Abstract
Current approaches to tissue regeneration are limited by the death of most transplanted cells and/or resultant poor integration of transplanted cells with host tissue. We hypothesized that transplanting progenitor cells within synthetic microenvironments that maintain viability, prevent terminal differentiation, and promote outward migration would significantly enhance their repopulation and regeneration of damaged host tissue. This hypothesis was addressed in the context of muscle regeneration by transplanting satellite cells to muscle laceration sites on a delivery vehicle releasing factors that induce cell activation and migration (hepatocyte growth factor and fibroblast growth factor 2) or transplantation on materials lacking factor release. Controls included direct cell injection into muscle, the implantation of blank scaffolds, and scaffolds releasing factors without cells. Injected cells demonstrated a limited repopulation of damaged muscle and led to a slight improvement in muscle regeneration, as expected. Delivery of cells on scaffolds that did not promote migration resulted in no improvement in muscle regeneration. Strikingly, delivery of cells on scaffolds that promoted myoblast activation and migration led to extensive repopulation of host muscle tissue and increased the regeneration of muscle fibers at the wound and the mass of the injured muscle. This previously undescribed strategy for cell transplantation significantly enhances muscle regeneration from transplanted cells and may be broadly applicable to the various tissues and organ systems in which provision and instruction of a cell population competent to participate in regeneration may be clinically useful.
Keywords: fibroblast growth factor 2, hepatocyte growth factor, myoblast, satellite cell, tissue engineering
Cell therapies have tremendous potential to treat a wide array of diseases and tissue defects, and the two major strategies of cell transplantation use either the direct introduction of cell suspensions, typically injected into the tissue of interest, or preculture and transplantation of cells on scaffolds intended to serve as templates for tissue formation (1, 2). However, poor cell survival and limited incorporation into host tissue are typical for directly injected cells, whereas integration of templated tissues with host tissues remains a significant challenge and does not repair damaged host tissue. For example, injected myoblasts enhance skeletal regeneration and reconstitute apparently normal fibers in dystrophic mice (3, 4), but the limited success of human myoblast transplantation is likely related to poor survival and spread of the transplanted cells (5). Three-dimensional skeletal muscle tissues are also frequently generated in vitro from cultured myoblasts (6–10), but it is unclear how to structurally and functionally integrate these new tissues with the host musculature.
We propose a previously undescribed approach to tissue regeneration that involves the transplantation of progenitor cells on scaffolds that are not intended to guide tissue formation around the scaffold but, in contrast, are designed to maintain the viability of passenger cells while simultaneously encouraging their activation and outward migration to repopulate the surrounding host-damaged tissue and enhance its regeneration. With this approach, the scaffold will mimic aspects of the special tissue microenvironments, termed niches, that maintain the potential of stem cell populations while allowing the daughter cells to migrate and attain specialized functions distant to the niche (11).
The potential of this approach to tissue regeneration was addressed specifically in the context of skeletal muscle regeneration, because there is great interest in developing new strategies to treat the six million Americans diagnosed with musculoskeletal diseases each year (12, 13). The process of skeletal muscle regeneration requires that quiescent satellite cells, which derive from the dermomyotome during development (14), become activated, proliferate, migrate to the site of injury, and fuse to form new fibers or to repair damaged fibers (15, 16). These activated proliferating skeletal muscle precursors are termed myoblasts, and it will be critical to appropriately design the physical and chemical aspects of the scaffold to successfully achieve host tissue repopulation and simultaneously prevent the premature terminal differentiation of these precursor cells in the scaffold. Myoblast fate is regulated by a variety of microenvironmental signals, including extracellular matrix molecules and growth factors. One may enhance myoblast survival and proliferation and regulate the extent of differentiation in vitro by providing a high density of arginine, glycine, aspartic acid (RGD)-containing cell adhesion ligands from polysaccharide gels used to encapsulate the cells (17). Trophic factors also clearly regulate myoblast fate and, whereas numerous factors regulate the proliferation and differentiation of satellite cells, hepatocyte growth factor (HGF) and fibroblast growth factor 2 (FGF2) have been specifically demonstrated to have a physiological role in skeletal muscle regeneration (18, 19). HGF is a primary initiator of satellite cell activation (20) and stimulates satellite cells to enter the cell cycle in vivo, and the receptor for HGF, c-met, is expressed in both quiescent and activated satellite cells (19, 21). FGF2 has been found to be present in the basement membrane surrounding developing myotubes, and Syndecan 3 and 4, which mediate FGF2 signaling, are present on quiescent and activated satellite cells (21). Interestingly, investigators have demonstrated that, whereas HGF and FGF increase myoblast numbers in vitro, both can inhibit muscle differentiation in vivo (22, 23).
