Abstract
The stress inoculation hypothesis presupposes that brief intermittent stress exposure early in life induces the development of subsequent stress resistance in human and nonhuman primates. Rodent studies, however, suggest a role for maternal care rather than stress exposure per se (i.e., the maternal mediation hypothesis). To investigate these two hypotheses, we examined maternal care and the development of stress resistance after exposure to brief intermittent infant stress (IS), mother–infant stress (MIS), or no stress (NS) protocols administered to 30 monkeys between postnatal weeks 17 and 27. Unlike rodents, the IS condition did not permanently increase primate maternal care, nor did measures of total maternal care predict subsequent offspring hypothalamic–pituitary–adrenal-axis responsivity. Although MIS infants received less maternal care than IS and NS infants, both IS and MIS monkeys developed subsequent stress resistance. These findings indicate that rearing differences in the development of stress resistance are more closely related to differences in prior stress exposure than to differences in maternal care. A second experiment confirmed this conclusion in a different cohort of 25 monkeys exposed as infants to high foraging-demand (HFD) or low foraging-demand (LFD) conditions. HFD infants exhibited intermittent elevations in cortisol levels and received less maternal care than LFD infants. In keeping with a key prediction of the stress inoculation hypothesis, HFD males responded to stress in adulthood with diminished hypothalamic–pituitary–adrenal-axis activation compared with LFD males. Results from both experiments demonstrate that stress inoculation, rather than high levels of maternal care, promotes the development of primate stress resistance.
Keywords: hypothalamic–pituitary–adrenal axis, monkey, maternal care, resilience
People with the capacity to maintain healthy emotional functioning in the aftermath of stressful experiences are said to be resilient, or stress resistant (1, 2). Researchers have sought to identify attributes associated with stress resistant individuals, with the expectation that understanding the etiology of stress resistance may lead to the prevention of stress-related psychiatric disorders. One intriguing finding to emerge from this retrospective research has been that stress resistance is associated with childhood exposure to mildly stressful events (1, 3). Prospective longitudinal studies of nonhuman primates support this finding, because monkeys exposed to brief, 1-h periods of maternal-separation stress exhibit diminished anxiety and attenuated hypothalamic–pituitary–adrenal (HPA)-axis responses to subsequent stressors compared with unmanipulated monkeys (4).
Little is known about the psychobiological mechanisms that promote the development of stress resistance in human or nonhuman primates, but studies of rodents suggest a possible role for maternal care. Similar to monkeys, neonatal rodents exposed to brief repeated maternal separations of 3–15 min in duration (i.e., postnatal handling) exhibit diminished emotionality and attenuated HPA-axis responses to subsequent stressors (5, 6). The development of rodent stress resistance is thought to be maternally mediated (7), because brief intermittent separations stimulate increased maternal licking and grooming not only at reunion but across pup development (8). These permanent changes in maternal behavior increase the expression of glucocorticoid receptors in the hippocampus, thereby enhancing sensitivity to glucocorticoid-feedback inhibition during stress exposure later in life (5). Importantly, the emotional and neuroendocrine stress resistance observed in briefly separated rodents can be replicated in unmanipulated offspring that naturally receive high levels of maternal care during development (9). That increased maternal care is sufficient to produce stress resistance in rodents is corroborated by evidence that the amount of maternal stimulation received by pups during development is negatively correlated with later stress-induced HPA-axis activation (9, 10).
The utility of rodent research in modeling the etiology of human stress resistance is limited, however, by critical developmental differences in rodent and primate HPA-axis physiology. The brief maternal separation paradigm coincides with the rodent stress-hyporesponsive period (SHRP), during which time mice and rats do not respond, or respond only weakly, to mild stressors with HPA-axis activation (11, 12). High levels of maternal stimulation maintain the pup’s SHRP status, with removal of maternal regulation resulting in gradual disinhibition of HPA-axis activity. Diminished maternal stimulation that fails to adequately inhibit activation of the pup’s HPA-axis during the SHRP, as documented for longer maternal separations of 3 h in duration (13), promotes stress hyperresponsivity later in life. Although it has not been systematically studied, human and nonhuman primate infants do not appear to exhibit a SHRP and are capable of exhibiting stress responses throughout lifespan development (14–17). Primate infants, unlike rodent pups, respond to brief intermittent maternal separations with immediate HPA-axis activation (18) and develop subsequent neuroendocrine stress resistance even after maternal separations of 4–6 h in duration (19). If the same psychobiological mechanisms “program” the stress response in rodents and primates, HPA-axis activation during primate development should lead to later stress hyperresponsivity. Yet, in primates, a paradigm that induces anxiety and activates the HPA-axis leads, instead, to emotional and neuroendocrine stress resistance (4, 19).
An alternative to the maternal mediation hypothesis is the stress inoculation hypothesis, which is based on the notion that mild stress, and the acute anxiety and HPA-axis activation that it engenders, is necessary for the development of subsequent stress resistance. Several theorists have likened the development of stress resistance to acquired immunity (1, 3). Immunity, whether naturally occurring or therapeutically induced by inoculation, derives from exposure to a mild version of a pathogen that strengthens immunological resistance. Similarly, early exposure to mild stress may “inoculate” the developing organism, thereby enhancing resistance to subsequent stressors. In the following experiments, we investigated the roles of maternal mediation and stress inoculation in the development of stress resistance in squirrel monkeys.
