Abstract
The phenotype of smooth muscle cells (SMCs) plays an important role in vascular function in health and disease. We investigated the mechanism of modulation of SMC phenotype (from contractile to synthetic) induced by the synergistic action of a growth factor (platelet-derived growth factor, PDGF-BB) and a cytokine (interleukin, IL-1β). Human aortic SMCs grown on polymerized collagen showed high expression levels of contractile markers (smooth muscle α-actin, myosin heavy chain, and calponin). These levels were not significantly affected by PDGF-BB and IL-1β individually, but decreased markedly after the combined usage of PDGF-BB and IL-1β. PDGF/IL-1β costimulation also induced a sustained phosphorylation of Akt and p70 ribosomal S6 kinase (p70S6K). The effects of PDGF/IL-1β costimulation on contractile marker expression and Akt and p70S6K phosphorylation were blocked by the phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 and by adenovirus expressing a dominant-negative Akt, and they were mimicked by constitutively active Akt. PDGF-BB/IL-1β induced a sustained phosphorylation of PDGF receptor (PDGFR)-β and its association with IL-1 receptor (IL-1R1). Such activation and association of receptors were blocked by a PDGFR-β neutralizing antibody (AF385), an IL-1R1 antagonist (IL-1ra), as well as a specific inhibitor of PDGFR-β phosphorylation (AG1295); these agents also eliminated the PDGF-BB/IL-1β-induced signaling and phenotypic modulation. PDGF-BB/IL-1β inhibited the polymerized collagen-induced serum response factor DNA binding activity in the nucleus, and this effect was mediated by the PDGFR-β/IL-1R1 association and phosphatidylinositol 3-kinase/Akt/p70S6K pathway. Our findings provide insights into the mechanism of SMC phenotypic modulation from contractile to synthetic, e.g., in atherosclerosis.
Keywords: signal transduction, smooth muscle phenotype, Akt, mTOR
Phenotypic modulation of smooth muscle cells (SMCs) is critical in the regulation of vascular function in health and disease (1). During the development of atherosclerotic plaques, vascular SMCs change from their physiological contractile phenotype to the pathophysiological synthetic phenotype and migrate into the intima, where they proliferate and produce extracellular matrix (ECM) (1). Growth factors, inflammatory cytokines, and ECM proteins have been implicated as factors that mediate such a phenotypic change of SMCs (1). These agonists are capable of stimulating SMCs by binding surface receptors and activating intracellular signaling pathways that ultimately regulate gene expression and cellular function. Understanding the interactions between these agonists may provide insights into the mechanism of phenotypic modulation of SMCs and the pathogenesis and progression of atherosclerosis.
When normal medial SMCs are placed in culture, they lose their contractility and develop features similar to those of synthetic SMCs in blood vessels harboring atherosclerotic lesions (2, 3). These alterations of SMCs in culture are accompanied by changes in their phenotypic markers, including a loss of expression of contractile markers such as smooth muscle α-actin (SMα-actin), myosin heavy chain (SM-MHC), and calponin (3). There is recent evidence that SMCs cultured on polymerized collagen retain their contractile phenotype and mimic many of the characteristics of medial SMCs in vivo (3); this provides a useful model for studying the mechanisms that control the modulation of SMCs from contractile to synthetic phenotype. Among the many growth factors and cytokines that can contribute to such a phenotypic modulation of SMCs, platelet-derived growth factor (PDGF)-BB and IL-1β possess the most potent mitogenic and inflammatory effects. PDGF-BB binds to the PDGF receptor (PDGFR)-β and subsequently activates several intracellular signaling cascades, including mitogen-activated protein kinases (MAPKs) and phosphatidylinositol 3-kinase/Akt (PI3K/Akt), which in turn activates the downstream targets mTOR and p70 ribosomal S6 kinase (p70S6K) (4). Culturing SMCs on polymerized collagen has been found to inhibit their responsiveness to PDGF-BB (3). In addition to being inflammatory, IL-1β can also be mitogenic (5). When used in combination with PDGF, IL-1β has been reported to have inhibitory as well as activating effects on SMC proliferation (6, 7).