In this report, scaffolds designed to promote myoblast survival and migration and prevent terminal differentiation were examined for their ability to enhance repopulation of injured muscle from transplanted myoblasts and increase regeneration. Scaffolds formed from arginine, glycine, aspartic acid (RGD)-presenting polymer, which also provide a sustained delivery of HGF and FGF2, were used in these studies. We have recently demonstrated that these materials dramatically increase the viability of seeded cells, increase outward migration to >100% of the original cell number, and simultaneously prevent the terminal differentiation of cells in the scaffold (24). A muscle laceration model was used in the current study to recapitulate injuries common in athletes and in trauma (25–27), because a variety of studies suggest the disparity between results in mouse and human studies is in part due to the indirect relation of the models often used in past mouse studies (irradiation, injection of cardiotoxin, or cryoinjury) to human injuries or disease (5, 28, 29). Further, donor myoblasts were obtained from Rosa26 mice that overexpress β-galactosidase and were transplanted into genetically matched normal mice to determine the participation of host versus donor cells in regeneration (30).
Results
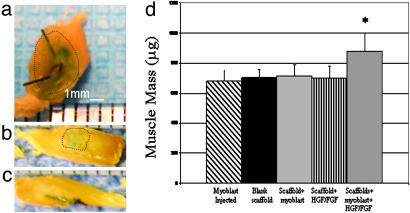
The tibialis muscle of each mouse was lacerated, the laceration was subsequently closed with suture, and one of five conditions was used to treat the laceration site: (i) myoblasts were directly injected into the muscle at the laceration site, (ii) blank scaffolds were placed over the laceration, (iii) scaffolds seeded with myoblasts were placed over the laceration, (iv) scaffolds releasing HGF and FGF2 (− cells) were placed over the laceration, and (v) scaffolds containing myoblasts and releasing HGF and FGF2 were placed. The implants were placed without the aid of any adhesive or glue and, upon retrieval at 10 and 30 days, 80% of the implants were in the same location as the day of surgery. The implants were attached to the injury site and the overlying epidermis by fascia-like tissue. A gross difference in the size of injured muscle treated with scaffolds containing growth factors and myoblasts, as compared with all other conditions, was observed at 30 days, because these muscles were larger in every dimension than the other conditions tested (Fig. 1 a–c). Quantification of the mass of these muscles revealed a statistically significant 30% higher mass compared with the other conditions (Fig. 1d). Gross observation also revealed that β-galactosidase activity, as indicated by LacZ staining, was noticeably more intense in muscles treated with the scaffolds containing myoblasts and growth factors (Fig. 1a) than in the other conditions in which myoblasts were transplanted, indicating a greater repopulation of the native muscle by cells transplanted in this condition.
Fig. 1.
Photographs of tibialis anterior muscles treated with scaffolds delivering cells and releasing HGF and FGF2 (a), scaffolds containing only HGF and FGF2 (b), and scaffolds containing only myoblasts (c). Muscles were stained to allow gross identification of regions containing donor cells (dotted lines outline positively stained tissue). Size bars are shown on the photomicrographs. (d) The mass of the muscle at 30 days after injury was greater when treated with scaffolds containing myoblasts and HGF and FGF2 (HGF/FGF2 cells in scaffold) as compared with injuries treated with an injection of myoblasts directly into the muscle [cells (injected)], blank scaffolds, scaffolds releasing growth factors without cells (HGF/FGF), or cells transplanted in scaffolds not releasing growth factors (cells in scaffold). Values represent mean and SD (n = 6). ∗, statistically significant difference (P < 0.001) compared with all other conditions.
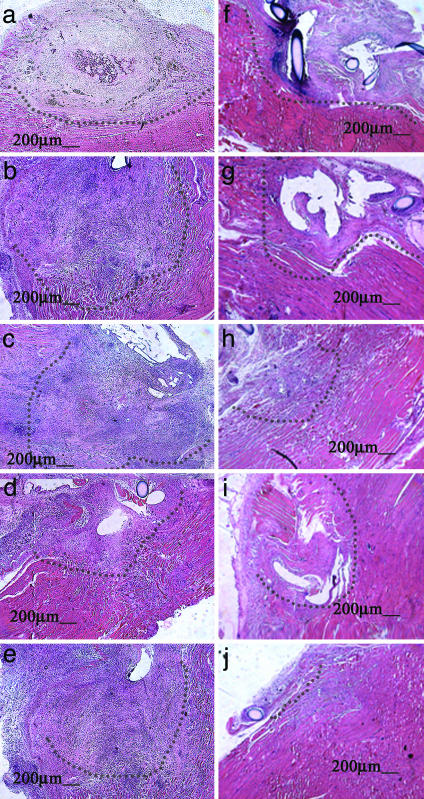
Analysis of tissue sections revealed a defect at 10 days that appeared largely necrotic in all conditions (Fig. 2 a–e). No normal muscle tissue appeared within the defect at this early time point. The defect was filled with cellular debris, blood, and basophilic cells. There were no myofibers that spanned the defect area. The muscle fibers that lined the borders of the defect were largely disorganized and contained centrally located nuclei. Interestingly, the muscle injury treated with a localized sustained delivery of growth factors alone had a larger defect area than any other condition at this time point, although this difference was statistically significant only when compared with the injury treated with sustained delivery of both myoblasts and growth factors. When sections from muscle defects treated with myoblast transplantation were viewed under high-power magnification, there were no gross differences observed in the number of LacZ(+) cells present in the tissue at this time (data not shown).
