Abstract
IL-1β was identified after a long search for the endogenous pyrogen. It acts by inducing synthesis of prostaglandin E2, which mediates the late phase of IL-1β-induced fever. Here we show by radiotelemetry that the early phase of the fever response to IL-1β is mediated by ceramide. Hypothalamic application of the cell-penetrating C2-ceramide mimics the rapid phase of the IL-1β-induced fever. Inhibition of ceramide synthesis blocks the rapid phase of fever but does not affect the slower prostaglandin E2-dependent phase, which is blocked by indomethacin or by null mutation of the EP3 prostanoid receptor. Electrophysiological experiments on preoptic area/anterior hypothalamic neurons show that C2-ceramide, but not dihydroceramide, mimics the rapid hyperpolarizing effects of IL-1β on the activity of warm-sensitive hypothalamic neurons. IL-1β-mediated hyperpolarization is blocked by PP2, the selective inhibitor of the protein tyrosine kinase Src, which is known to be activated by ceramide. These in vivo and in vitro data suggest that ceramide fulfills the criteria for an endogenous pyrogen.
Keywords: fever, neutral sphingomyelinase, preoptic area
A multitude of microbial and inorganic substances of widely varying origin and chemical composition are able to cause fever, a regulated rise in core body temperature (CBT) (1, 2). Root and Wolff (3) have pointed out that the uniformity of the fever response to all of these pyrogens, with respect to peak temperature and duration, suggests there may be an endogenous common mediator, an endogenous pyrogen. A long search has identified IL-1 as the endogenous pyrogen that mediates the common action of a broad variety of exogenous substances (4). Another line of investigation for the endogenous pyrogens was based on the pharmacological identification of antipyretic substances such as salicylates more than a century ago. Because this class of antipyretic agents are effective in reducing fever of almost any cause, it was implied there must exist an endogenous pyrogen whose synthesis and/or effects are blocked by salicylates. Experiments with purified and later with recombinant IL-1β showed that indeed the fever response to LPS, an exogenous pyrogen from Gram-negative bacteria wall, was mimicked by IL-1β (reviewed in ref. 1) and blocked by salicylates (5). The identification of prostaglandin (PG) E2 (PGE2) as another endogenous pyrogen and the recognition of the role of salicylates and aspirin-like antiinflammatory drugs as inhibitors of PG biosynthesis have produced a working hypothesis for the fever response to Gram-negative bacteria: Bacterial cell wall → LPS → IL-1β → cyclooxygenase (COX)2 → PGE2 → fever response.
This cascade also provided an explanation for the antipyretic effects of PG synthesis inhibitors. Subsequent studies using null mutated mice strains for prostanoid receptor subtypes, as well as selective drugs for prostanoid receptors, identified the EP3 prostanoid receptor (EP3R) and EP1R receptors as mediators of the pyrogenic effects of PGE2 (6–9). This scheme has been useful in explaining all but the first rapid rise of CBT initiated within the first 15–40 min after IL-1β administration, although the biphasic nature of the LPS and IL-1β-mediated fever has been described (10, 11). The fast rise in hypothalamic temperature as well as CBT in response to IL-1β is not consistent with the transcription-dependent action of IL-1β, which requires the NF-κB-dependent induction of the synthesis of COX2 and the subsequent production of PGH and PGE2.
We show here that, in the preoptic area (POA)/anterior hypothalamus (AH), IL-1β signaling through a rapid nontranscription-dependent enzymatic pathway, including the type 1 IL-1 receptor (IL-1R1)-mediated activation of the Neutral sphingomyelinase (N-Smase) is involved in the rapid rise of fever. We demonstrate that the product of N-Smase, ceramide, may be the second messenger mediating the effects on the activity of POA/AH warm-sensitive neurons. Our results suggest that the IL-1β effects on hypothalamic neurons involve the activity of protein tyrosine kinase Src. Furthermore, we show that inhibition of N-Smase blocks the rapid first phase of fever but does not affect the slower PGE2-dependent fever peak. Conversely, the inhibitors of PGE2 synthesis or the null mutation of EP3R do not affect the rapid first phase of IL-1β-induced fever but significantly attenuate the slower phase of the fever response to IL-1β.