Because SMCs are exposed to both growth factors and cytokines during lesion development, we postulated that these two types of agonists may interplay and exert synergistic effects on phenotypic modulation of SMCs. In this study, we found that PDGF-BB and IL-1β were cooperative in inducing phenotypic modulation of human aortic SMCs cultured on polymerized collagen from a contractile toward a synthetic phenotype. This synergistic effect of PDGF-BB and IL-1β on SMC phenotypic modulation involves a crosstalk between their corresponding receptors PDGFR-β and IL-1 receptor (IL-1R1) and is mediated through the PI3K/Akt/p70S6K signaling pathway. This study presents evidence for a mechanism of signal regulation in which growth factors and cytokines act synergistically through the interaction of their receptors to induce phenotypic modulation of SMCs.
Results
PDGF-BB and IL-1β Synergistically Induce Contractile-to-Synthetic Phenotype Modulation of SMCs Cultured on Polymerized Collagen.
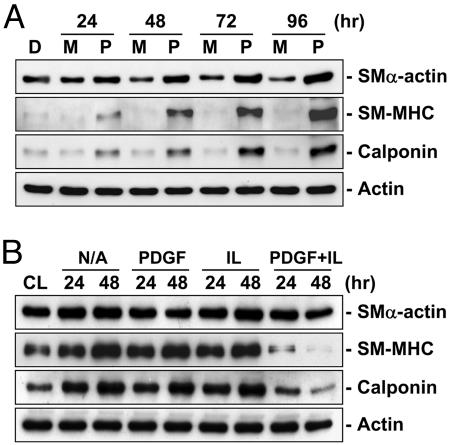
The levels of expression of contractile marker proteins SMα-actin, SM-MHC, and calponin were higher in SMCs grown on polymerized collagen than those grown on monomeric collagen, over the 96-h period tested (Fig. 1A). Concomitantly, the level of expression of the synthetic marker vimentin was lower in SMCs on polymerized collagen than those on monomeric collagen (Fig. 9A, which is published as supporting information on the PNAS web site). Treatments of SMCs on polymerized collagen with PDGF-BB (25 ng/ml) or IL-1β (10 ng/ml) individually for 24 or 48 h did not alter the expressions of the contractile marker proteins or the synthetic marker. However, the combined usage of these two agents (PDGF-BB/IL-1β costimulation) markedly inhibited the expressions of the contractile marker proteins (Fig. 1B) and markedly increased the expression of the synthetic marker vimentin (Fig. 9B). This contractile-to-synthetic phenotype modulation of SMCs by PDGF-BB/IL-1β was substantiated by the BrdUrd incorporation and flow cytometric assays, which showed that PDGF-BB/IL-1β costimulation resulted in an increase in the percentage of SMCs (on polymerized collagen) in the synthetic phase as compared with the unstimulated control (Supporting Text and Fig. 10, which are published as supporting information on the PNAS web site). In additional experiments, after 24 h of PDGF-BB/IL-1β costimulation, we replaced the medium with one that did not contain these agonists; another 24 h later, their contractile and synthetic marker protein expressions rose and fell, respectively, to become similar to those in the untreated controls (Fig. 11, which is published as supporting information on the PNAS web site), indicating that the phenotypic modulation by PDGF-BB/IL-1β is reversible.
Fig. 1.
PDGF-BB and IL-1β synergistically induce SMCs on polymerized collagen to change from a contractile toward a synthetic phenotype. (A) SMCs were kept in Petri dishes (D) or cultured on monomeric (M) or polymerized collagen (P) for the times indicated. (B) After culturing on polymerized collagen for 48 h (CL), the cells were kept without any treatment (N/A) or treated with PDGF-BB (PDGF; 25 ng/ml) or IL-1β (IL; 10 ng/ml) individually or in combination (PDGF+IL) for 24 or 48 h, and their protein expressions of SMα-actin, SM-MHC, and calponin were determined by using Western blot analysis as described in Materials and Methods. Results are representative of triplicate experiments with similar results.