Experiment 1
The goals of this experiment were twofold: (i) to determine whether brief intermittent separations permanently increase primate maternal behavior during development, and (ii) to test whether maternal care received during development predicts subsequent offspring HPA-axis responsivity. Thirty infants from three rearing conditions were studied. In the first condition, only infants were exposed to brief intermittent separations from the natal group for 10 weekly 1-h sessions [infant stress (IS)]. In the second condition, mothers and infants were removed together from the natal group and exposed to an otherwise identical stress protocol [mother–infant stress (MIS)]. Both IS and MIS protocols acutely induce anxiety and activate the HPA-axis in young monkeys (18, 20). In the third condition, mothers and infants remained undisturbed in their natal groups [no stress (NS)]. Maternal care was assessed every other week during three distinct periods in the home cage (i.e., before stress exposure, immediately after stress exposure, and 24 h after stress exposure). Eleven weeks after completion of the rearing protocols, a 30-min novelty stress test was administered to examine rearing differences in offspring HPA-axis responsivity.
Research has shown that IS infants develop neuroendocrine stress resistance, whereas NS infants do not (19). The maternal-mediation hypothesis therefore predicts that IS infants will consistently receive more maternal care than NS infants. Although it is unknown whether MIS infants develop stress resistance, this condition was included because maternal stress diminishes primate maternal care (21). If MIS infants receive less or equal amounts of maternal care compared with control infants, the maternal mediation hypothesis predicts that MIS infants will not develop neuroendocrine stress resistance. The stress inoculation hypothesis, in contrast, predicts the development of neuroendocrine stress resistance in all offspring exposed to brief intermittent stress protocols, because these protocols acutely induce anxiety and activate the HPA-axis.
Results.
Rearing differences in the frequency and duration of maternal behavior.
Rearing protocols did not permanently alter the frequency or duration of any maternal behavior measure across development, because no rearing differences were evident in the periods immediately before or 24 h after stress exposure. Several transient effects were observed immediately after stress exposure, however, including a main effect of rearing condition on the duration of dorsal contact (i.e., the species-typical infant riding posture on the mother’s shoulders and upper back) (F2,26 = 5.304, P = 0.012). Upon returning to the home cage, IS infants spent more time clinging to their mothers than did NS infants (39 ± 3 vs. 19 ± 6 min; P = 0.011). IS infants also clung to their mothers more than did MIS infants (MIS = 26 ± 4 min), but this difference failed to reach statistical significance. MIS and NS infants did not differ from one another. A significant protocol-week by rearing-condition interaction was found for nursing duration immediately after stress exposure (F8,104 = 4.37, P = 0.004). However, post hoc tests at each week revealed no consistent rearing differences, because both IS and MIS infants differed from NS infants (and not each other) at only two of the five time points (i.e., weeks 3 and 5). These data indicate that IS infants generally received more maternal care than NS infants immediately after stress exposure, whereas MIS infants did not significantly differ from either group.
Rearing differences in mother–infant social transactions.
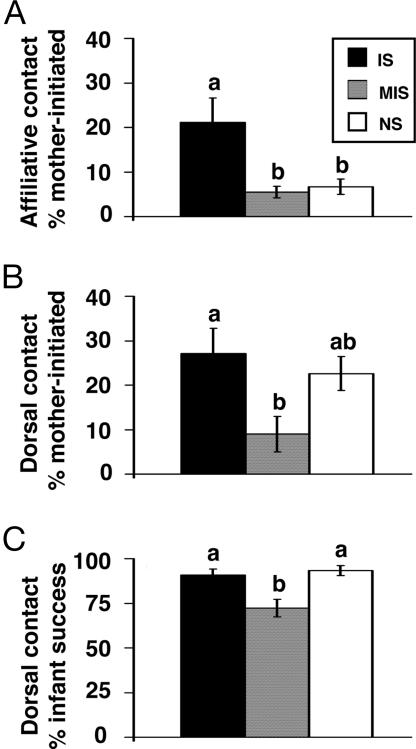
Enduring rearing differences in mother–infant transactions were observed across all three observation period types (Fig. 1). A significant effect of rearing condition was found for the percentage of total affiliative contact attempts initiated by mothers relative to their infants (F2,26 = 4.964, P = 0.015). IS mothers made more affiliative contact attempts (21%) than either MIS (6%; P = 0.02) or NS (7%; P = 0.049) mothers, whereas MIS and NS mothers did not differ. Similarly, rearing differences were found for the percentage of total dorsal contact attempts initiated by mothers relative to infants (F2,26 = 3.787, P = 0.036). IS mothers initiated dorsal contact with infants 27% of the time, whereas MIS mothers initiated dorsal contact only 9% of the time (P = 0.043). Although NS mothers’ dorsal contact initiatives more closely resembled those of IS mothers (23%), they did not significantly differ from either group. Finally, a rearing difference in the percentage of total infant-initiated dorsal contact attempts that were accommodated by mothers was found (F2,26 = 8.340, P = 0.002). MIS infants’ attempts to attain dorsal contact were accommodated 72% of the time, whereas IS and NS infants dorsal contact attempts were, respectively, accommodated 91% (P = 0.009) and 93% (P = 0.003) of the time. These results indicate that, across observation periods, MIS infants received diminished maternal care relative to IS and, at times, NS infants.
