Abstract
Understanding the mechanisms that regulate germ-cell development is crucial to reproductive medicine and animal production. Animal gametes originally derive from sexually undifferentiated primordial germ cells (PGCs), which develop into mitotic germ cells (oogonia or spermatogonia) before proceeding to meiosis [Wylie, C. (1999) Cell 96, 165–174]. Spermatogonia are thought to include a population of cells with stem cell activity, which proliferate throughout the lifespan of male animals and produce spermatozoa [Zhao, G. Q. & Garbers, D. L. (2002) Dev. Cell 2, 537–547]. However, the functional differences between PGCs and spermatogonial stem cells are poorly understood. Here we show that transplanted adult testicular germ cells can colonize sexually undifferentiated embryonic gonads and resume gametogenesis. Testicular germ cells containing spermatogonial stem cells isolated from adult male rainbow trout (Oncorhynchus mykiss) were transplanted into the peritoneal cavity of newly hatched embryos of both sexes, and the behavior of the donor cells was observed. The testicular germ cells differentiated into spermatozoa in male recipients and fully functional eggs in female recipients. Furthermore, the donor-derived spermatozoa and eggs obtained from the recipient fish were able to produce normal offspring. These findings indicate that fish testicular germ cells, probably spermatogonial stem cells, possess a high level of developmental plasticity and sexual bipotency, even after the animal reaches maturity. Furthermore, our results suggest that spermatogonial stem cells are at least partly functionally similar to PGCs.
Keywords: developmental plasticity, germ cell transplantation, sexual bipotency, spermatogonia, stem cell
Primordial germ cells (PGCs) differentiate into eggs or spermatozoa in the ovary or testis, respectively. Animal PGCs emerge in extragonadal areas and migrate to the genital ridges, which are anlagen of the gonads. During migration, mammalian PGCs divide and increase in number (1), whereas fish PGCs proliferate after migration (2). During sexual differentiation, oogonia in female gonads commence meiosis immediately, whereas spermatogonia in male gonads continue with mitosis and enter meiosis at a later developmental stage (3, 4).
Spermatogonia are basically classified in two types: undifferentiated spermatogonial stem cells and differentiated spermatogonia committed to mitotic proliferation leading to meiosis. The former possess an ability to self-renew throughout the lifetime of animals and continuously supply spermatozoa (3). These unique stem cells transmit genetic information to the next generation through maturation and fertilization. Embryonic PGCs were recently reported to colonize the postnatal mouse testis and undergo spermatogenesis (5). Although the genomic methylation status of mouse PGCs and spermatogonial stem cells are distinct (6), the physiological differences between them remain unclear. Morphologically, spermatogonial stem cells are smaller than their sexually undifferentiated progenitor PGCs and possess distinctive microstructures (7). Furthermore, the differentiation of mouse PGCs into spermatogonial stem cells alters the expression of various genes, including alkaline phosphatase, c-kit (8), nanos homologue 2 (nanos2), and nanos homologue 3 (nanos3) (9). Yet it remains unclear how these shifts in gene expression induce functional differences between vertebrate PGCs and spermatogonial stem cells. Furthermore, functional studies in nonmammalian vertebrates are limited and, particularly in fish, there is no direct evidence that spermatogonia include a stem-cell population.
Germ-cell transplantation is useful for the functional characterization of these cells at different developmental stages. The only technique to our knowledge for transplanting vertebrate germ cells into sexually undifferentiated gonads is that established with rainbow trout in our laboratory (10, 11). The current study investigated the physiological characteristics, particularly the developmental plasticity, of spermatogonial stem cells. We transplanted adult rainbow trout testicular cells, which were predicted to contain a spermatogonial stem-cell population, into newly hatched trout embryos and observed their fates.