Results
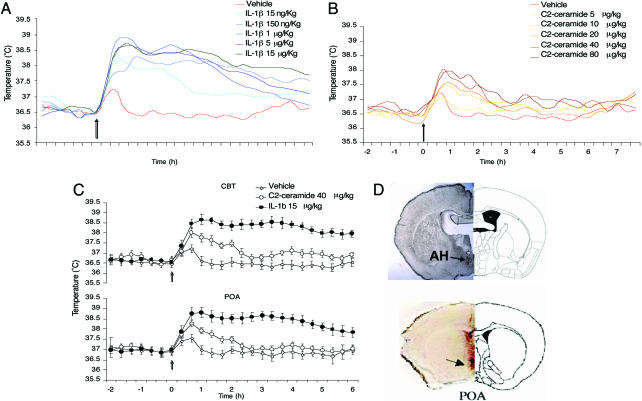
Injection of IL-1β into the preoptic area of the AH causes a rapid fever response in wild-type mice (Fig. 1A and C). This fever response is followed by radiotelemetric devices implanted into the peritoneum and thermoprobes implanted into the preoptic area, respectively. For location of the cannula and termistor, see Fig. 1D. Changes in brain temperature and CBT follow each other closely (Fig. 1C). The response to IL-1β consists of two phases: a rapid rise of hypothalamic temperature and CBT peaking at 30–60 min and a long-lasting phase that continues at peak fever temperature (≈38.5°C) over the next 3 h (Fig. 1A). The amplitude of the fever response is dose-dependent in the IL-1β dose range of 15 ng/kg to 15 μg/kg.
Fig. 1.
Dose–response curve for IL-1β and C2-ceramide on CBT after microinjection in the AH. (A) Dose–response curve for IL-1β-induced fever. CBT increased significantly at 60 min in IL-1β treated at 1, 5 and 15 μg/kg compared with IL-1β 15 ng/kg and 150 ng/kg or vehicle (∗, P < 0.05; data are averaged at 20-min intervals). (B) Dose–response curve for C2-ceramide-induced fever. CBT increased significantly at 40 min in C2-ceramide treated at doses 10, 20, 40, and 80 μg/kg (∗, P < 0.05, compared with vehicle). (C) Simultaneous recording of temperature in the POA and CBT comparing C2-ceramide 40 μg/kg, IL-1β 15 μg/kg, and vehicle control. C2-ceramide-induced rise in CBT peaks at 40 min, whereas the maximum rise in CBT caused by IL-1β peaks at 60 min. The effect of C2-ceramide declines after 60 min, reaching the baseline at ≈2 h, whereas the IL-1β-induced rise in CBT at 60 min remains at a constant level for the following 3 h, after which it declines but is significantly above the baseline (8–12 h) after injection. (A–C) All data points are from six animals each. (D) Location of cannulae aiming the AH and track of the thermisor to the POA; see arrows. Slices correspond to anterior–posterior coordinates from Bregma, −0.46 for AH and 0.38 for POA.