Fig. 2.
Photomicrographs of defects 10 (a–e) and 30 days after injury (f–j). Conditions included injuries treated with an injection of myoblasts directly into the muscle (a and f), blank scaffolds (b and g), scaffolds releasing growth factors without cells (c and h), cells transplanted in scaffolds not releasing growth factors (d and i), and scaffolds delivering myoblasts and HGF and FGF2 (e and j). Defects are outlined with dotted lines. At 10 days, defects were unresolved and filled with necrotic debris in all conditions. At 30 days, the laceration injuries began to resolve in all conditions, but myoblasts delivered on scaffolds in combination with growth factors led to virtually complete resolution of the defect at this time point. Size bars are shown on the photomicrographs.
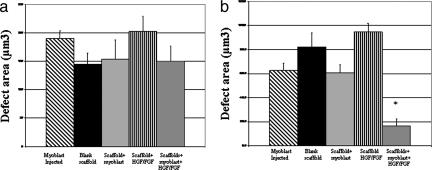
In contrast to early results, the defects in the muscles treated with sustained localized delivery of myoblasts and growth factors were largely resolved at 30 days (Fig. 2j). In many of these animals, the only remaining defect was that caused by the closing suture. In addition, there were few areas of fat deposit and virtually no scar tissue at this time point. The unresolved defect areas in the other experimental conditions had also decreased in size (Fig. 2 f–i), as compared with the defect area at 10 days, but were much larger than the cell/HGF and FGF2 delivery condition. In addition, scar tissue or fat deposits were apparent in these other conditions. When the areas of unresolved defects were quantified, there were no statistically significant differences between the conditions at 10 days (Fig. 3a). However, at 30 days after injury, the defects in muscles treated with scaffolds delivering cells and growth factors were significantly smaller than in any other condition (Fig. 3b). A lesser reduction in defect size was also seen in muscles treated with injected cells or scaffolds delivering HGF and FGF2.
Fig. 3.
Quantitative analysis of the remaining defect area 10 (a) and 30 days after injury (b). Conditions included an injection of myoblasts directly into the muscle [cells (injected)], blank scaffolds, scaffolds releasing growth factors without cells (HGF/FGF), cells transplanted in scaffolds not releasing growth factors (cells in scaffold), and scaffolds delivering myoblasts and HGF and FGF2 (HGF/FGF2 cells in scaffold). No significant resolution of the defects occurred in any condition at 10 days. In contrast, at 30 days after injury, the defects in muscles treated with scaffolds delivering cells and growth factors were significantly smaller than in any other condition (∗, P < 0.05, as compared with all other conditions). A less-pronounced but still significant reduction in defect size was also seen in muscles treated with injected cells or scaffolds delivering HGF and FGF2 (#, P < 0.01 compared with blank scaffolds or cells transplanted on scaffolds not releasing growth factors). Values represent mean and SD (n = 6).
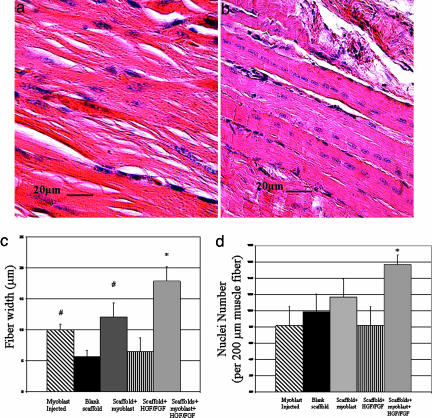
To further analyze muscle regeneration, the mean width of regenerated myofibers and number of postmitotic centrally located nuclei per length of myofiber in the region proximal to the resolving muscle defects were quantified. The mean width of regenerating fibers and density of centrally located nuclei were qualitatively greater in muscles treated with scaffold delivery of cells and growth factors (Fig. 4b) as compared with scaffolds delivering only growth factors (Fig. 4a) or any other experimental condition. Determination of the mean width of fibers 30 days after injury confirmed that muscles treated with myoblasts in combination with growth factors exhibited a 3-fold increase in fiber size as compared with the blank scaffolds, injected cells, or cells transplanted alone in scaffolds (Fig. 4c). The fiber width also increased in the experimental group involving HGF and FGF2 delivery but not as dramatically. In addition, the muscle fibers in the injury group treated with myoblasts and growth factors via scaffold delivery contained 30% more centrally located nuclei than any other condition at 30 days after injury (Fig. 4d), indicating more fusion of myoblasts into the fibers, which is consistent with the larger size of these fibers.