Results
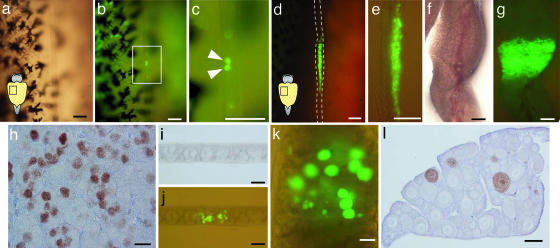
A testicular cell suspension was prepared from 9-month-old transgenic rainbow trout carrying a GFP gene driven by the regulatory elements of the germ-line-specific rainbow trout vasa-like gene (12). Approximately 18,000 cells including 10,000 germ cells were transplanted i.p. into newly hatched (32–35 days postfertilization) allogenic rainbow trout fry, which possess sexually undifferentiated genital ridges containing PGCs. By 20 days posttransplantation (pt), the transplanted germ cells had incorporated into the genital ridges of 43% (12/28) of the recipient fish (Fig. 1a–c). The mean (± SEM) number of incorporated GFP-positive germ cells was 4.6 ± 2.2 (range from 1 to 9). Immediately after morphological sexual differentiation (2 months pt), colonies of proliferated GFP-labeled germ cells were observed in the testes of 39% (31/80) of the recipients (Fig. 1 d and e). By 7 months pt, vastly increased numbers of spermatogonia showed green fluorescence (Fig. 1 f and g). Immunohistological analysis with GFP-specific antibody revealed that the GFP-positive cells resembled undifferentiated spermatogonia surrounded by Sertoli cells (Fig. 1h). Surprisingly, 2 months pt, the donor-derived testicular germ cells also colonized the ovaries of 37% (26/45) of the female recipients and differentiated into oocytes (≈19 μm in diameter; Fig. 1 i and j). By 7 months pt, these had developed into perinucleolus-stage oocytes (≈100 μm in diameter) similar to the allogenic recipient oocytes. It was noteworthy that testicular germ cells remained able to colonize embryonic gonads and differentiate into male or female germ cells, even when isolated from the postspermiation testis (28 months old). Furthermore, there were no significant differences in colonization efficiency in male and female recipients (data not shown).
Fig. 1.
Donor germ cells in recipient gonads after transplantation. (a–c) Incorporation of GFP-labeled donor germ cells into a recipient gonad at 20 days pt in bright-field view (a), fluorescent view (b), and highly magnified image of a flame in b (c). Arrowheads indicate donor-derived germ cells showing green fluorescence. (d and e) Proliferation of donor germ cells in a recipient testis at 2 months pt showing a ventral view of a recipient peritoneal cavity (d) and an isolated testis (e). (f–h) Vast proliferation of donor germ cells in a recipient testis at 7 months pt in bright-field view (f), fluorescent view (g), and according to immunohistochemistry using a GFP-specific antibody (h). (i and j) Proliferation of donor testicular germ cells in a recipient ovary at 2 months pt in bright-field view (i) and fluorescent view (j). (k and l) Donor testicular germ cell-derived oocytes in a recipient ovary at 7 months pt in fluorescent view (k) and according to immunohistochemistry using a GFP-specific antibody (l). (Bars: 100 μm, a–e and i–l; 1 mm, f and g; and 20 μm, h.)
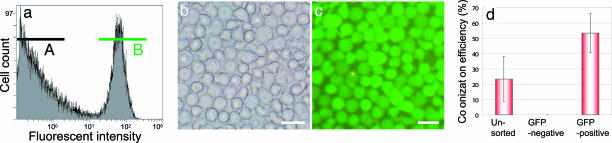
To clarify which testis cell population repopulated the embryonic gonads, we performed cell transplantation studies by using GFP-dependent flow-cytometric cell sorting (Fig. 2a–c). We previously reported that only PGCs and spermatogonia showed green fluorescence throughout the lifespan of vasa-GFP transgenic males (13). Thus, the GFP-positive population of adult fish comprises spermatogonia alone. Our current results revealed that only GFP-positive spermatogonia formed green fluorescent colonies in the recipient embryonic gonads after transplantation (Fig. 2d). Although the GFP-positive germ cells were distributed everywhere in the recipient body cavity right after the transplantation, PCR using various recipient tissues showed that donor cells carrying the vasa-GFP gene were present only in gonads possessing green fluorescent germ cells at 3 months pt and later stages (data not shown). Thus, somatic donor cells without green fluorescence did not survive or differentiate into germ cells in the allogenic recipients. These findings demonstrate that only spermatogonia can repopulate embryonic gonads.