PDGF-BB/IL-1β-Induced Phenotypic Modulation of SMCs on Polymerized Collagen Is Mediated by the PI3K/Akt/p70S6K Pathway.
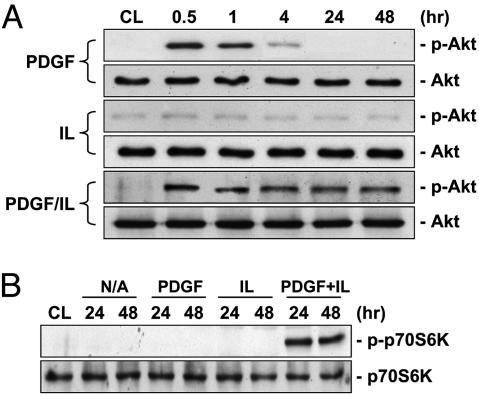
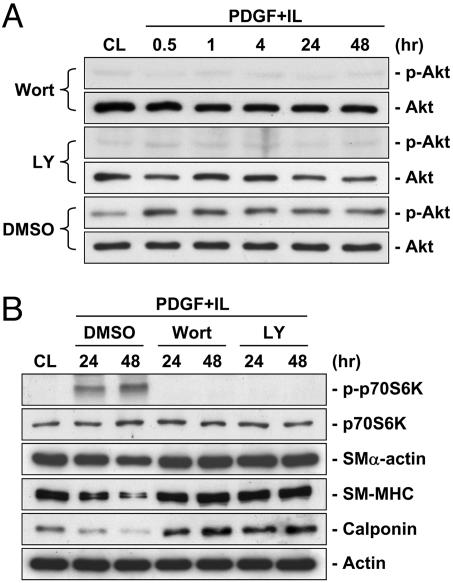
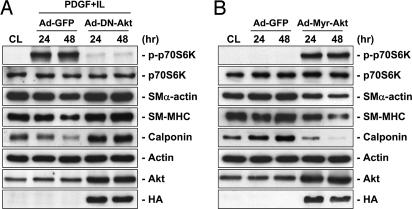
The PI3K/Akt/p70S6K pathway has been shown to regulate a number of cellular processes, including cell cycle progression and proliferation (8). IL-1β alone had no effect on Akt phosphorylation; PDGF-BB alone induced a transient increase in Akt phosphorylation, reaching a maximal level within 30 min and then declining to nearly the basal level within 4 h (Fig. 2A). However, PDGF-BB/IL-1β costimulation induced a sustained increase in Akt phosphorylation throughout the 48-h period tested. Phosphorylation of p70S6K was not affected by individual treatments with PDGF-BB or IL-1β, but was markedly enhanced by PDGF-BB/IL-1β costimulation (Fig. 2B). Wortmannin (200 nM) and LY294002 (30 μM), two specific inhibitors of PI3K, eliminated the PDGF-BB/IL-1β-induced phosphorylation of Akt (Fig. 3A) and p70S6K (Fig. 3B) and down-regulation of contractile marker proteins in SMCs on polymerized collagen (Fig. 3B). In contrast, rapamycin (50 nM), a specific inhibitor of mTOR, inhibited the PDGF-BB/IL-1β-induced phosphorylation of p70S6K, but not Akt, and elevated the contractile marker protein expressions (Fig. 12, which is published as supporting information on the PNAS web site). SMCs on polymerized collagen infected with adenovirus expressing the dominant-negative Akt before PDGF-BB/IL-1β costimulation abrogated the PDGF-BB/IL-1β-induced increases in p70S6K phosphorylation and decreases in contractile marker protein expressions (Fig. 4A). Furthermore, the SMCs expressing constitutively active myristoylated Akt mimicked the synergistic effects of PDGF-BB/IL-1β (in the absence of this costimulation) in increasing p70S6K phosphorylation and decreasing contractile marker protein expressions in comparison to the control infected cells (Fig. 4B). These results indicate that Akt is necessary and sufficient for these synergistic effects of PDGF-BB/IL-1β.