Fig. 1.
Rearing differences in mother–infant social transactions. Affiliative (A) and dorsal (B) contact attempts initiated by mothers relative to infants and total infant-initiated dorsal contact attempts accommodated by mothers (C) are presented as mean ± SEM for monkeys previously exposed to IS (n = 11), MIS (n = 10), or NS (n = 9) rearing conditions. Groups without shared letters differ significantly (P < 0.05), whereas groups with shared letters do not.
Neuroendocrine stress responsivity and maternal care.
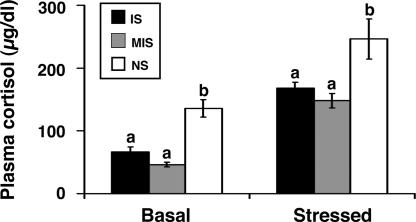
Rearing differences were found for baseline cortisol levels (F2,26 = 10.739, P < 0.0001), such that both IS (P = 0.048) and MIS (P < 0.0001) offspring exhibited diminished baseline cortisol levels compared with NS offspring. To control for these differences, baseline cortisol levels were used as a statistical covariate to analyze rearing differences in poststress cortisol levels. Consistent with the stress inoculation hypothesis, rearing differences in stress resistance were observed (F2,25 = 5.124, P = 0.014). Specifically, previously stress-exposed IS (P = 0.033) and MIS (P = 0.013) offspring exhibited diminished cortisol responses to the novelty stress test compared with NS offspring (Fig. 2).
Fig. 2.
Plasma cortisol levels at baseline and after stress exposure are presented as mean ± SEM for juvenile monkeys previously exposed to IS (n = 11), MIS (n = 10), or NS (n = 9) rearing conditions. Groups without shared letters (a, b) differ significantly (P < 0.05).
Total maternal care did not predict subsequent offspring cortisol levels at baseline or after novelty stress exposure. Although enduring rearing differences in several social-transaction measures were found, as was a transient increase in dorsal contact immediately after stress exposure, none of these measures predicted subsequent baseline- or stress-levels of cortisol. Nursing duration in the period immediately after stress exposure, however, did predict cortisol levels at baseline [β = −0.525 (df = 28); P = 0.003] and after novelty stress exposure [β = −0.397 (df = 28), P = 0.030]. To test the hypothesis that nursing duration mediates the effects of rearing condition on baseline- and stress-levels of cortisol, nursing duration and rearing condition were examined together as independent variables in the same regression equation (22, 23). Rearing-related differences in baseline (F2,26 = 4.79, P = 0.017) and stress (F2,26 = 3.86, P = 0.034) levels of cortisol remained significant, whereas nursing duration no longer predicted either cortisol measure. These results do not support a role for nursing as a mediator of the effect of rearing condition on cortisol levels.
Experiment 2
Findings from experiment 1 suggest that rearing differences in neuroendocrine stress resistance are more closely related to differences in prior stress exposure than to differences in maternal care. The primary goal of experiment 2 was to assess the generality of these findings by testing the maternal mediation and stress inoculation hypotheses in 25 monkeys exposed to a different type of mild early life stress. The protocols used in this experiment consisted of high foraging-demand (HFD) and low foraging-demand (LFD) conditions that differed in the effort required by mothers to obtain food. These daily foraging protocols did not alter infant growth, weight, nursing behavior, or solid-food consumption, but HFD infants exhibited intermittently elevated cortisol levels and received diminished maternal care compared with LFD infants across development (24, 25).
At 8 years of age, monkeys were restrained in primate chairs for two 30-min sessions that were administered 7 days apart. An intramuscular injection of saline was given 60 min before the first restraint test to examine rearing differences in HPA-axis responsivity and poststress recovery. Because neuroendocrine stress resistance in rats arises from enhanced glucocorticoid-feedback regulation of the HPA-axis stress response (5), we also tested in this experiment whether the same was true for monkeys. Thus, exogenous cortisol (i.e., 2.5 mg/kg hydrocortisone sodium succinate) was intramuscularly injected 60 min before the second restraint test. This dose of hydrocortisone inhibits stress-induced increases in squirrel monkey adrenocorticotropic hormone (ACTH) (26) and was used to assess rearing differences in glucocorticoid-feedback inhibition of the HPA-axis stress response.
The maternal mediation and stress inoculation hypotheses make different predictions about HPA-axis responsivity in HFD and LFD monkeys. The maternal mediation hypothesis predicts that the diminished maternal behavior and elevated cortisol levels that characterize the HFD condition will promote increased stress responsivity in HFD compared with LFD monkeys. Conversely, the stress inoculation hypothesis predicts that the mild stress and elevated cortisol levels that typify the HFD condition will produce stress resistance in HFD but not LFD monkeys.
Baseline ACTH and cortisol levels did not vary across repeated samples, nor did either differ by rearing condition. Although no effect of gender on baseline ACTH levels was found, gender did influence baseline cortisol levels (F1,21 = 14.785, P = 0.001). Specifically, females had baseline cortisol levels that were 2-fold higher than males (186 ± 24 vs. 87 ± 9).