Fig. 2.
Transplantation of spermatogonia after GFP-dependent flow cytometry. (a) Fluorescent intensity patterns of total testicular cells. The x axis represents the GFP intensity (logarithmic scale), and the y axis indicates the cell counts (linear scale). (b and c) Bright (b) and fluorescent (c) views of cells isolated with gate B. (Bars = 20 μm.) (d) The frequency to produce a GFP-positive germ-cell colony after the transplantation of GFP-positive or GFP-negative cell populations into allogenic recipients. Unsorted testicular cells were also transplanted as a control.
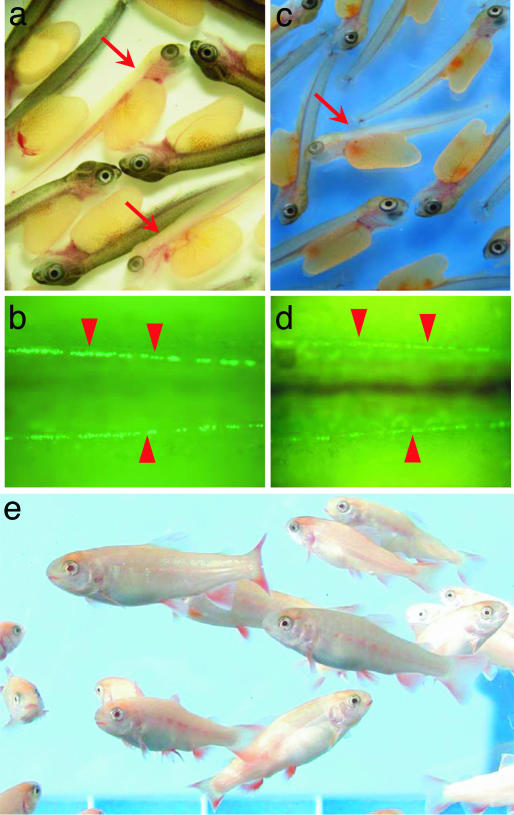
Progeny tests investigated whether donor-derived germ cells matured and produced functional gametes in recipient gonads. Dominant albino transgenic trout carrying the vasa-GFP gene and nontransgenic wild-type trout functioned as donors and recipients, respectively. The latter were reared to maturity, and milt samples were obtained 1 year pt from 26 males. Milt samples from 13 GFP-positive males identified by PCR were used to artificially inseminate eggs from wild-type nontransgenic females (Table 1). Offspring showing albino body color and green fluorescent germ cells were obtained from most crosses (Fig. 3a and b), suggesting that donor-derived germ cells could differentiate into fully functional spermatozoa in the allogenic recipient testes. Donor-derived offspring also were obtained from these recipients the next spawning season (Table 1). The average germ-line transmission rate was 5.46 ± 3.34% (range from 0.16% to 40.49%). Differentiated rainbow trout spermatogonia reportedly undergo mitosis seven times, followed by two consecutive cycles of meiosis (14); thus, one founder spermatogonium can produce up to 512 spermatozoa. In the current study, the average number of donor-derived spermatozoa produced in the recipient testes was 20.1 × 107 (range from 0.3 to 143.1 × 107). The average number of incorporated donor cells was 4.6 ± 2.2. These values imply that the donor-derived germ cells underwent mitosis 24 times on average (range from 18 to 27), which was far more than the predicted proliferation rate. In addition, the recipients produced donor-derived spermatozoa during two consecutive spawning seasons. These data suggest that the colonized germ cells possessed stem-cell activity, could differentiate into spermatozoa, and had a high or even unlimited capacity for self-renewal.