Fig. 2.
PDGF-BB/IL-1β induce sustained phosphorylations of Akt and p70S6K in SMCs on polymerized collagen. (A) SMCs were cultured on polymerized collagen for 48 h (CL) and then stimulated with PDGF-BB (PDGF) or IL-1β (IL) individually or in combination (PDGF + IL) for the times indicated. The cells were lysed, and their Akt phosphorylation was determined by using Western blot analysis as described in Materials and Methods. (B) After culturing on polymerized collagen for 48 h (CL), the cells were kept without any treatment (N/A) or treated with PDGF-BB or IL-1β individually or in combination for 24 or 48 h, and their p70S6K phosphorylation was determined. Representative results are shown from four independent experiments with similar results.
Fig. 3.
Wortmannin and LY294002 inhibit the PDGF-BB/IL-1β-induced phosphorylation of Akt and p70S6K and reduction of contractile marker protein expression in SMCs on polymerized collagen. (A and B) SMCs were cultured on polymerized collagen for 48 h (CL) and then costimulated with PDGF-BB and IL-1β (PDGF+IL) for the times indicated (A) or for 24 and 48 h (B). Before costimulation, the cells were pretreated with wortmannin (200 nM; Wort), LY294002 (30 μM; LY) or DMSO (as control) for 1 h. The cells were lysed and their Akt (A) and p70S6K phosphorylations and SMα-actin, SM-MHC, and calponin protein (B) expressions were determined by using Western blot analysis with the respective antibodies. Results are representative of triplicate experiments with similar results.
Fig. 4.
Akt mediates p70S6K phosphorylation and marker protein expression in SMCs on polymerized collagen. SMCs were cultured on polymerized collagen for 48 h (CL), and then costimulated with PDGF-BB and IL-1β (PDGF+IL) (A) or kept without being stimulated by these agonists (B) for 24 or 48 h. Before culturing on polymerized collagen, the cells were infected with adenoviruses encoding control green fluorescence protein (Ad-GFP), or hemagglutinin (HA)-tagged dominant-negative (Ad-DN-Akt) or dominant-positive Akt (Ad-Myr-Akt), as described in Materials and Methods. The cells were lysed, and their p70S6K phosphorylation and SMα-actin, SM-MHC, and calponin protein expressions were determined by using Western blot analysis. Infection was confirmed by the increases in Akt and HA expression in the Ad-DN-Akt- and Ad-Myr-Akt-infected cells. Results are representative of triplicate experiments with similar results.
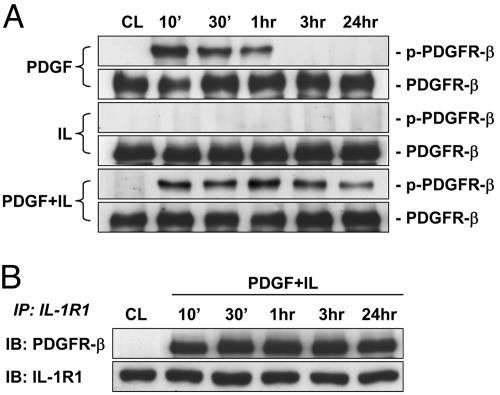
PDGF-BB/IL-1β Induce a Sustained Phosphorylation of PDGFR-β and Its Association with IL-1R1 in SMCs on Polymerized Collagen.