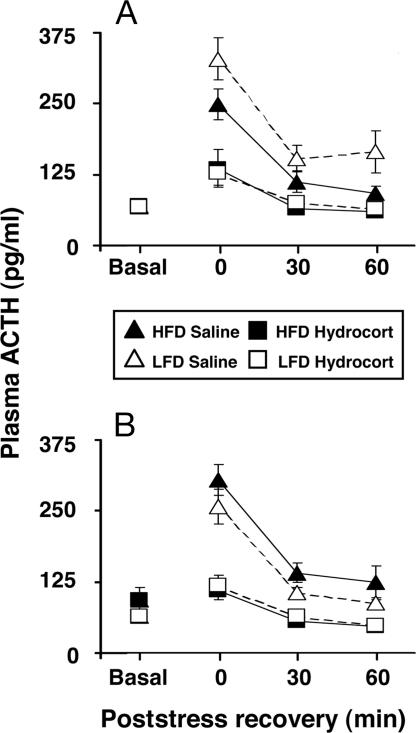
A significant rearing-condition by gender interaction across the saline-pretreatment poststress sample time points for ACTH was found (F1,20 = 4.476, P = 0.047). Further analysis revealed a main effect of rearing condition on stress responsivity for male, but not female, monkeys (Fig. 3). Although ACTH levels decreased significantly over time in all monkeys (F2,40 = 22.461, P < 0.0001), only adult males previously exposed to the HFD condition responded to restraint stress with diminished ACTH levels compared with males from the LFD condition (F1,10 = 6.253, P = 0.031). This rearing effect was also apparent for time-integrated poststress measures of ACTH in male (F1,10 = 5.851, P = 0.036), but not female, monkeys.
Fig. 3.
Plasma ACTH levels after pretreatment with saline or hydrocortisone 0, 30, and 60 min after stress exposure are presented as mean ± SEM for male (A) and female (B) monkeys previously exposed to HFD (n = 6 males, 6 females) or LFD (n = 7 males, 6 females) rearing conditions.
Unlike ACTH, cortisol levels after the saline pretreatment continued to rise significantly across the poststress measurement period. In keeping with the gender differences in baseline cortisol levels described above, time-integrated poststress measures of cortisol in females (306 ± 25 μg/dl) were 67% greater than in males (184 ± 14 μg/dl; F1,21 = 18.358, P < 0.0001). However, the magnitude of poststress increases in cortisol levels above baseline did not differ by gender. Cortisol levels at 0, 30, and 60 min after stress were, respectively, 68 ± 8, 100 ± 10, and 118 ± 8 μg/dl higher than baseline for males, and 79 ± 26, 126 ± 23, and 148 ± 26 μg/dl higher than baseline for females. No significant gender or rearing effects on poststress cortisol levels were discerned.
As expected, the hydrocortisone pretreatment robustly suppressed stress levels of ACTH relative to the saline pretreatment (Fig. 3). Hydrocortisone suppressed time-integrated poststress levels of ACTH in both males (F1,11 = 99.83, P < 0.0001) and females (F1,10 = 69.47, P < 0.0001). Within 30 min of stressor termination, ACTH levels were equal to or less than baseline in all monkeys, and glucocorticoid-feedback inhibition did not differ significantly by rearing condition.
Discussion
Findings from experiment 1 indicate that, unlike rodents, brief maternal separations do not permanently increase primate maternal behavior, nor does total maternal care predict later offspring HPA-axis stress responsivity. Although young MIS infants receive less maternal care across development than IS or NS infants, both IS and MIS monkeys develop stress resistance. Despite the fact that nursing duration in the period immediately after stress exposure predicted later cortisol measures, subsequent analyses did not support a role for nursing as a mediator of the effect of rearing condition on cortisol levels. These findings suggest that rearing differences in the development of stress resistance are more closely related to differences in prior stress exposure than to differences in maternal care. Experiment 2 confirmed these findings in a second cohort of monkeys exposed to a different type of mild early life stress. HFD infants exhibit modestly elevated cortisol levels and receive less maternal care than LFD infants during development. As adults, HFD males subsequently demonstrate diminished stress-induced HPA-axis activation compared with LFD males. Results from these two experiments suggest that stress inoculation, rather than high levels of maternal care, promotes the development of primate stress resistance.
Our findings from monkeys parallel data from rabbits that indicate that brief separations from the natal nest, independent of maternal care, are sufficient to produce stress resistance. Mother rabbits provide minimal maternal care, however, and stress resistance develops in briefly separated rabbit pups whether they are raised with or without their mothers (27). In contrast, stress resistance does not develop in motherless monkeys (28) or orphaned children (29). Combined with findings from rodent studies reviewed above, this collective evidence suggests that species differences in parental behavior (i.e., minimal vs. extensive) and HPA-axis development (i.e., presence vs. absence of the SHRP) may determine how stress resistance is programmed on a species-by-species basis.
The notion that high levels of maternal care are not required to produce stress resistance does not negate the critical role of parenting in primate development nor does it indicate that aspects of lifelong health are impervious to parental care. Indeed, mother–infant attachment relationships were firmly established before the initiation of both of our experiments, and all infants received adequate maternal care, well within the range of what is typical for squirrel monkeys (30). In humans, socioemotional support derived from close relationships clearly promotes effective coping (31). It seems likely, then, that some threshold amount of maternal care during or after stress exposure is required to bolster the young primate’s coping capacity, thereby contributing to the development of stress resistance.