Table 1.
Appearance rate of donor-derived offspring among F1 generation
| Recipient | Age, year | Total no. of F1 offspring | No. of donor-derived F1 offspring | Donor-derived/total, % | Recipient | Age, year | Total no. of F1 offspring | No. of donor-derived F1 offspring | Donor-derived/total, % |
|---|---|---|---|---|---|---|---|---|---|
| Male 2 | 1 | 1,591 | 8 | 0.50 | Female 9 | 2 | 2,632 | 6 | 0.23 |
| 2 | 1,042 | 0 | 0.00 | ||||||
| Male 5 | 1 | 4,352 | 1,762 | 40.49 | Female 10 | 2 | 2,176 | 118 | 5.42 |
| 2 | 1,030 | 286 | 27.77 | ||||||
| Male 8 | 1 | 4,299 | 28 | 0.65 | Female 11 | 2 | 3,204 | 2 | 0.06 |
| 2 | 946 | 8 | 0.85 | ||||||
| Male 10 | 1 | 2,984 | 18 | 0.60 | Female 13 | 2 | 3,133 | 10 | 0.32 |
| 2 | 777 | 6 | 0.77 | ||||||
| Male 11 | 1 | 3,770 | 484 | 12.84 | Female 15 | 2 | 3,181 | 50 | 1.57 |
| 2 | 1,365 | 312 | 22.86 | ||||||
| Male 14 | 1 | 3,319 | 90 | 2.71 | Female 17 | 2 | 1,616 | 160 | 9.90 |
| 2 | 1,381 | 52 | 3.77 | ||||||
| Male 15 | 1 | 3,602 | 10 | 0.28 | Female 18 | 2 | 2,164 | 138 | 6.38 |
| 2 | 1,213 | 2 | 0.16 | ||||||
| Male 17 | 1 | 2,725 | 28 | 1.03 | Female 19 | 2 | 3,243 | 86 | 2.65 |
| 2 | 1,448 | 20 | 1.38 | ||||||
| Male 18 | 1 | 2,286 | 6 | 0.26 | Female 26 | 2 | 2,148 | 2 | 0.09 |
| 2 | 926 | 2 | 0.22 | ||||||
| Male 19 | 1 | 2,344 | 4 | 0.17 | Female 30 | 2 | 2,636 | 22 | 0.83 |
| 2 | 1,068 | 0 | 0.00 | ||||||
| Male 22 | 1 | 3,535 | 118 | 3.34 | Female 32 | 2 | 3,308 | 2 | 0.06 |
| 2 | 1,291 | 104 | 8.06 | ||||||
| Male 24 | 1 | 3,219 | 86 | 2.67 | Female 36 | 2 | 2,900 | 2 | 0.07 |
| 2 | ND* | ND* | ND* | ||||||
| Female 1 | 2 | 2,094 | 44 | 2.10 | Female 38 | 2 | 2,886 | 62 | 2.15 |
| Female 5 | 2 | 2,725 | 50 | 1.83 | Female 40 | 2 | 2,290 | 12 | 0.52 |
*Recipient male 24 died after the first spawning season.
Fig. 3.
Donor-derived offspring obtained from progeny tests. (a) Donor-derived F1 offspring showing albino body color (arrow) derived from a male recipient. (b) Genital ridges (arrowheads) containing GFP-labeled germ cells in the donor-derived F1 offspring shown in a. (c) Donor-derived F1 offspring showing albino body color (arrow) derived from a female recipient. (d) Genital ridges (arrowheads) containing GFP-labeled germ cells in the donor-derived F1 offspring shown in c. (e) F1 offspring at 8 months old developed from testicular cell-derived eggs.