Fig. 4.
The width of regenerating fibers and number of centrally located nuclei at 30 days were significantly greater in muscles treated with scaffolds delivering cells and growth factors (b) as compared with scaffolds delivering only growth factors (a) or any of the other conditions. Fiber width was quantified (c), as was the number of centrally located nuclei per fiber length (d). Fiber width was increased with myoblast injection or treatment with scaffolds releasing HGF and FGF2 (#, P < 0.01 compared with blank scaffolds or scaffolds transplanting cells without growth factors) and was most dramatically increased by treatment with scaffolds delivering myoblasts and growth factors (∗, P < 0.001 compared with all other conditions). Increased centrally located nuclei per muscle length were observed only when scaffolds containing myoblasts and HGF/FGF2 were used to treat muscle injury. Values represent mean and SD (n = 6).
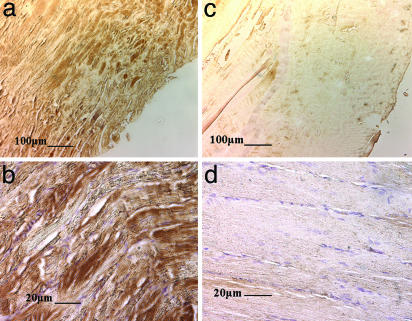
Finally, immunostaining of tissue sections from the tibialis anterior muscle 30 days after injury revealed that the increases in the muscle size, fiber width, and fiber nuclei were accompanied by robust engraftment of transplanted myoblasts into host-regenerating muscle, when cells were transplanted on scaffolds releasing HGF/FGF2 (Fig. 5 a and c). A more limited number of engrafted donor cells was noted in the condition by using direct myoblast injection (Fig. 5 b and d). No LacZ(+) cells were noted in the other experimental and control conditions.
Fig. 5.
Photomicrographs at low (a and c) and high power (b and d) of tissue sections immunostained to identify donor myoblasts (positive staining for β-galactosidase) in the regenerating tissues. Injection of cells (c and d) leads to minimal donor cell incorporation into host musculature. In contrast, transplantation of cells on scaffolds releasing growth factors leads to extensive incorporation of donor cells into the regenerating muscle tissue (a and b). Size bars are shown on the photomicrographs.
Discussion
Modulation of tissue regeneration subsequent to injury by cell transplantation requires the survival of donor cells and their stable incorporation into the host tissue. Transplantation of cells on scaffolds, which activates the cells promotes their outward migration and prevents premature terminal differentiation, combines the advantages of tissue regeneration obtained with direct cell injection with the control over transplanted cell fate made possible with the use of cell-instructive scaffolds. The results of this study indicate specifically that direct injection of myoblasts into injured muscles enhances regeneration, as does localized delivery of HGF and FGF2 from a scaffold. However, transplanting the cells with a scaffold that simultaneously delivers HGF and FGF2 dramatically enhanced the participation of transplanted cells in muscle regeneration and the overall extent of regeneration. This general approach to tissue regeneration is anticipated to find broad utility in the transplantation of many cell types and regeneration of multiple tissues.
Transplantation of myoblasts via direct injection and delivery with a scaffold not releasing growth factors led to distinct outcomes in this model system. Injection of myoblasts, in support of many past reports (5), enhanced muscle regeneration, although to a modest extent. The injected cells participated in muscle fiber formation, as evidenced by identification of donor-derived cells in the defect site, decreased mean defect size at 30 days and increased skeletal muscle fiber width. In contrast, transplantation of the same cell number on the scaffolds without growth factor release led to no detectable changes in muscle regeneration as compared with implantation of blank scaffolds at the defect site. This finding is not surprising, because cell migration out of scaffolds is low in the absence of the activating effects of HGF and FGF2 (20–30% of seeded cells migrate from scaffolds over 4 days in vitro) (24). This condition likely provides few cells that can participate in regeneration of the surrounding tissue.