The importance of maternal care notwithstanding, results from our studies implicate a role for stress inoculation in the development of primate stress resistance. Whether these findings reflect specific or general stress inoculation effects merits consideration. Specific inoculation refers to the notion that exposure to a given stressor confers subsequent resistance to the same or similar stressor. General inoculation, in contrast, refers to the notion that prior exposure to one stressor strengthens resistance to a variety of stressors (4). In experiment 1, the IS and MIS protocols and the subsequent stress resistance test had several elements in common. These elements include exposure to an unfamiliar environment and separation from the natal group. IS monkeys, however, also exhibit emotional stress resistance in their home cage in the presence of the natal group (4) and demonstrate enhanced performance on an emotionally challenging cognitive test compared with NS monkeys (32). There were, likewise, considerable differences between the HFD protocol and the restraint test used in experiment 2. Although further research is required to determine whether mild early life stress exposure also confers resistance to subsequent stressors of a physiological nature (e.g., immune challenge or insulin-induced hypoglycemia), the initial results from our experiments point to some form of general stress inoculation.
Several rearing-related discrepancies were apparent both between and within experiments. A rearing difference in baseline cortisol levels, for example, was found in experiment 1 but not experiment 2. This disparity is likely attributable to age, because rearing differences in baseline cortisol levels become less detectable over development (32) and are no longer discerned by the late-juvenile period (ref. 19; and K.J.P., K. L. Rainwater, C.L.B., A.F.S., S. E. Lindley, and D.M.L., unpublished work). It is not clear why this change occurs, nor is the function of this rearing effect understood. A second disparity was evident within experiment 2. Significant rearing differences in ACTH, but not cortisol, levels were found in adult male monkeys after restraint-stress exposure. In the hour after restraint stress termination, ACTH levels steadily decreased toward baseline, whereas cortisol levels continued to rise. Because the adrenal response to stress temporally follows that of the pituitary, it is likely that our assessment period was too short to capture rearing differences in adrenal recovery. The restraint test as used in experiment 2 thus appears more suitable for detecting rearing differences in the temporal dynamics of primate pituitary compared with adrenal stress physiology.
Several gender differences were found in experiment 2. These include 2-fold higher cortisol levels in adult females compared with males at baseline and after stress exposure and demonstration of neuroendocrine stress resistance only in adult males. Stress resistance is reliably expressed equally in males and females throughout adolescence (19), but evidence from experiment 2 indicates that gender differences in stress resistance may emerge after the peripubertal period. Higher baseline glucocorticoid levels and enhanced stress responsivity have been reported for adult female rats and humans, and ovarian steroids are thought to modulate both phenomena (33, 34). In contrast, androgens, which are more prevalent in adult males than females, inhibit HPA-axis activation (35). One might conclude that pubertal changes in specific gonadal steroid levels may contribute to gender differences in adult stress resistance. An alternative possibility is that stress-inoculated female monkeys do not “lose” their stress resistant status at puberty, but rather, ovarian hormones and elevated baseline cortisol levels mask this phenomenon at certain points in the ovarian cycle. Careful monitoring of ovarian function in cycling and noncycling adult females with respect to HPA-axis physiology is required to accurately test whether female stress resistance persists into adulthood.
Neuroendocrine stress resistance in rodents is mediated, in part, by enhanced glucocorticoid-feedback sensitivity (5), but we failed to find a similar effect in primates. These results suggest that the neural basis of stress resistance in primates may differ from that in rodents. This possibility has important implications for guiding future research, particularly in humans, which more closely resemble monkeys than rats. It should be noted, however, that only one dose of hydrocortisone was used in our study. Although the dose was carefully selected based on previous research (26), examination of multiple hydrocortisone doses is required to more fully evaluate rearing differences in primate sensitivity to glucocorticoid negative feedback.
A final topic that warrants comment is that the skewed sex ratio in experiment 1 (23 females and 7 males) limits the generalizability of the cortisol and maternal care data to males. In a separate cohort, however, IS compared with NS males were previously shown to exhibit diminished stress-induced cortisol levels (19). We likewise documented in experiment 2 that, similar to MIS males, mildly stressed HFD males that received less maternal care than control males in infancy develop subsequent stress resistance. Aspects of experiment 1 nevertheless remain to be replicated, especially the effects of maternal care on subsequent HPA-axis stress responsivity in IS and NS male monkeys.
In conclusion, it is likely that certain forms of primate stress resistance arise from manageable exposure to mild stress that stimulates anxiety and activates the HPA-axis. This formulation presupposes that anxiety and HPA-axis activation during stress exposure play key roles in promoting the development of stress resistance. With continued investigation of this hypothesis, a comprehensive understanding of the etiology and neurobiology of stress resistance may ultimately provide a foundation for improved treatment and prevention of stress-related psychiatric disorders.
Methods
Experiment 1.
Subjects.
Thirty squirrel monkey infants (Samiri sciureus) were born at Stanford University and served as subjects. Subjects were housed in wire-mesh cages (1.8 × 1.2 × 1.8 m) in 10 natal groups composed of three to four mother–infant dyads. Group composition was determined primarily by birth date to minimize developmental differences between cohabitating infants. Monkeys were housed on a 12/12 h light/dark cycle in rooms with an ambient temperature of 26°C. Cages were cleaned daily, and monkeys had ad libitum access to water, food, and toys. A sliding door in each home cage facilitated access to a portable capture cage. Monkeys were trained to enter the capture cage to facilitate experimental manipulations. All procedures were approved by Stanford University’s Administrative Panel on Laboratory Animal Care.