Our results on sustained activation of Akt by PDGF-BB/IL-1β, but not either alone, suggest that PDGF-BB/IL-1β may exert synergistic effects on tyrosine kinase phosphorylation of PDGFR-β, which has been shown to form complexes with PI3K to activate the PI3K/Akt signaling pathway (9). We tested whether costimulation of SMCs on polymerized collagen with PDGF-BB and IL-1β induces phosphorylation of PDGFR-β and its association with IL-1R1. PDGF-BB alone induced a transient phosphorylation of PDGFR-β in SMCs on polymerized collagen; IL-1β alone did not have any effect (Fig. 5A). Costimulation with PDGF-BB and IL-1β induced a sustained phosphorylation of PDGFR-β over the 24-h period tested. This sustained phosphorylation of PDGFR-β was eliminated by pretreating the cells with an IL-1R1 antagonist IL-1ra (Fig. 13, which is published as supporting information on the PNAS web site), suggesting that the effect of IL-1β was mediated by binding to IL-1R1. To obtain evidence for the association of PDGFR-β and IL-1R1, we immunoprecipitated IL-1R1 from membrane preparations of the PDGF-BB/IL-1β-treated cells and probed for the presence of PDGFR-β in the immunoprecipitates with an anti-PDGFR-β antibody. Costimulation with PDGF-BB/IL-1β induced a rapid association between PDGFR-β and IL-1R1 within 10 min, and this association remained for at least 24 h (Fig. 5B). In contrast, we did not observed association of these receptors in unstimulated cells or in cells stimulated with PDGF-BB or IL-1β alone (Fig. 14, which is published as supporting information on the PNAS web site). The association between the receptors after costimulation with PDGF-BB/IL-1β was further confirmed by performing immunoprecipitation of the membrane preparations of the PDGF-BB/IL-1β-treated cells with an anti-PDGFR-β antibody followed by detection of IL-1R1 in the immunoprecipitates by using an anti-IL-1R1 antibody (data not shown).
Fig. 5.
PDGF-BB/IL-1β induce a sustained phosphorylation of PDGFR-β and its association with IL-1R1 in SMCs on polymerized collagen. SMCs were cultured on polymerized collagen for 48 h (CL), and then stimulated with PDGF-BB (PDGF) or IL-1β (IL) individually or in combination (PDGF+IL) for the times indicated. The cells were lysed and the phosphorylation of PDGFR-β (A) and its association with IL-1R1 on the cell membrane (B) were determined by using Western blot analysis and immunoprecipitation assay, as described in Materials and Methods. Results are representative of triplicate experiments with similar results.
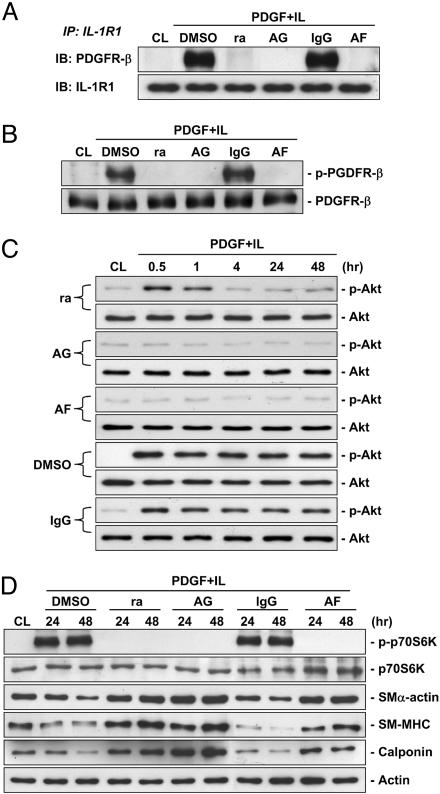
Sustained Phosphorylation of PDGFR-β and Its Association with IL-1R1 Are Required for the PDGF-BB/IL-1β-Induced Activation of Akt and p70S6K and Phenotypic Modulation of SMCs on Polymerized Collagen.