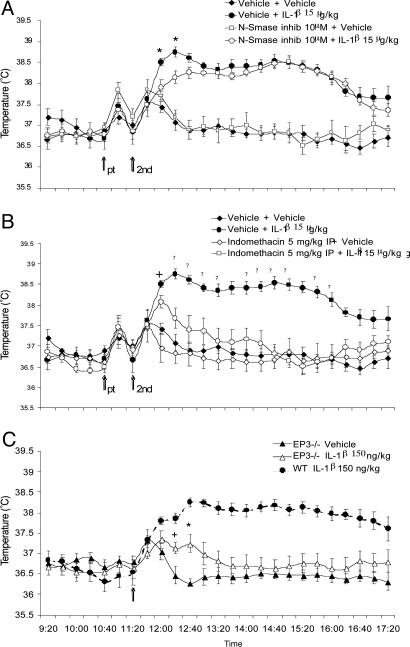
Ceramide is a soluble product of N-Smase that can be rapidly activated by IL-1β acting at IL-1R1 (ref. 12; see also Fig. 4). Injection of C2-ceramide (the cell-penetrating analog of ceramide) at doses ranging from 5 to 80 μg/kg into the AH caused a dose-dependent rapid rise in the temperature of both the preoptic area and CBT (Fig. 1B). The slope of the rise of the temperature in response to C2-ceramide was almost identical to that produced by IL-1β (Fig. 1 A and B). The amplitude of the C2-ceramide-mediated fever never reaches that of IL-1β-induced fever, although supramaximal doses of both C2-ceramide and IL-1β were used, but clearly C2-ceramide-mediated fever accounts for ≈80% of the amplitude of the IL-1β-caused fever. The C2-ceramide-mediated fever response declined after 60 min (Fig. 1B). The intrahypothalamic application of the inhibitor of the N-Smase, spiroepoxide (10 μM), abolished almost completely the rapid phase (Fig. 2A), having no effect on the later and slower phase of the fever response to IL-1β. Administration of the PG synthesis inhibitor indomethacin 5 mg/kg i.p. injection had no effect on the early rapid rise of CBT (Fig. 2B), whereas it almost completely inhibited the slower and longer-lasting phase of the fever response to IL-1β. Similarly, the rapid rise in CBT in response to IL-1β is unaffected by the null mutation of the prostanoid receptor EP3R, mediating much of the pyrogenic effects of IL-1β in the hypothalamus (9), whereas the same null mutation attenuates the later (60-min-postinjection) phase of the fever response to IL-1β (Fig. 2C).
Fig. 4.
Schematic model of the fast and slow phases of IL-1β-induced fever. The fast response (0–30 min) to IL-1β to a large extent is mediated by ceramide. The covalent modification of ion channels by ceramide-activated Src could be responsible for the fast neuronal effect of the cytokine. The slow phase of the IL-1β-induced fever depends on NF-κB-mediated transcription of COX2 and production PGE2 that activates prostanoid receptors on neurons. Indomethacin, a COX1/2 inhibitor, blocks PGE2 synthesis; spiroepoxide, an N-Smase inhibitor, inhibits the production of ceramide; and PP2, a selective inhibitor of Src, inhibits protein tyrosine phosphorylation by Src.
Fig. 2.
Inhibition of the different phases of fever response by intrahypothalamic application of N-Smase inhibitor, spiroepoxide, or i.p.-administered indomethacin. Pretreatment (pt) was followed by second treatment (2nd) (see arrows). (A) N-Smase inhibitor 10 μM pretreatment inhibited partially the effect of IL-1β at 40 and 60 min; F(3, 16) = 13.887; ∗, P < 0.01, vehicle + IL-1β vs. vehicle + vehicle, N-Smase inhibitor 10 μM + IL-1β 15 μg/kg, and N-Smase inhibitor 10 μM + vehicle. After 60 min, the vehicle + IL-1β and N-Smase inhibitor 10 μM + IL-1β 15 μg/kg differ with vehicle + vehicle and N-Smase inhibitor 10 μM + vehicle groups; F(3, 16) = 38.741, P < 0.01. (B) Pretreatment with indomethacin does not have an effect on the early phase of the IL-1β increase on CBT at 40 min (see +, F(3, 16) = 18.541, P < 0.05, vehicle + IL-1β vs. indomethacin + vehicle, vehicle + vehicle) but interferes with the development of the fever response after 60 min (F(3, 16) = 21.342; ▾, P < 0.01). (C) The rapid rise in CBT in response to IL-1β is unaffected by the null mutation of the prostanoid receptor type 3; EP3R (paired t test, +, P < 0.05 at 60 min and ∗, P < 0.01 at 80 min, vehicle vs. IL-1β 150 ng/kg).