Rearing protocols.
Subjects remained undisturbed in their natal groups through postnatal week 16, at which time natal groups were assigned to one of three conditions. The IS condition included 11 offspring (7 females and 4 males). From postnatal weeks 17–27, each infant was removed from its mother and natal group for a 1-h period once a week, placed in a cage (46 × 46 × 46 cm) adjacent to unfamiliar monkeys in a different room, and temporarily deprived of all forms of maternal and natal-group contact. After the 1-h separation period, each infant was returned to the home cage. No more than one infant from each natal group was separated on a given day, and all manipulations occurred between 1400 and 1800 h. The MIS condition included 10 offspring (8 females and 2 males). This rearing protocol was identical to the IS protocol in all ways, except that each infant and its mother were removed together for the 1-h period. In the third condition, nine offspring (eight females and one male) remained undisturbed as NS controls.
Behavioral observations.
Each IS and MIS mother–infant dyad was observed during three distinct 15-min periods in the home cage: (i) before stress exposure, (ii) immediately after stress exposure, and (iii) 24 h after stress exposure. These observations were conducted for the first, third, fifth, seventh, and ninth stress exposures, for a total of 15 observation periods per dyad. Observations of NS mother–infant dyads occurred at matched time points to facilitate comparisons between rearing conditions.
Squirrel monkey mothers, unlike rodent mothers, do not lick or groom their infants. An established squirrel monkey ethogram was thus used to select maternal behavior measures (30). During postnatal weeks 17–27, infants are attaining physical, if not psychological, independence from their mothers. Infants at these ages locomote independently, consume solid foods, and nurse infrequently (36). Although no longer in constant physical contact, mothers still provide maternal care and engage in a variety of complex social interactions with their infants.
By using a computer-aided recording program, mother–infant behavior was collected by a trained observer. Durations of a given behavior were calculated by summing the elapsed period between initiation and termination. Bouts of a given behavior were distinct if separated by at least 3 seconds. The following measures were summarized for each observation period: (i) dorsal contact (infant observed in the species-typical riding posture on the mother’s shoulders and upper back), (ii) affiliative contact (mother and infant observed in side-by-side huddling), and (iii) nursing contact (infant observed in the species-typical ventral nursing position). A composite measure of maternal care was created by combining dorsal, affiliative, and nursing contact scores.
Social transactions between mothers and infants were also recorded for each observation period (24). Transactions were initiated by attempts to change the immediate state of association between two individuals by means of either make-or-break dorsal contact initiatives or make-or-break affiliative contact initiatives. Successful attempts were scored whenever make-or-break contact initiatives were accommodated by the target. Failed attempts were scored when either initiative by an actor was resisted by the target.
Neuroendocrine stress responsivity and hormone quantification.
At an average of 38 weeks of age, a 30-min novelty stress test was administered to examine rearing differences in offspring HPA-axis responsivity. Each mother–offspring dyad was transported to a cage (60 × 60 × 90 cm) in an unfamiliar room that did not contain other monkeys. Both the cage and room used for stress testing were different from those used for the rearing protocols. Tests occurred between 1500 and 1800 h.
Blood samples were collected from offspring 10 days before the experimental manipulations to establish cortisol measures in an undisturbed state. Blood samples were also collected immediately after testing to examine cortisol levels after novelty exposure, and a subset of these data was reported in ref. 4. All blood samples were collected between 1530 and 1800 h to control for circadian variation (37). Femoral blood samples were collected within 3 minutes of capture from manually restrained monkeys with single-use syringes containing 20 μl of ethylenediamine tetraacetic acid. Each sample was transferred to a chilled tube and centrifuged at 4°C, and the plasma fraction was stored at −80°C. Cortisol was measured in duplicate by using an established radioimmunoassay as described in ref. 37.
Data analysis.
The effects of rearing condition on mother–infant-behavior measures and cortisol levels were assessed with ANOVA. Rearing condition and gender were between-subjects factors, and protocol week (1, 3, 5, 7, and 9), and observation period (before stress exposure, immediately after stress exposure, and 24 h after stress exposure) were within-subjects, repeated-measures factors. The Geisser–Greenhouse correction was used to adjust for multiple comparisons across repeated-measures factors. Tukey post hoc comparisons were used to discern significant rearing differences. Simple and multiple linear-regression models were used to investigate whether maternal behavior predicted offspring cortisol levels and to test whether rearing condition effects on cortisol levels were mediated by maternal behavior, respectively (22, 23). All test statistics were evaluated with two-tail probabilities (P < 0.05), and descriptive statistics are presented as mean ± SEM.
Experiment 2.
Subjects, rearing protocol, and subsequent husbandry.
Twenty-five squirrel monkeys born at Stanford University served as subjects. Subjects were housed under standard laboratory conditions in eight natal groups, each composed of three to four mother–infant dyads. Groups were randomized at 10 weeks of age to HFD (six male and six female infants) or LFD (seven male and six female infants) rearing conditions. The HFD condition consisted of 120% (by weight), and the LFD condition consisted of 600% (by weight) of normal daily food intake buried in foraging boards as described in detail in ref. 24. These foraging protocols were administered daily and lasted 12 weeks. All monkeys were housed under standard laboratory conditions thereafter. Mothers were removed after weaning at 36 weeks, and offspring were housed with two to three same-sex peers.