To test whether sustained phosphorylation of PDGFR-β and its association with IL-1R1 are essential elements in the PDGF-BB/IL-1β-induced signaling and phenotypic modulation, we performed the following experiments on SMCs on polymerized collagen. Pretreatment with a neutralizing antibody against PDGFR-β (AF385, 20 μg/ml), an antagonist of IL-1R1 (IL-1ra, 10 μg/ml), or a specific inhibitor of PDGFR-β phosphorylation (AG1295, 10 μM) for 1 h before the addition of PDGF-BB/IL-1β abolished the PDGF-BB/IL-1β-induced PDGFR-β/IL-1R1 association (Fig. 6A) and PDGFR-β phosphorylation (Fig. 6B), which were observed in the control cells pretreated with DMSO or isotype-matched IgG. Plotting of the time course of Akt phosphorylation in these costimulated cells (Fig. 6C) shows that AF385 and AG1295 caused complete inhibition at all time points over the 48-h period studied, but the cells pretreated with IL-1ra showed a transient increase in Akt phosphorylation. Thus, the effects of blocking IL-1β with PDGF-BB/IL-1β costimulation yielded the same result as the use of PDGF-BB alone (Fig. 2A). Pretreatments with AF385, IL-1ra, or AG1295 abolished the PDGF-BB/IL-1β-induced p70S6K phosphorylation at 24 and 48 h (Fig. 6D), which was accompanied by increases in the expressions of contractile marker proteins; these changes are to be contrasted with the results on control cells pretreated with DMSO or isotype-matched IgG (Fig. 6D).
Fig. 6.
Sustained PDGFR-β/IL-1R1 association and PDGFR-β phosphorylation are required for PDGF-BB/IL-1β-induced activation of Akt and p70S6K and phenotypic modulation of SMCs on polymerized collagen. SMCs were cultured on polymerized collagen for 48 h (CL), and then costimulated with PDGF-BB/IL-1β (PDGF+IL) for 24 h (A and B) or for the times indicated (C and D). Before costimulation, the cells were pretreated with IL-1ra (ra; 10 μg/ml), AG1295 (AG; 10 μM), AF385 (AF, 20 μg/ml), or DMSO or IgG (as controls) for 1 h (A, B, and D). The cells were lysed; the association of PDGFR-β with IL-1R1 on the cell membrane (A), phosphorylations of PDGFR-β (B), Akt (C), and p70S6K (D), and protein expressions of SMα-actin, SM-MHC, and calponin (D) were determined by using immunoprecipitation assay and Western blot analysis with the respective antibodies, as described in Materials and Methods. Results are representative of triplicate experiments with similar results.
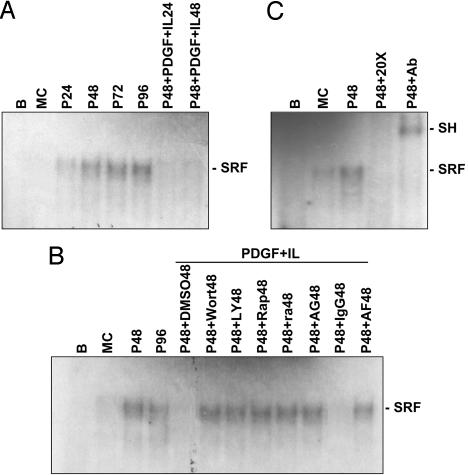
PDGF-BB/IL-1β Inhibited Polymerized Collagen-Induced Serum Response Factor (SRF)-DNA-Binding Activity in SMCs Through PDGFR-β/IL-1R1 Association and the PI3K/Akt/p70S6K Pathway.
The binding of SRF to CArG (CC[A/T]6GG) box in the SMC promoters plays an indispensable role in the expression of almost all SMC-contractile marker genes characterized so far (1). We thus investigated whether PDGF-BB/IL-1β can modulate SRF-DNA binding activity in the nucleus of SMCs cultured on polymerized collagen. We performed EMSA by using the nuclear protein extracts from the conditioned SMCs and the oligonucleotide-containing sequence between −97 and −76 of the SM-MHC promoter as a probe. This sequence of the SM-MHC promoter contains one CArG box (5′-CCATATTATGG-3′) located at −91. SMCs grown on polymerized collagen for 24, 48, 72, and 96 h showed a sustained increase in SRF-DNA binding activity in the nucleus in comparison to the cells on monomeric collagen (Fig. 7A). PDGF-BB/IL-1β costimulation inhibited this polymerized collagen-induced binding activity; this inhibitory effect was eliminated by pretreating the cells with AF385, IL-1ra, AG1295, wortmannin, LY294002, or rapamycin (Fig. 7B), suggesting that the PDGFR-β/IL-1R1 association and PDGFR-β phosphorylation modulate the transcriptional activation of the contractile marker proteins via the PI3K/Akt/p70S6K pathway. The specificity of this binding for SRF was substantiated by its abolition by coincubation of nuclear proteins with 20-fold unlabeled oligonucleotides and by the supershifting in gel mobility of the SRF–oligonucleotide complex after preincubating nuclear proteins with an antibody to SRF (Fig. 7C).