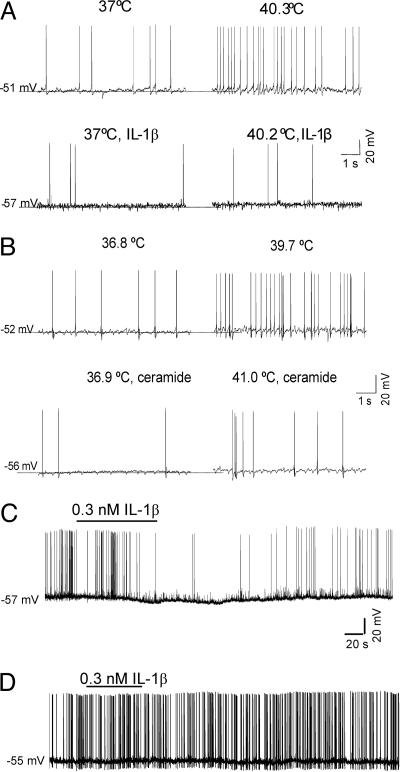
It has been postulated that one major mechanism by which pyrogens act to raise the CBT is through the inhibition of the warm-sensitive neurons in the POA/AH that are involved in the regulation of the control of CBT (13). Indeed, multiple studies show that IL-1β, both in vivo and in vitro, can rapidly inhibit the spontaneous firing of warm-sensitive neurons (14, 15), which, together with cold-sensitive and heat-insensitive POA/AH neurons, are thought to regulate the body temperature set point (13). Because the cell-penetrating C2-ceramide mimicked the effects of IL-1β in vivo, we compared the effects of IL-1β and C2-ceramide on the activity and thermosensitivity of cultured warm-sensitive POA/AH neurons, characterized earlier in detail (16, 17). Bath application of IL-1β or C2-ceramide, in the presence of the PG synthesis inhibitor indomethacin (10 μM), resulted in hyperpolarization and decreased the firing rate of these neurons (effects that developed within 1–2 min) (Fig. 3C). The amplitude of the hyperpolarization averaged 6.3 ± 2.4 mV (n = 7) for IL-1β and 7.7 ± 4.1 mV (n = 5) for C2-ceramide. In the presence of IL-1β or C2-ceramide, the firing rate of the neurons was less sensitive to increases in temperature. Their thermosensitivity decreased significantly: from 0.9 ± 0.1 spikes per s−1°C−1 to 0.2 ± 0.4 spikes per s−1°C−1 (n = 7) and from 0.9 ± 0.2 spikes per s−1°C−1 to 0.3 ± 0.2 spikes per s−1°C−1 (n = 5) for IL-1β and C2-ceramide, respectively (paired t test, P < 0.01). In contrast, dihydroceramide, a membrane-impermeable ceramide analog, did not hyperpolarize the POA/AH neurons and did not change their thermosensitivity (n = 9). Because the actions of ceramide may be mediated by activation of the protein tyrosine kinase Src, as suggested by studies in other cell types (18), we have examined whether the selective Src inhibitor PP2 (10 μM) affected the response to IL-1β. We found that preincubation of POA/AH neurons with this inhibitor for 20 min abolished the effects of the cytokine (Fig. 3D, n = 15).
Fig. 3.
IL-1β and C2-ceramide decrease the firing rate and reduce the thermosensitivity of warm-sensitive POA/AH neurons. (A) Current-clamp recordings showing the response of a warm-sensitive neuron to a temperature increase from 37 to 40.3°C before (Upper) and after application of 0.3 nM IL-1β (Lower). IL-1β hyperpolarized the neuron by ≈6 mV and reduced its thermosensitivity from 0.9 spikes per s−1°C−1 to 0.1 spikes per s−1°C−1. (B) Current-clamp recordings showing the response of a warm-sensitive neuron to a temperature increase from 36.8 to 39.7°C before (Upper) and after (Lower) application of 10 μM C2-ceramide. C2-ceramide hyperpolarized the neuron by ≈4 mV and reduced its thermosensitivity from 1.1 spikes per s−1°C−1 to 0.2 spikes per s−1°C−1. (C) Time course of the IL-1β (0.3 nM) hyperpolarization. (D) Preincubation of the neurons with the selective Src inhibitor PP2 (10 μM) for 20 min prevents the effect of IL-1β.