Delivery of a combination of HGF and FGF2 from scaffolds, in the absence of transplanted cells, had a modest effect on muscle regeneration. The width of regenerating fibers was increased in this condition, as compared with blank scaffolds, and the number of centrally located nuclei in these fibers, a hallmark of regenerating myofibers (16), was increased as well. These effects were consistent with the modest decrease in defect area noted at 30 days. Past studies of local HGF and FGF2 delivery to sites of muscle regeneration, however, have led to results distinct from those obtained in this study. Local HGF delivery has been documented to increase the number of activated myoblasts within injured muscle, consistent with its well recognized role in activating satellite cells, but repeated presentation of HGF actually inhibited regeneration (22). The high dose of HGF in that study may have retarded the ability of host myoblasts to withdraw from the cell cycle and terminally differentiate. In addition, application of endogenous FGF2 has been reported to not enhance muscle regeneration (23). However, the system used in the current study, in contrast to the previous studies, delivered very small quantities of the factors (5 ng), continually released the factors over a time period of 3–10 days (24), and delivered a combination of the two factors in place of a single factor; the type of muscle injury was also different. This system likely yields concentrations of growth factor sufficient to enhance myoblast viability and migration within the scaffold and to mobilize myoblasts in host tissue but insufficient to inhibit myogenesis outside the scaffold. Delivery of growth factors alone, both in this and previous studies (22, 31), led to no significant increase in muscle mass.
Transplanting myoblasts on a scaffold that promoted their outward migration significantly enhanced muscle regeneration by every measure examined in this study. The number of transplanted cells participating in muscle regeneration, as indicated by immunohistochemical staining for β-galactosidase, dramatically increased. The width of regenerating fibers was significantly enhanced, as was the number of centrally located nuclei in the fibers, which are both consistent with an increased number of myoblasts participating in muscle formation. The enhanced regeneration led to almost complete resolution of the injury defect by 30 days and to a significant recovery of muscle mass after the atrophy induced by the injury. Cells placed in these growth factor-releasing scaffolds very efficiently migrate out from the scaffolds in vitro (100% migration in 4 days), and the growth factor release maintains the cells in an activated, proliferating, but nondifferentiated state (myoD-positive, myogenin-negative) in the scaffold (24). Myoblasts injected into muscle likely have poor survival due to the lack of an adhesion substrate and the inflammatory environment present in the injury. Transplantation of cells in scaffolds likely maintains the viability of the transplanted cells [cells within scaffolds in vitro still ≈50% viable at 4 days (24)], while partially protecting them from the inflammatory environment. Further, activation of myoblasts by exposure to HGF and FGF2 increases their migration and proliferation (32, 33) and thus likely enhances their ability to populate host musculature. The increase in muscle mass, muscle fiber size, and the number of myonuclei per fiber, all events that resemble the normal regeneration of muscle tissue, give hope for the utility of this approach in the treatment of muscle lacerations.
This strategy to enhance skeletal muscle regeneration with cell transplantation is extremely flexible and may be widely useful. A number of stem cell populations can participate in muscle regeneration (34), and any of the populations could be used with this approach. Similarly, a wide variety of other factors play a role in regulating the proliferation and differentiation of satellite cells (31, 35–40) and could readily be incorporated into this system. Finally, this approach may be broadly applicable to any tissue or organ system in which provision and instruction of a cell population competent to participate in regeneration of damaged tissues may be clinically useful (e.g., applications ranging from hematopoietic system reconstitution to neural regeneration).
Materials and Methods
Scaffold Preparation.
Ultrapure MVG alginate powder (Pronova, Oslo) was irradiated with a cobalt-60 source for 4 h at a γ-dose of 5.0 Mrad (Phoenix Laboratory, University of Michigan) to produce low-molecular-weight alginate [weight average molecular weight (Mw) = 5.3 × 104 g/mol] (41). Alginates were further modified with covalently conjugated oligopeptides with a sequence of G4RGDSP (Commonwealth Biotechnologies, Richmond, VA) at an average density of 3.4 mM peptide/mol of alginate monomer, by using carbodiimide chemistry, as described (42). High-molecular-weight ultrapure alginate (MVG; Pronova; Mw = 2.7 × 105 g/mol) was also covalently modified with this oligopeptide.