Fig. 7.
PDGF-BB/IL-1β inhibited polymerized collagen-induced SRF-DNA binding activity in SMCs through PDGFR-β/IL-1R1 association and the PI3K/Akt/p70S6K pathway. SMCs were cultured on polymerized collagen for 24, 48, 72, and 96 h (P24, P48, P72, and P96, respectively). SMCs cultured on monomeric collagen for 48 h were used as control (MC). SMCs were cultured on polymerized collagen for 48 h, and then costimulated with PDGF-BB and IL-1β for 24 h (P48+PDGF+IL24) or 48 h (P48+PDGF+IL48) (A). In parallel experiments, SMCs were pretreated with wortmannin (P48+Wort48), LY294002 (P48+LY48), rapamycin (P48+Rap48), IL-1ra (P48+ra48), AG1295 (P48+AG48), AF385 (P48+AF48), DMSO (P48+DMSO48), or IgG (P48+IgG48) for 1 h before costimulation with PDGF-BB/IL-1β for 48 h in the presence of these pretreatment reagents (B). Total nuclear extracts were prepared and analyzed by EMSA using a 32P-labeled oligonucleotide probe containing the CArG (CC[A/T]6GG) box, which serves as a binding site for SRF. The specificity of the retarded complexes (SRF) was assessed by preincubating the nuclear extracts either with excess unlabeled oligonucleotides containing SRF binding sequences as a competitor (P48 + 20×) or with SRF antibodies (1 μg) (P48 + Ab) (C). EMSA analyzed from nuclear extracts preincubated with the SRF antibody shows a super shift band (SH). 32P-labeled oligonucleotide probe incubated with reaction buffer only was used as a negative control (B). Results are representative of triplicate experiments with similar results.
Discussion
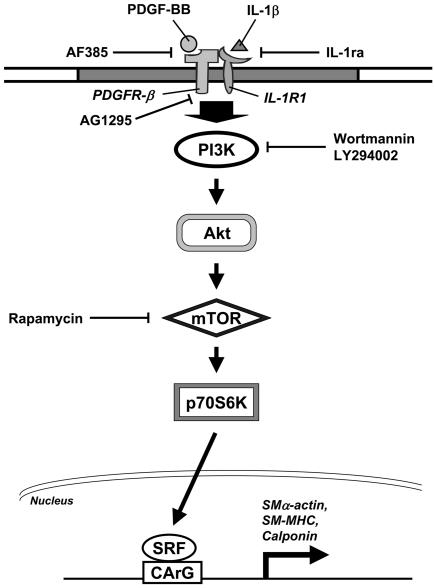
The present study has characterized a novel mechanism (summarized in Fig. 8) by which PDGF-BB and IL-1β play a synergistic role in phenotypic modulation (from a contractile toward a synthetic state) of human aortic SMCs grown on polymerized collagen, characterized by decreases in the expression of contractile phenotypic markers SMα-actin, SM-MHC, and calponin, and an increase in the expression of synthetic phenotypic marker vimentin, as well as cell proliferation. Several lines of evidence indicate that this synergistic effect of PDGF-BB/IL-1β on SMC phenotypic modulation was mediated by the sustained phosphorylation of PDGFR-β and its association with IL-1R1 and through the PI3K/Akt/p70S6K pathway. First, PDGF-BB/IL-1β costimulation, but not their separate stimulations, inhibited the polymerized collagen-induced SMα-actin, SM-MHC, and calponin protein expressions, but increased vimentin expression. Second, PDGF-BB/IL-1β costimulation of SMCs on polymerized collagen induced sustained phosphorylation of Akt and p70S6K. This sustained increase in phosphorylation was abolished by the PI3K inhibitors wortmannin and LY294002, which consequently increased the contractile marker protein expression. Moreover, the mTOR inhibitor rapamycin also eliminated the effect of PDGF-BB/IL-1β on p70S6K and marker protein expression. Third, adenovirus with dominant-negative Akt abolished the PDGF-BB/IL-1β-induced increases in p70S6K phosphorylation and decreases in contractile marker protein expressions. Complementary results were obtained showing that constitutively active Akt mimicked the effect of PDGF-BB/IL-1β on p70S6K and contractile marker protein expressions. Fourth, PDGF-BB/IL-1β induced a sustained phosphorylation of PDGFR-β and its association with IL-1R1. These effects on receptor association and activation were abolished by a PDGFR-β neutralizing antibody (i.e., AF385), an IL-1R1 antagonist (i.e., IL-1ra), and a specific inhibitor of PDGFR-β phosphorylation (i.e., AG1295), which also abrogated the PDGF-BB/IL-1β-mediated signaling and contractile marker protein expression. Finally, PDGF-BB/IL-1β inhibited the polymerized collagen-induced SRF-DNA binding activity, and this effect was eliminated by AF385, IL-1ra, AG1295, wortmannin, LY294002, and rapamycin.