Additional progeny tests were performed with 40 mature (2-year-old) female recipients. Of these, 16 (40%) yielded donor-derived offspring with green fluorescent germ cells and albino phenotype (Fig. 3 c and d). The average contribution rate of the transplanted testicular germ cells to the female germ line was 2.14 ± 0.70% (range from 0.06% to 9.90%). This value was not significantly different from the germ-line transmission rate of male recipients. The early survival rates of donor-derived and control offspring were similar, and the hatched fry grew up normally to date (Fig. 3e). Thus, testicular germ cells could colonize the embryonic gonads of female recipients and produce functional eggs.
Discussion
In this study, the transplanted germ cells resumed gametogenesis in the recipient somatic environments concurrently with endogenous germ cells. These adult testicular germ cells interacted with somatic counterparts in the recipient embryonic gonads that originally involve sexually undifferentiated PGCs. Thus, testicular germ cells contain a cell population possessing a high level of developmental plasticity. Alternatively, the adult testis could have retained germ cells with functional characteristics similar to those of PGCs.
We also confirmed that testicular germ cells contain a population with stem-cell activity. Spermatogonial stem cells are assumed to be present in the fish testis because of the occurrence of spermatogonia, which are morphologically similar to mammalian spermatogonial stem cells, along with repetitive waves of spermatogenesis in seasonally reproducing species (15). The current study produced direct evidence of germ-line stem cells in the fish testis.
Several previous reports have described natural and artificial sex reversal induced by exogenous steroids in fish, but they did not clarify whether it was caused by differentiated germ cells changing their sexual characteristics or undifferentiated germ cells within the gonad being recruited to initiate a new course of differentiation (16). Further, most of them have described sex reversal induced by exogenous sex steroids treated before or around the sex-differentiation period (16). It was also demonstrated that transplantation of male or female PGCs into embryonic recipients of opposite sex leads sex reversal of germ cells in chickens; however, donor germ cells were isolated from sexually undifferentiated chick gonads but not from adult gonads (17). Our study demonstrated that spermatogonia isolated from the adult testis contained a cell population that could differentiate into fully functional eggs. The results obtained in this study confirmed that spermatogonia, probably spermatogonial stem cells, are sexually bipotent and sexual differentiation of germ cells is controlled solely by the somatic microenvironment, rather than being cell autonomous.
In summary, spermatogonia are developmentally plastic, include a stem-cell population, and are sexually bipotent. These facts indicate that early germ-cell development in fish is highly flexible and/or spermatogonia are at least partly functionally similar to sexually undifferentiated PGCs, despite their distinctive morphology.
Drosophila male germ-line stem cells can regenerate by spermatogonial dedifferentiation (18). Therefore, transplanted spermatogonia might dedifferentiate into PGCs in recipient fish embryos. No molecular markers are available to distinguish PGCs and spermatogonia, making the possibility of dedifferentiation impossible to clarify at present. This important question should be addressed in future studies.
This report describes spermatogonial transplantation in nonmammalian species. Although it was unclear how transplanted spermatogonia were incorporated into recipient gonads, i.p. transplanted PGCs can migrate by extending pseudopodia (10) and isolated spermatogonia can extend pseudopodia in vitro (S. Shikina and G.Y., unpublished data). Spermatogonia might therefore actively migrate to the recipient gonads in response to chemotactic signals.
Our approach to producing functional spermatozoa and eggs derived from adult testicular germ cells represents a powerful tool for breeding domestic animals and fish. Furthermore, self-fertilization could be achieved by mating female and male recipients carrying germ cells derived from the same donor. This process would allow the production of inbred strains with desirable genetic traits in fewer generations than existing strategies.
Materials and Methods
Germ-Cell Transplantation.