Discussion
The search for endogenous pyrogen has led to the identification of IL-1 (1), a pluripotent proinflammatory cytokine that, after binding to the IL-1R1, a Toll receptor family member, can induce via an NF-κβ-dependent mechanism the transcription of many genes, including that of IL-6, TNF-α, inducible NO synthase, and COX2, to name a few. These genes code for proteins that are also endogenous pyrogens, like TNF-α or, as in the case of COX2, for an enzyme that catalyzes a key step in the biosynthesis of PGE2, another endogenous pyrogen. Because the pyrogenic action of these mediators requires transcription, translation, posttranslational modifications, subsequent release of the mediators, and binding to their cognate receptors, these mediators cannot account for the rapidly rising phase of the febrile response occurring within minutes, which is seen when IL-1β is applied directly in the POA/AH (Figs. 1–3). The biphasic nature of the fever responses to LPS (11) and IL-1 (10, 19), which present a rapid rise followed by a second peak, has been described. Whereas PGE2 synthesis inhibitors block the long-lasting fever, the nature of the pyrogen responsible for the first phase of the response remained unknown. It should be emphasized that the kinetics and amplitude of febrile response can vary between species and with age and gender (19). Thus, the rabbit is very sensitive to pyrogens and has a very rapid response, which may reflect a somewhat different PG metabolism than those of rat or human. In the latter, the induction of COX2 is an important event that generates larger amounts of PGE2 than those produced by the constitutively expressed COX1.
It should also be noted that not all transcription-mediated events are slow; IL-1β- and LPS-mediated synthesis of some cytokines in lymphocytes and macrophages can be very rapid and can occur within 15 min (20, 21). Nevertheless, in the case of the IL-1R1-MyD88-dependent NF-κβ-mediated induction of COX2 (22) and subsequent production of PGE2 in the brain, there is a lag (23), thus the rapid rising phase of the IL-1β-induced fever could have a mediator other than PGE2. We have shown earlier that in central neurons, IL-1β can activate the N-Smase via the IL-1R1, leading to the formation of the soluble mediator ceramide (12). The activation of N-Smase is transcription-independent, fast, and MyD88-dependent. Ceramide production is thus fast, and its effects on neuronal activity are likely be mediated by the rapid activation of the protein tyrosine kinase Src (24, 25) and the Src-mediated modulation of the phosphorylation state and activity of several ion channels (26–29). Indeed, Fig. 2 shows that the N-Smase inhibitor spiroepoxide slows down the first rapid phase of fever. Unfortunately, this compound is rather toxic, limiting its dosing in vivo. Furthermore, studies on spiroepoxide show that, at higher concentrations than required for N-Smase inhibition, it acts as partial agonist in the activation of Src (C. Davis and T.B., unpublished observations). Nevertheless, at the doses used here, it slowed the first phase of the IL-1β-induced fever. On the other hand, this phase of fever is faithfully mimicked by C2-ceramide, suggesting that ceramide production by the IL-1β/IL-1R1-activated N-Smase is responsible for the first phase of the fever to IL-1β (Figs. 1 and 2).
The electrophysiological data are in line with the in vivo observations on CBT changes, showing that C2-ceramide mimics the rapid neuronal effects of IL-1β (Fig. 3). Furthermore, in this experimental setting, we observed no effects of dihydroceramide (a membrane-impermeable ceramide analog) on neuronal responses, suggesting that the lipid mediator ceramide acts intracellularly. The proposed action of ceramide in nonneuronal cells is the activation of several proteins, such as KSR and tyrosine kinase Src (24, 25). We have shown that the selective Src inhibitor PP2 inhibits the response to IL-1β, strongly implying a role of Src activation. It has been shown in many other systems, where Src is activated by other stimuli, that Src modulates the phosphorylation state and activity of various ion channels (26–28). The toxicity of PP2 prevents the in vivo demonstration of Src involvement in the rapid rise of IL-1β/ceramide-mediated fever.
The successful therapeutic intervention to control fever in a multitude of settings is based on the inhibition of the slower and longer-lasting PGE2-dependent branch of IL-1β signaling (Fig. 4). The rapid neuronal ceramide-mediated actions of IL-1β, however, should not be neglected, because these might explain the fast response to IL-1β in several neuronal contexts such as long-term potentiation (LTP) and febrile seizures (30, 31) and during the first phase of fever response, as shown here. Because these rapid neuronal effects of IL-1β are independent of PGE2 production and are mediated to a large extent by ceramide, this sphingolipid could be considered as an endogenous pyrogen.
Materials and Methods
Anterior Hypothalamic Microinjection.