To fabricate alginate scaffolds that were highly porous, molds (2 × 5×5 mm) were constructed from polyvinylsulfoxane (PVS) (Kerr, Orange, CA). Porogens were constructed from size 14 stainless-steel orthodontic straight wire cut to 10-mm lengths. The orthodontic wire was aligned in two sets of parallel rows 500 μm apart, sterilized, and placed in the scaffold mold. A solution containing equal concentrations of irradiated low-molecular-weight (1%, wt/vol) and nonirradiated high-molecular-weight (1%, wt/vol) modified alginate was prepared in calcium-free DMEM (Invitrogen). HGF (Santa Cruz Biotechnology) and FGF2 (BD Biosciences, Franklin Lakes, NJ) were added to the alginate solution (final concentrations, 100 ng/ml). A calcium sulfate slurry (0.41 g of CaSO4/ml double-distilled H2O) (Aldrich) was added at a ratio of 40 μl of CaSO4/1 ml of alginate and vigorously mixed. The resulting solution was immediately expressed into the PVS mold containing the wire porogens. A sterile glass plate was placed over the mold and, after the alginate had completely gelled (30 min), the gel containing the wire porogens was carefully lifted from the PVS mold and placed in a 100-cm3 Petri dish. To produce macroporous scaffolds with open interconnected pores, the gels were cooled to −70°C, the wire porogens were carefully removed, and the gels were lyophilized and stored at −20°C until needed.
Cell Culture and Seeding.
Four-month-old B6.129S7-Gt(ROSA)26Sor/J mice (The Jackson Laboratory) were killed, and the satellite cells were isolated from hindlimbs, as described (43). Briefly, hindlimb skeletal musculature was surgically excised, finely minced, and disassociated in 0.02% Trypsin (GIBCO) and 2% Collagenase type 4 (Worthington) for 60 min at 37°C/5% CO2 while agitating on an orbital shaker. Disassociated muscle was strained in a 70-μm sieve, centrifuged at 1,600 rpm (Eppendorf 5810R) for 5 min, and resuspended in 10-ml-high glucose DMEM, supplemented with pyruvate (GIBCO). Media were further supplemented with 10% FBS and 1% penicillin/streptomycin (GIBCO). Resuspended cells were plated in 75-cm2 tissue-culture flasks (Fisher), and HGF (50 ng/ml) and FGF2 (50 ng/ml) were added to the medium. After 7 days, cultures were passaged, and purified satellite cell suspensions were obtained via Percoll fractionation, as described (44). Purified cultures were incubated for 7 days at 37°C until 80% confluent and then collected via trypsinization and seeded at 107 cells/ml onto modified open-pore polymer scaffolds.
Surgical Procedure.
Four-week-old C57BL/6 mice were anesthetized via intraperitoneal injection of ketamine (0.5 ml/kg) and xylazine (0.25 ml/kg). Bilateral incisions were made to expose the tibialis anterior muscle of both hindlimbs. Once exposed, the muscle was completely lacerated at the midlength ventral dorsally. The proximal ends of the lacerated muscle were then closed by using a no. 4 black silk continuous suture, and scaffolds were placed over the wound or myoblasts injected into the muscle. In all conditions using myoblast transplantation, a total of 5 × 105 cells were delivered. The surgical site was closed with no. 4 black silk interrupted suture and left undisturbed until the muscle was retrieved at 10 or 30 days.
Analysis.
Tibialis anterior muscle was excised and fixed in 4% paraformaldehyde for 2 h and rinsed for 1 h in PBS. Whole muscle was then incubated overnight in β-galactosidase staining solution containing 25 μl/ml X-Gal stock solution. The muscle was paraffin-embedded, cut into serial sections (5 μm thick), and placed on glass slides for histological analysis. Sections were deparaffinized through descending series of EtOH, rehydrated in H2O, and washed for 5 min in 3% H2O2 (Sigma) in PBS to quench any endogenous peroxidase activity. Sections were stained with Gill's 3 hematoxylin (Sigma) and aqueous eosin solution (Sigma) to visualize tissue morphology. Finally, serial sections were incubated with a monoclonal anti-β-galactosidase antibody (1:1,000) (Chemicon) for 1 h and then incubated with a horseradish peroxidase-conjugated secondary antibody (1:1,000) (DakoCytomation, Dako). Samples were rinsed and mounted with Permount (Fisher). Controls for immunohistochemistry included tissue sections with no transplanted cells (untreated lacerations) and staining of sections from lacerations treated with peptide modified, macroporous, myoblast containing scaffolds only with secondary antibody. Both controls demonstrated minimal to no positive staining.
Defect size was determined via histological analysis of 10- to 12-μm paraffin-embedded serial sections of isolated tibialis anterior muscle. Longitudinal sections across the central third of the entire length of the muscle were stained with hematoxylin/eosin, and the section that contained the largest defect area was selected for analysis (22, 45). Analysis was performed by using adobe photoshop (Adobe Systems, San Jose, CA) and image pro plus software (Media Cybernetics, Silver Spring, MD). Images (×100) were obtained by using a Leica CTR 5000 light microscope and open lab software (Improvision, Lexington, MA). Six samples were analyzed for each condition. Areas of muscle defect were identified in hematoxylin/eosin-stained sections via their lack of organized muscle fibers and the presence of centrally located myofiber nuclei. Fiber size and nuclei number were determined via high-powered microscopic analysis of 10 random fields of regenerating muscle fibers adjacent to the muscle defect. Only centrally located nuclei were counted in the quantification of number of nuclei.