Donor cells were prepared from dominant albino transgenic rainbow trout carrying a GFP gene driven by vasa gene-regulatory regions (13). Spermatogonia in the testis of the transgenic trout were labeled by indelible green fluorescence (19). Freshly isolated testes were minced and incubated with 1 ml of 0.5% trypsin (Worthington) in PBS (pH 8.2) containing 5% FBS and 1 mM Ca2+ for 2 h at 20°C. During incubation, gentle pipetting was applied to physically disperse any remaining intact portions of the testis. The resultant cell suspension was filtered through a 42-μm pore-size nylon screen to eliminate nondissociated cell clumps and then stored on ice until the time of transplantation. The allogenic transplantation of donor germ cells was performed i.p. according to the method of Takeuchi and colleagues (10).
Fluorescent Observation of Donor Germ Cells.
Recipient embryos were observed under a fluorescent microscope 20 days pt to evaluate the incorporation rate of donor germ cells into the genital ridges. The proliferation and differentiation of the donor-derived germ cells in the gonads of the recipients were observed at 2 and 7 months pt.
Immunohistochemistry.
Tissues were prepared for immunohistochemical analysis with a GFP-specific antibody according to the method described by Yoshizaki and colleagues (12). The immunoreactions were performed with anti-GFP (Hoffmann-La Roche) and visualized with a Vectastain Elite ABC kit (Vector Laboratories).
Cell Sorting by Flow Cytometry Followed by Transplantation.
GFP-dependent cell sorting was used to isolate spermatogonia according to the method of Kobayashi and colleagues (20). Two linear gates were set for the analysis of GFP intensity: gate A was for GFP-negative somatic cells, and gate B covered the remaining signals with stronger fluorescence (Fig. 2a). The sorted cells were resuspended in DMEM (Life Technologies, Grand Island, NY) with 5% FBS and transplanted into recipient embryos. The colonization efficiency of donor-derived cells in the recipient genital ridges was calculated by the following formula: colonization efficiency (%) = number of embryos incorporating GFP-labeled cells in their genital ridges at 20 days pt/number of embryos used for cell transplantation × 100. All transplantation experiments involved a minimum of 30 embryos and were repeated at least in triplicate.
PCR Detection of Donor-Derived Cells.
PCR was performed with GFP-specific primers as described (21), to examine the distribution of donor-derived cells in recipient fish 3 and 20 months pt. Template DNA was extracted from the following tissues: gill, kidney, spleen, liver, muscle, intestine, gonads containing donor-derived germ cells, and contralateral gonads without GFP-positive germ cells.
Progeny Tests.
Recipients transplanted with testicular cells were reared until maturity. Semen was collected from 1- and 2-year-old male recipients. DNA was extracted from 1 μl of semen and subjected to PCR with GFP-specific primers, according to the method of Takeuchi and colleagues (21). To determine the production of offspring from spermatozoa derived from donor testicular cells, semen from GFP-positive fish was used to fertilize nontransgenic eggs from wild-type females. The milts produced by recipients were extracted by using abdominal pressure and their volumes were measured. In addition, the milt samples were diluted 1,000 times with PBS and the spermatozoa were counted with a hemocytometer. The number of donor-derived spermatozoa was obtained by using the following formula: whole milt volume (ml) × spermatozoa density (number per ml) × germ-line-transmission rate of donor-derived haplotype (%)/100. To determine whether transplanted testicular germ cells could differentiate into functional gametes, eggs obtained from 2-year-old female recipients were fertilized with spermatozoa from wild-type males.
Acknowledgments
This study was supported in part by a Grant-in-Aid from the Ministry of Agriculture, Forestry, and Fisheries.
