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogt B. A. Nat. Rev. Neurosci. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liotti M., Brannan S., Egan G., Shade R., Madden L., Abplanalp B., Robillard R., Lancaster J., Zamarripa F. E., Fox P. T., Denton D. Proc. Natl. Acad. Sci. USA. 2001;98:2035–2040. doi: 10.1073/pnas.98.4.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vogt B. A., Berger G. R., Derbyshire S. W. Eur. J. Neurosci. 2003;18:3134–3144. doi: 10.1111/j.1460-9568.2003.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ploghaus A., Narain C., Beckmann C. F., Clare S., Bantick S., Wise R., Matthews P. M., Rawlins J. N., Tracey I. J. Neurosci. 2001;21:9896–9903. doi: 10.1523/JNEUROSCI.21-24-09896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrovic P., Kalso E., Petersson K. M., Ingvar M. Science. 2002;295:1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- 19.Bantick S. J., Wise R. G., Ploghaus A., Clare S., Smith S. M., Tracey I. Brain. 2002;125:310–319. doi: 10.1093/brain/awf022. [DOI] [PubMed] [Google Scholar]
- 20.Lorenz J., Cross D., Minoshima S., Morrow T., Paulson P., Casey K. Neuron. 2002;35:383–393. doi: 10.1016/s0896-6273(02)00767-5. [DOI] [PubMed] [Google Scholar]
- 21.Valet M., Sprenger T., Boecker H., Willoch F., Rummeny E., Conrad B., Erhard P., Tolle T. R. Pain. 2004;109:399–408. doi: 10.1016/j.pain.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 22.Hutchison W. D., Harfa L., Dostrovsky J. O. Neuroscience. 1996;70:391–407. doi: 10.1016/0306-4522(95)00372-x. [DOI] [PubMed] [Google Scholar]
- 23.Ringler R., Greiner M., Kohlloeffel L., Handwerker H. O., Forster C. Pain. 2003;105:445–453. doi: 10.1016/S0304-3959(03)00258-6. [DOI] [PubMed] [Google Scholar]
- 24.Bornhovd K., Quante M., Glauche V., Bromm B., Weiller C., Buchel C. Brain. 2002;125:1326–1336. doi: 10.1093/brain/awf137. [DOI] [PubMed] [Google Scholar]
- 25.Coghill R. C., Sang C. N., Maisog J. M., Iadarola M. J. J. Neurophysiol. 1999;82:1934–1943. doi: 10.1152/jn.1999.82.4.1934. [DOI] [PubMed] [Google Scholar]
- 26.Derbyshire S. W., Jones A. K., Gyulai F., Clark S., Townsend D., Firestone L. L. Pain. 1997;73:431–445. doi: 10.1016/S0304-3959(97)00138-3. [DOI] [PubMed] [Google Scholar]
- 27.Iannetti G. D., Zambreanu L., Cruccu G., Tracey I. Neuroscience. 2005;131:199–208. doi: 10.1016/j.neuroscience.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 28.Gracely R. H., Petzke F., Wolf J. M., Clauw D. J. Arthritis Rheum. 2002;46:1333–1343. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- 29.Sedan O., Sprecher E., Yarnitsky D. Pain. 2005;113:354–359. doi: 10.1016/j.pain.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 30.Murphy E. P., Porter J. H., Heath G. F. Behav. Neural Biol. 1985;43:86–99. doi: 10.1016/s0163-1047(85)91524-9. [DOI] [PubMed] [Google Scholar]
- 31.Nishino T., Shimoyama N., Ide T., Isono S. Anesthesiology. 1999;91:1633–1638. doi: 10.1097/00000542-199912000-00014. [DOI] [PubMed] [Google Scholar]
- 32.LaGraize S. C., Borzan J., Rinker M. M., Kopp J. L., Fuchs P. N. Neurosci. Lett. 2004;372:30–34. doi: 10.1016/j.neulet.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 33.Gracely R. H., Dubner R., McGrath P. A. Science. 1979;203:1261–1263. doi: 10.1126/science.424753. [DOI] [PubMed] [Google Scholar]
- 34.Gracely R. H., Lota L., Walter D. J., Dubner R. Pain. 1988;32:55–63. doi: 10.1016/0304-3959(88)90023-1. [DOI] [PubMed] [Google Scholar]
- 35.Friston K. J., Frith C. D., Liddle P. F., Dolan R. J., Lammertsma A. A., Frackowiak R. S. J. Cereb. Blood Flow Metab. 1990;10:458–466. doi: 10.1038/jcbfm.1990.88. [DOI] [PubMed] [Google Scholar]
- 36.Fox P. T., Mintun M. A., Reiman E. M., Raichle M. E. J. Cereb. Blood Flow Metab. 1988;8:642–653. doi: 10.1038/jcbfm.1988.111. [DOI] [PubMed] [Google Scholar]
- 37.Raichle M. E., Martin W. R., Herscovitch P., Mintun M. A., Markham J. J. Nucl. Med. 1983;24:790–798. [PubMed] [Google Scholar]
- 38.Jenkinson M., Bannister P., Brady M., Smith S. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 39.Talairach P., Tournoux J. A Stereotactic Coplanar Atlas of the Human Brain. Stuttgart, Germany: Thieme; 1988. [Google Scholar]
- 40.Lancaster J., Glass T. G., Lankipalli B. R., Downs H., Mayberg H., Fox P. Hum. Brain Mapp. 1995;3:209–223. [Google Scholar]
- 41.Lancaster J. L., Fox P. T., Downs H., Nickerson D. S., Hander T. A., El Mallah M., Kochunov P. V., Zamarripa F. J. Nucl. Med. 1999;40:942–955. [PubMed] [Google Scholar]