The two-tailed Student's t test was used to analyze data, and all data were plotted as the mean ± standard deviation of the mean.
Acknowledgments
This work was supported by National Institutes of Health/National Institute for Dental and Craniofacial Research Grant R01 DE13349.
Abbreviations
- HGF
hepatocyte growth factor
- FGF
fibroblast growth factor.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Langer R., Vacanti J. P. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Griffith L. G., Naughton G. Science. 2002;295:1009–1014. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- 3.Lipton B. H., Schultz E. Science. 1979;205:1292–1294. doi: 10.1126/science.472747. [DOI] [PubMed] [Google Scholar]
- 4.Partridge T. A., Morgan J. E., Coulton G. R., Hoffman E. P., Kunkel L. M. Nature. 1989;337:176–179. doi: 10.1038/337176a0. [DOI] [PubMed] [Google Scholar]
- 5.Skuk D., Tremblay J. P. J. Muscle Res. Cell Motil. 2003;24:285–300. [PubMed] [Google Scholar]
- 6.Saxena A. K., Marler J., Benvenuto M., Willital G. H., Vacanti J. P. Tissue Eng. 1999;5:525–532. doi: 10.1089/ten.1999.5.525. [DOI] [PubMed] [Google Scholar]
- 7.Chromiak J. A., Shansky J., Perrone C., Vandenburgh H. H. In Vitro Cell Dev. Biol. Anim. 1998;34:694–703. doi: 10.1007/s11626-998-0065-2. [DOI] [PubMed] [Google Scholar]
- 8.Maley M. A., Davies M. J., Grounds M. D. Exp. Cell Res. 1995;219:169–179. doi: 10.1006/excr.1995.1217. [DOI] [PubMed] [Google Scholar]
- 9.Dennis R. G., Kosnik P. E., 2nd In Vitro Cell Dev. Biol. Anim. 2000;36:327–335. doi: 10.1290/1071-2690(2000)036<0327:EAICPO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 10.Levenberg S., Rouwkema J., Macdonald M., Garfein E. S., Kohane D. S., Darland D. C., Marini R., van Blitterswijk C. A., Mulligan R. C., D'Amore P. A., et al. Nat. Biotechnol. 2005;23:879–884. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- 11.Ohlstein B., Kai T., Decotto E., Spradling A. Curr. Opin. Cell Biol. 2004;16:693–699. doi: 10.1016/j.ceb.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Lubeck D. P. Best Pract. Res. Clin. Rheumatol. 2003;17:529–539. doi: 10.1016/s1521-6942(03)00023-8. [DOI] [PubMed] [Google Scholar]
- 13.McClatchey K. D. Arch Pathol. Lab. Med. 2004;128:480. doi: 10.5858/2004-128-480-MCAM. [DOI] [PubMed] [Google Scholar]
- 14.Gros J., Manceau M., Thome V., Marcelle C. Nature. 2005;435:954–958. doi: 10.1038/nature03572. [DOI] [PubMed] [Google Scholar]
- 15.Allen R. E., Rankin L. L. Proc. Soc. Exp. Biol. Med; 1990. pp. 81–86. [DOI] [PubMed] [Google Scholar]
- 16.Hawke T. J., Garry D. J. J. Appl. Physiol. 2001;91:534–551. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- 17.Rowley J., Sun Z., Goldman D., Mooney D. Adv. Mater. 2002;14:886–889. [Google Scholar]
- 18.Kablar B., Krastel K., Ying C., Asakura A., Tapscott S. J., Rudnicki M. A. Development (Cambridge, U.K.) 1997;124:4729–4738. doi: 10.1242/dev.124.23.4729. [DOI] [PubMed] [Google Scholar]
- 19.Rubin J. S., Day R. M., Breckenridge D., Atabey N., Taylor W. G., Stahl S. J., Wingfield P. T., Kaufman J. D., Schwall R., Bottaro D. P. J. Biol. Chem. 2001;276:32977–32983. doi: 10.1074/jbc.M105486200. [DOI] [PubMed] [Google Scholar]
- 20.Tatsumi R., Anderson J. E., Nevoret C. J., Halevy O., Allen R. E. Dev. Biol. 1998;194:114–128. doi: 10.1006/dbio.1997.8803. [DOI] [PubMed] [Google Scholar]
- 21.Cornelison D. D., Filla M. S., Stanley H. M., Rapraeger A. C., Olwin B. B. Dev. Biol. 2001;239:79–94. doi: 10.1006/dbio.2001.0416. [DOI] [PubMed] [Google Scholar]