Abbreviations
- PGC
primordial germ cell
- pt
posttransplantation.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Wylie C. Cell. 1999;96:165–174. doi: 10.1016/s0092-8674(00)80557-7. [DOI] [PubMed] [Google Scholar]
- 2.Yoshizaki G., Takeuchi Y., Kobayashi T., Ihara S., Takeuchi T. Fish Physiol. Biochem. 2002;26:3–12. [Google Scholar]
- 3.Zhao G. Q., Garbers D. L. . Dev. Cell. 2002;2:537–547. doi: 10.1016/s1534-5807(02)00173-9. [DOI] [PubMed] [Google Scholar]
- 4.Matova N., Cooley L. Dev. Biol. 2001;231:291–320. doi: 10.1006/dbio.2000.0120. [DOI] [PubMed] [Google Scholar]
- 5.Chuma S., Kanatsu-Shinohara M., Inoue K., Ogonuki N., Miki H., Toyokuni S., Hosokawa M., Nakatsuji N., Ogura A., Shinohara T. Development (Cambridge, U.K.) 2005;132:117–122. doi: 10.1242/dev.01555. [DOI] [PubMed] [Google Scholar]
- 6.Li J. Y., Lees-Murdock D. J., Xu G. L., Walsh C. P. Genomics. 2004;84:952–960. doi: 10.1016/j.ygeno.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Patiño R. In: An Atlas of Fish Histology: Normal and Pathological Features. Takashima F., Hibiya T., editors. Tokyo: Kodansha; 1995. pp. 144–150. [Google Scholar]
- 8.Lacham-Kaplan O. Reproduction. 2004;128:147–152. doi: 10.1530/rep.1.00220. [DOI] [PubMed] [Google Scholar]
- 9.Tsuda M., Sasaoka Y., Kiso M., Abe K., Haraguchi S., Kobayashi S., Saga Y. Science. 2003;301:1239–1241. doi: 10.1126/science.1085222. [DOI] [PubMed] [Google Scholar]
- 10.Takeuchi Y., Yoshizaki G., Takeuchi T. Biol. Reprod. 2003;69:1142–1149. doi: 10.1095/biolreprod.103.017624. [DOI] [PubMed] [Google Scholar]
- 11.Takeuchi Y., Yoshizaki G., Takeuchi T. Nature. 2004;430:629–630. doi: 10.1038/430629a. [DOI] [PubMed] [Google Scholar]
- 12.Yoshizaki G., Takeuchi Y., Sakatani S., Takeuchi T. Int. J. Dev. Biol. 2000;44:323–326. [PubMed] [Google Scholar]
- 13.Takeuchi Y., Yoshizaki G., Kobayashi T., Takeuchi T. Biol. Reprod. 2002;67:1087–1092. doi: 10.1095/biolreprod67.4.1087. [DOI] [PubMed] [Google Scholar]
- 14.Loir M. Mol. Reprod. Dev. 1999;53:422–433. doi: 10.1002/(SICI)1098-2795(199908)53:4<422::AID-MRD8>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 15.Schulz R. W., Miura T. Fish Physiol. Biochem. 2002;26:43–56. [Google Scholar]
- 16.Devlin R., Nagahama Y. Aquaculture. 2002;208:191–364. [Google Scholar]
- 17.Kagami H., Clark M. E., Verrinder Gibbins A. M., Etches R. J. Mol. Reprod. Dev. 1995;42:379–387. doi: 10.1002/mrd.1080420403. [DOI] [PubMed] [Google Scholar]
- 18.Brawley C., Matunis E. Science. 2004;304:1331–1334. doi: 10.1126/science.1097676. [DOI] [PubMed] [Google Scholar]
- 19.Yoshizaki G., Takeuchi Y., Kobayashi T., Takeuchi T. Fish Physiol. Biochem. 2003;28:453–457. [Google Scholar]
- 20.Kobayashi T., Yoshizaki G., Takeuchi Y., Takeuchi T. Mol. Reprod. Dev. 2004;67:91–100. doi: 10.1002/mrd.20003. [DOI] [PubMed] [Google Scholar]
- 21.Takeuchi Y., Yoshizaki G., Takeuchi T. Mar. Biotechnol. 1999;1:448–457. doi: 10.1007/pl00011801. [DOI] [PubMed] [Google Scholar]