Murine recombinant IL-1β was from R&D Systems (Minneapolis). C2-ceramide, dihydroceramide, indomethacin, and the N-Smase inhibitor spiroepoxide (all from Sigma-Aldrich) were dissolved in anhydrous DMSO to obtain ceramide/DMSO, indomethacin/DMSO, and N-Smase inhibitor/DMSO stock. Then, to maintain experimental conditions, these substances were dissolved with artificial cerebrospinal fluid (aCSF) to reach a final concentration of 10% vol/vol DMSO (vehicle). Completion of dissolution of the ceramide required 20 min of stirring at room temperature. To maintain experimental conditions, the other lyophilized substances were reconstituted in pyrogen-free aCSF (145 mM Na+/2.7 mM K+/1.0 mM Mg2+/1.2 mM Ca2+/1.5 mM Cl−, pH 7.2) containing 0.1% vol/vol BSA, aliquoted, and stored at −20°C. Immediately before use, substances were brought to appropriate concentrations by adding aCSF and reducing the final concentration of DMSO at 10%. Each aliquot was used only once. C57BL/6J male mice, 20–25 g (12–14 weeks old), were anesthetized with halothane (induction 3–5%, maintenance 0.9–1.5%) and implanted with transmitter devices (TA10TA-F20; Data Sciences, Arden Hills, MN) into the peritoneal cavity for CBT measurement. Mice were allowed to recover for 1 week and then submitted to a second surgery. After anesthesia (induction 3–5%, maintenance 0.9–1.5%), mice were placed into a stereotaxic apparatus. The skull was exposed, and holes were drilled to accommodate stereotaxic placement of a guide cannula aiming at the AH (anterior–posterior from bregma, 0.3 mm; lateral, 0.3 mm; and ventral, 4.1 mm). Additional groups of mice were implanted with a thermisor (Physitemp, Clifton, NJ) into the POA anterior–posterior from bregma, 0.38 mm; lateral, 0.4 mm; and ventral, 4.4 mm. Mice were individually housed in a Plexiglas cage in a room maintained at 30 ± 0.25°C on a 12:12 h light–dark cycle (lights on at 7:00 a.m.) with ad libitum access to food and water. The cages were positioned onto the receiver plates (RPC-1; Data Sciences), and mice with thermisor were connected via individual sockets to an electric swivel commutator (Dragonfly; R&D Systems) and acquisition software (thermes-16; Physitemp Instruments). CBT and motor activity (MA) were recorded for at least 72 h before anterior hypothalamic treatment to ascertain that baseline levels of temperature are stable and that no ongoing febrile response confounds the results. During this period, the animals were habituated to the experimental conditions and handled for 10 min every day (10:30–11:30 a.m.). POA temperature was recorded only during the day of treatment. On the day of injection, an internal cannula (33 Ga, 1.7-cm length) connected to plastic tubing and a microsyringe (1 μl) was used to deliver the drugs into the AH. A volume of 0.5 μl was injected in a period of 5 min to allow a diffusion of the drug. For combined treatments, we applied a pretreatment (10:40 a.m.) followed by a 20-min interval and then by a second treatment (11:20 a.m.). After this procedure, mice were returned to the home cage to continue the telemetry recording.
Histology.
Mice received an intracardiac perfusion with 4% paraformaldehyde. The brain was then removed, incubated for 24 h in 4% paraformaldehyde for an additional 24 h in 30% sucrose at 4°C, and frozen. Tissue sections of 40 or 10 μm were cut after fixation on a freezing-stage microtome. Location of injection and thermistor electrodes were determined (Fig. 1D).
Data Analysis.
Data were grouped and analyzed in a 20-min interval. Significance was evaluated by using a paired t test or ANOVA with repeated measures followed by post hoc Sheffe tests comparing 20-min intervals.
Neuronal Cultures.
Mixed POA/AH cultures (containing neurons and glia) were established from embryonic day 14 Swiss–Webster mice. After mechanical dissociation, cells were plated onto poly-d-lysine-coated coverslips at a density of one to two POAs per anterior hypothalami per milliliter and allowed to develop in vitro. Cultures were kept in Minimal Essential Media with Earle’s salts (Invitrogen) supplemented with 5% (vol/vol) FBS/5% (vol/vol) horse serum/glucose (20 mM)/glutamine (2 mM). Five days after plating, nonneuronal development was halted by addition of 10 μM cytosine arabinoside. Thereafter, cultures were fed every 4 days and used for electrophysiology experiments between 24 and 45 days in vitro.