- 22.Miller K. J., Thaloor D., Matteson S., Pavlath G. K. Am. J. Physiol. 2000;278:C174–C181. doi: 10.1152/ajpcell.2000.278.1.C174. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell C. A., McGeachie J. K., Grounds M. D. Growth Factors. 1996;13:37–55. doi: 10.3109/08977199609034565. [DOI] [PubMed] [Google Scholar]
- 24.Hill E., Boontheekul T., Mooney D. J. Tissue Eng. 2005 doi: 10.1089/ten.2006.12.1295. in press. [DOI] [PubMed] [Google Scholar]
- 25.Chan Y. S., Li Y., Foster W., Horaguchi T., Somogyi G., Fu F. H., Huard J. J. Appl. Physiol. 2003;95:771–780. doi: 10.1152/japplphysiol.00915.2002. [DOI] [PubMed] [Google Scholar]
- 26.Fukushima K., Badlani N., Usas A., Riano F., Fu F., Huard J. Am J. Sports Med. 2001;29:394–402. doi: 10.1177/03635465010290040201. [DOI] [PubMed] [Google Scholar]
- 27.Menetrey J., Kasemkijwattana C., Fu F. H., Moreland M. S., Huard J. Am. J. Sports Med. 1999;27:222–229. doi: 10.1177/03635465990270021801. [DOI] [PubMed] [Google Scholar]
- 28.Beauchamp J. R., Morgan J. E., Pagel C. N., Partridge T. A. J. Cell Biol. 1999;144:1113–1121. doi: 10.1083/jcb.144.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skuk D., Goulet M., Roy B., Tremblay J. P. Exp. Neurol. 2002;175:112–126. doi: 10.1006/exnr.2002.7899. [DOI] [PubMed] [Google Scholar]
- 30.Friedrich G., Soriano P. Genes Dev. 1991;5:1513–1523. doi: 10.1101/gad.5.9.1513. [DOI] [PubMed] [Google Scholar]
- 31.Leshem Y., Spicer D. B., Gal-Levi R., Halevy O. J. Cell Physiol. 2000;184:101–109. doi: 10.1002/(SICI)1097-4652(200007)184:1<101::AID-JCP11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 32.Bischoff R. Dev. Biol. 1986;115:129–139. doi: 10.1016/0012-1606(86)90234-4. [DOI] [PubMed] [Google Scholar]
- 33.Gospodarowicz D., Weseman J., Moran J. S., Lindstrom J. J. Cell Biol. 1976;70:395–405. doi: 10.1083/jcb.70.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J. C., Goldhamer D. J. Reprod. Biol. Endocrinol. 2003;1:101. doi: 10.1186/1477-7827-1-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson J. E. Mol. Biol. Cell. 2000;11:1859–1874. doi: 10.1091/mbc.11.5.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kinoshita I., Vilquin J. T., Roy T., Tremblay J. P. Neuromuscul. Disord. 1996;6:187–193. doi: 10.1016/0960-8966(96)00004-1. [DOI] [PubMed] [Google Scholar]
- 37.Li Y. P. Am. J. Physiol. 2003;285:C370–C376. doi: 10.1152/ajpcell.00453.2002. [DOI] [PubMed] [Google Scholar]
- 38.McPherron A. C., Lawler A. M., Lee S. J. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 39.Palacio J., Galdiz J. B., Bech J. J., Marinan M., Casadevall C., Martinez P., Gea J. Arch. Bronconeumol. 2002;38:311–316. doi: 10.1016/s0300-2896(02)75224-1. [DOI] [PubMed] [Google Scholar]
- 40.Ryten M., Dunn P. M., Neary J. T., Burnstock G. J. Cell Biol. 2002;158:345–355. doi: 10.1083/jcb.200202025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alsberg E., Anderson K. W., Albeiruti A., Franceschi R. T., Mooney D. J. J. Dent. Res. 2001;80:2025–2029. doi: 10.1177/00220345010800111501. [DOI] [PubMed] [Google Scholar]
- 42.Rowley J. A., Madlambayan G., Mooney D. J. Biomaterials. 1999;20:45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 43.Irintchev A., Zweyer M., Pfendtner M., Wernig A. Eur. J. Neurosci. 1998;10:366–366. [Google Scholar]
- 44.McKinney-Freeman S. L., Jackson K. A., Camargo F. D., Ferrari G., Mavilio F., Goodell M. A. Proc. Natl. Acad. Sci. USA. 2002;99:1341–1346. doi: 10.1073/pnas.032438799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hill M., Wernig A., Goldspink G. J. Anat. 2003;203:89–99. doi: 10.1046/j.1469-7580.2003.00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]