Neuroendocrine stress responsivity and hormone quantification.
At an average of 8.4 years of age, adult monkeys were restrained for two separate 30-min sessions in a standard primate chair. Chair restraint allows for slight movement and does not induce pain or inflict tissue damage (26). Restraint stress was used because adult squirrel monkeys do not respond to novelty stress as robustly as do infants (38). Tests were administered during the breeding season, when females exhibit ovarian hormone cyclicity, to best approximate adult human physiology (39).
The first test was preceded 60 min earlier by an intramuscular injection of saline to examine rearing differences in HPA-axis stress responsivity. Seven days later, an intramuscular injection of exogenous cortisol (i.e., 2.5 mg/kg hydrocortisone sodium succinate; Upjohn, Kalamazoo, MI) was likewise administered 60 min before the second test to examine rearing differences in glucocorticoid-feedback inhibition of the HPA-axis stress response.
Immediately after completion of each test, blood samples were collected, and monkeys were returned to the home cage. Additional samples were then collected 30 and 60 min later to provide posttest measures of recovery. Blood samples were also collected from otherwise undisturbed monkeys in the home cage 7 days before and 7 days after experimental manipulations to assess baseline hormone levels.
Blood samples were obtained as described in experiment 1 between 1330 and 1430 h to control for circadian variation (37). Plasma levels of ACTH and cortisol were measured in duplicate by using established radioimmunoassays (37). The cortisol assay does not distinguish between endogenous and exogenous cortisol (i.e., hydrocortisone), and, therefore, ACTH levels served as the primary index of stress responsivity and glucocorticoid-feedback regulation (26).
Data analysis.
Baseline hormone measures were analyzed for rearing condition, gender, and time effects by using repeated-measures ANOVA. Time-integrated poststress hormone measures were determined with the trapezoidal rule to estimate the area under each monkey’s saline and hydrocortisone time course curve (26). Time course (0, 30, and 60 min after restraint) and time-integrated poststress measures were then analyzed for rearing condition, gender, and hydrocortisone-pretreatment effects with baseline hormone levels controlled as a statistical covariate. All test statistics were evaluated with two-tailed probabilities (P < 0.05), and descriptive statistics are presented as mean ± SEM.
Acknowledgments
We thank Dr. Seymour Levine, Dr. Shelly Flagel, and Michael Heller for their thoughtful comments on this manuscript and Dr. Helena Kraemer for statistical guidance. This work was supported by National Institute of Mental Health Grants MH66537, MH47573, and MH50605; National Institute on Drug Abuse Grant DA16902; the Nancy Pritzker Network (New York); and a Stanford University School of Medicine Dean’s Fellowship.
Abbreviations
- ACTH
adrenocorticotropic hormone
- HFD
high foraging-demand
- HPA
hypothalamic–pituitary–adrenal
- IS
infant stress
- LFD
low foraging-demand
- MIS
mother–infant stress
- NS
no stress
- SHRP
stress-hyporesponsive period.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Rutter M. J. Adolesc. Health. 1993;14:626–631. doi: 10.1016/1054-139x(93)90196-v. 690–696. [DOI] [PubMed] [Google Scholar]
- 2.Masten A. S. Am. Psychol. 2001;56:227–238. doi: 10.1037//0003-066x.56.3.227. [DOI] [PubMed] [Google Scholar]
- 3.Boyce W. T., Chesterman E. J. Dev. Behav. Pediatr. 1990;11:105–111. [PubMed] [Google Scholar]
- 4.Parker K. J., Buckmaster C. L., Schatzberg A. F., Lyons D. M. Arch. Gen. Psychiatry. 2004;61:933–941. doi: 10.1001/archpsyc.61.9.933. [DOI] [PubMed] [Google Scholar]
- 5.Meaney M. J. Annu. Rev. Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 6.Levine S. Science. 1957;126:405. doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- 7.Smotherman W. P., Bell R. W. In: Maternal Influences and Early Behavior. Bell R. W., Smotherman W. P., editors. New York: Spectrum; 1980. pp. 201–210. [Google Scholar]
- 8.Lee M. H., Williams D. I. Anim. Behav. 1974;22:679–681. [Google Scholar]