Patch-Clamp Recording.
Standard tight-seal recordings in current-clamp mode (I-clamp fast) were performed with an Axopatch 200B amplifier (Molecular Devices). The external recording solution was 155 mM NaCl /3.5 mM KCl/2 mM CaCl2/1.5 MgSO4 mM/10 mM glucose/10 mM Hepes, pH 7.4, 300–305 mosM. Indomethacin (10 μM) was added to all of the extracellular solutions to block COX1 and COX2 and prevent PGE2 production. The pipette solution was 130 mM K-gluconate/10 mM KCl/10 mM Hepes/2 mM MgCl2/0.5 mM EGTA/2 mM ATP/1 mM GTP, pH 7.4. Glass micropipettes were pulled with a horizontal puller (P-87; Sutter Instruments, Novato, CA) using borosilicate glass. The electrode resistance after back-filling was 2–6 MΩ. All voltage measurements were corrected for the liquid junction potential (approximately −12 mV). The recording chamber was perfused at 3 ml/min, and its content was fully changed within 1 min. The temperature of the external solution was controlled with an HCC-100A heating/cooling bath temperature controller (Dagan Instruments, Minneapolis). The usual temperature during the recordings was 36–37°C. To prevent changes induced in the electrode reference potential, the ground electrode was thermally isolated in a separate bath connected to the recording bath by a filter-paper bridge. The thermal coefficient was determined as described (17). Data are presented as means ± SD.
Acknowledgments
We thank Bruno Conti, Chris Davis, and Henri Korn for helpful comments on and input into these experiments. This work was supported from funds from National Institutes of Health Grant NIHR01NS04350103.
Abbreviations
- PG
prostaglandin
- PGE2
PG E2
- COX
cyclooxygenase
- CBT
core body temperature
- POA
preoptic area
- AH
anterior hypothalamus
- N-Smase
neutral sphingomyelinase
- EP3R
EP3 prostanoid receptor
- IL-1R1
type 1 IL-1 receptor.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Dinarello C. A. J. Endotox. Res. 2004;10:201–222. doi: 10.1179/096805104225006129. [DOI] [PubMed] [Google Scholar]
- 2.Sheagren J. N., Wolff S. M. Nature. 1966;210:539–540. doi: 10.1038/210539a0. [DOI] [PubMed] [Google Scholar]
- 3.Root R. K., Wolff S. M. J. Exp. Med. 1968;128:309–323. doi: 10.1084/jem.128.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dinarello C. A. Rev. Infect. Dis. 1984;6:51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- 5.Vane J. R., Botting R. M. Thromb. Res. 2003;110:255–258. doi: 10.1016/s0049-3848(03)00379-7. [DOI] [PubMed] [Google Scholar]
- 6.Oka T. Front. Biosci. 2004;1:3046–3057. doi: 10.2741/1458. [DOI] [PubMed] [Google Scholar]
- 7.Oka T., Oka K., Kobayashi T., Sugimoto Y., Ichikawa A., Ushikubi F., Narumiya S., Saper C. B. J. Physiol. 2003;551:945–954. doi: 10.1113/jphysiol.2003.048140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oka T., Oka K., Saper C. B. Brain Res. 2003;968:256–262. doi: 10.1016/s0006-8993(03)02268-6. [DOI] [PubMed] [Google Scholar]
- 9.Ushikubi F., Segi E., Sugimoto Y., Murata T., Matsuoka T., Kobayashi T., Hizaki H., Tuboi K., Katsuyama M., Ichikawa A., et al. Nature. 1998;395:281–284. doi: 10.1038/26233. [DOI] [PubMed] [Google Scholar]
- 10.Lesnikov V. A., Efremov O. M., Korneva E. A., Van Damme J., Billiau A. Cytokine. 1991;3:195–198. doi: 10.1016/1043-4666(91)90016-7. [DOI] [PubMed] [Google Scholar]