- 9.Liu D., Diorio J., Tannenbaum B., Caldji C., Francis D., Freedman A., Sharma S., Pearson D., Plotsky P. M., Meaney M. J. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg K. M., Denenberg V. H., Zarrow M. X. Anim. Behav. 1970;18:138–143. doi: 10.1016/0003-3472(70)90082-5. [DOI] [PubMed] [Google Scholar]
- 11.Rosenfeld P., Suchecki D., Levine S. Neurosci. Biobehav. Rev. 1992;16:553–568. doi: 10.1016/s0149-7634(05)80196-4. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt M., Enthoven L., van der Mark M., Levine S., de Kloet E. R., Oitzl M. S. Int. J. Dev. Neurosci. 2003;21:125–132. doi: 10.1016/s0736-5748(03)00030-3. [DOI] [PubMed] [Google Scholar]
- 13.Huot R. L., Plotsky P. M., Lenox R. H., McNamara R. K. Brain Res. 2002;950:52–63. doi: 10.1016/s0006-8993(02)02985-2. [DOI] [PubMed] [Google Scholar]
- 14.Bowman R. E., Wolf R. C. Proc. Soc. Exp. Biol. Med; 1965. pp. 133–135. [DOI] [PubMed] [Google Scholar]
- 15.Pryce C. R., Palme R., Feldon J. J. Clin. Endocrinol. Metab. 2002;87:691–699. doi: 10.1210/jcem.87.2.8244. [DOI] [PubMed] [Google Scholar]
- 16.Buss K. A., Schumacher J. R., Dolski I., Kalin N. H., Goldsmith H. H., Davidson R. J. Behav. Neurosci. 2003;117:11–20. doi: 10.1037//0735-7044.117.1.11. [DOI] [PubMed] [Google Scholar]
- 17.Gunnar M. R. Pediatrics. 1992;90:491–497. [PubMed] [Google Scholar]
- 18.Coe C. L., Glass J. C., Wiener S. G., Levine S. Psychoneuroendocrinology. 1983;8:401–409. doi: 10.1016/0306-4530(83)90019-7. [DOI] [PubMed] [Google Scholar]
- 19.Levine S., Mody T. Neurosci. Biobehav. Rev. 2003;27:83–89. doi: 10.1016/s0149-7634(03)00011-3. [DOI] [PubMed] [Google Scholar]
- 20.Jordan T. C., Hennessy M. B., Gonzalez C. A., Levine S. Physiol. Behav. 1985;34:489–493. doi: 10.1016/0031-9384(85)90038-1. [DOI] [PubMed] [Google Scholar]
- 21.Brent L., Koban T., Ramirez S. Biol. Psychiatry. 2002;52:1047–1056. doi: 10.1016/s0006-3223(02)01540-8. [DOI] [PubMed] [Google Scholar]
- 22.Baron R. M., Kenny D. A. J. Pers. Soc. Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 23.Kraemer H. C., Stice E., Kazdin A., Offord D., Kupfer D. Am. J. Psychiatry. 2001;158:848–856. doi: 10.1176/appi.ajp.158.6.848. [DOI] [PubMed] [Google Scholar]
- 24.Lyons D. M., Kim S., Schatzberg A. F., Levine S. Dev. Psychobiol. 1998;32:285–291. [PubMed] [Google Scholar]
- 25.Champoux M., Hwang L., Lang O., Levine S. Psychoneuroendocrinology. 2001;26:461–477. doi: 10.1016/s0306-4530(01)00006-3. [DOI] [PubMed] [Google Scholar]
- 26.Lyons D. M., Yang C., Eliez S., Reiss A. L., Schatzberg A. F. J. Neurosci. 2004;24:3655–3662. doi: 10.1523/JNEUROSCI.0324-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denenberg V. H. Dev. Psychobiol. 1999;34:1–3. doi: 10.1002/(sici)1098-2302(199901)34:1<1::aid-dev2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 28.Suomi S. J. Br. Med. Bull. 1997;53:170–184. doi: 10.1093/oxfordjournals.bmb.a011598. [DOI] [PubMed] [Google Scholar]
- 29.Kaler S. R., Freeman B. J. J. Child Psychol. Psychiatry. 1994;35:769–781. doi: 10.1111/j.1469-7610.1994.tb01220.x. [DOI] [PubMed] [Google Scholar]
- 30.Hopf S., Hartmann-Wiesner E., Kuhlmorgen B., Mayer S. Folia Primatologica. 1974;21:225–249. doi: 10.1159/000155602. [DOI] [PubMed] [Google Scholar]
- 31.Cohen S., Wills T. A. Psychol. Bull. 1985;98:310–357. [PubMed] [Google Scholar]
- 32.Parker K. J., Buckmaster C. L., Justus K. R., Schatzberg A. F., Lyons D. M. Biol. Psychiatry. 2005;57:848–855. doi: 10.1016/j.biopsych.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 33.Young E. A., Altemus M. Ann. N.Y. Acad. Sci. 2004;1021:124–133. doi: 10.1196/annals.1308.013. [DOI] [PubMed] [Google Scholar]
- 34.Kitay J. I. Endocrinology. 1961;68:818–824. doi: 10.1210/endo-68-5-818. [DOI] [PubMed] [Google Scholar]
- 35.Handa R. J., Burgess L. H., Kerr J. E., O’Keefe J. A. Horm. Behav. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- 36.Boinski S., Fragaszy D. M. Anim. Behav. 1989;37:415–428. [Google Scholar]
- 37.Lyons D. M., Ha C. M., Levine S. Horm. Behav. 1995;29:177–190. doi: 10.1006/hbeh.1995.1013. [DOI] [PubMed] [Google Scholar]
- 38.Vogt J. L., Levine S. Physiol. Behav. 1980;24:829–832. doi: 10.1016/0031-9384(80)90135-3. [DOI] [PubMed] [Google Scholar]
- 39.Mendoza S. P., Lowe E. L., Resko J. A., Levine S. Physiol. Behav. 1978;20:515–522. doi: 10.1016/0031-9384(78)90240-8. [DOI] [PubMed] [Google Scholar]