- 11.Skarnes R. C., Brown S. K., Hull S. S., McCracken J. A. J. Exp. Med. 1981;154:1212–1224. doi: 10.1084/jem.154.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nalivaeva N. N., Rybakina E. G., Pivanovich I., Kozinets I. A., Shanin S. N., Bartfai T. Cytokine. 2000;12:229–232. doi: 10.1006/cyto.1999.0547. [DOI] [PubMed] [Google Scholar]
- 13.Heller H. C., Crawshaw L. I., Hammel H. T. Sci. Am. 1978;239:112–113. doi: 10.1038/scientificamerican0878-102. 102- 10. [DOI] [PubMed] [Google Scholar]
- 14.Nakashima T., Hori T., Mori T., Kuriyama K., Mizuno K. Brain Res. Bull. 1989;23:209–213. doi: 10.1016/0361-9230(89)90149-4. [DOI] [PubMed] [Google Scholar]
- 15.Vasilenko V. Y., Petruchuk T. A., Gourine V. N., Pierau F. K. Neurosci. Lett. 2000;292:207–210. doi: 10.1016/s0304-3940(00)01470-1. [DOI] [PubMed] [Google Scholar]
- 16.Tabarean I. V., Behrens M. M., Bartfai T., Korn H. Proc. Natl. Acad. Sci. USA. 2004;101:2590–2595. doi: 10.1073/pnas.0308718101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tabarean I. V., Conti B., Behrens M., Korn H., T. B. Neuroscience. 2005;135:433–449. doi: 10.1016/j.neuroscience.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 18.Gulbins E., Szabo I., Baltzer K., Lang F. Proc. Natl. Acad. Sci. USA. 1997;94:7661–7666. doi: 10.1073/pnas.94.14.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morimoto A., Murakami N., Sakata Y., Watanabe T., Yamaguchi K. J. Physiol. 1990;427:227–239. doi: 10.1113/jphysiol.1990.sp018169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawai T., Takeuchi O., Fujita T., Inoue J., Muhlradt P. F., Sato S., Hoshino K., Akira S. J. Immunol. 2001;167:5887–5894. doi: 10.4049/jimmunol.167.10.5887. [DOI] [PubMed] [Google Scholar]
- 21.Zughaier S. M., Zimmer S. M., Datta A., Carlson R. W., Stephens D. S. Infect. Immun. 2005;73:2940–2950. doi: 10.1128/IAI.73.5.2940-2950.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akira S., Takeda K., Kaisho T. Nat. Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 23.Coceani F., Bishai I., Dinarello C. A., Fitzpatrick F. A. Am. J. Physiol. 1983;244:R785–R793. doi: 10.1152/ajpregu.1983.244.6.R785. [DOI] [PubMed] [Google Scholar]
- 24.Kolesnick R., Golde D. W. Cell. 1994;77:325–328. doi: 10.1016/0092-8674(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 25.Saklatvala J. Br. Med. Bull. 1995;51:402–418. doi: 10.1093/oxfordjournals.bmb.a072969. [DOI] [PubMed] [Google Scholar]
- 26.Kalia L. V., Gingrich J. R., Salter M. W. Oncogene. 2004;23:8007–8016. doi: 10.1038/sj.onc.1208158. [DOI] [PubMed] [Google Scholar]
- 27.Salter M. W., Kalia L. V. Nat. Rev. Neurosci. 2004;5:317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- 28.Viviani B., Bartesaghi S., Gardoni F., Vezzani A., Behrens M. M., Bartfai T., Binaglia M., Corsini E., Di Luca M., Galli C. L., et al. J. Neurosci. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiong Z. G., Pelkey K. A., Lu W. Y., Lu Y. M., Roder J. C., MacDonald J. F., Salter M. W. J. Neurosci. 1999;19:RC37. doi: 10.1523/JNEUROSCI.19-21-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dube C., Vezzani A., Behrens M., Bartfai T., Baram T. Z. Ann. Neurol. 2005;57:152–155. doi: 10.1002/ana.20358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pang L., Holland E., Knox A. J. Br. J. Pharmacol. 1998;125:1320–1328. doi: 10.1038/sj.bjp.0702193. [DOI] [PMC free article] [PubMed] [Google Scholar]