Fig. 8.
Schematic representation of the signaling pathway regulating the expressions of SMα-actin, SM-MHC, and calponin in SMCs on polymerized collagen in response to PDGF-BB/IL-1β costimulation.
Because of the lack of a primary culture system for SMCs or SMC-derived cell lines in which they can maintain fully contractile phenotype, the factors or signaling pathways regulating the phenotypic modulation of SMCs from a contractile to a synthetic state had not been characterized. By culturing SMCs on polymerized collagen that promotes the maintenance of SMC contractile phenotype (2, 3), we have been able to elucidate the factors that signal and control the modulation of SMCs from contractile toward synthetic phenotype. The results from the present study may provide insights into the mechanisms regulating progression of disorders involving vascular smooth muscle, e.g., atherosclerosis.
It has been reported that PDGF-BB-activated PDGFR-β can be coupled to several downstream intracellular signaling molecules, including PI3K (9), to initiate a series of events culminating in changes in SMC function (4). However, recent studies have shown that SMCs cultured on polymerized collagen significantly attenuate their PDGF-BB-responsiveness (3). We found that PDGF-BB alone induced only a transient activation of PDGFR-β and Akt in SMCs on polymerized collagen, and it did not activate p70S6K and modulate SMC marker protein expression. However, PDGF-BB/IL-1β costimulation induced sustained activations of PDGFR-β and Akt, followed by the activation of p70S6K and the down-regulation of contractile marker protein expressions. The blockade of Akt activation by AG1295 and AF385 (data not shown) indicate that PDGFR-β plays a role. The effect of IL-1β is mediated by binding to IL-1R1, as demonstrated by the inhibitory effect of IL-1ra on PDGF-BB/IL-1β-mediated activation of PDGFR-β/Akt/p70S6K and expression of contractile marker proteins.
Our present study has provided direct evidence for a synergism between PDGF-BB and IL-1β based on the PDGFR-β and IL-1R1 receptor/receptor interactions. This is a true synergism rather than the summation of the effects of submaximal doses of PDGF-BB and IL-1β, because IL-1β was effective in eliciting these effects at saturating concentrations of PDGF-BB, which by itself was not able to do so (data not shown). The results obtained from coimmunoprecipitation and Western blotting experiments using membrane preparations of the PDGF-BB/IL-1β-treated cells demonstrated that PDGFR-β and IL-1R1 can form heteromeric complexes on the cell membrane. It remains to be resolved whether PDGFR-β and IL-1R1 are physically associated by means of direct protein–protein interactions (heterodimers) or they are linked by additional cytosolic adaptor proteins, such as c-Src, which has recently been shown to be involved in the coupling between PDGFR-β and G protein-coupled receptors (10).
The existence of PDGFR-β/IL-1R1 heteromeric complex provides the possibility for close crosstalk between PDGFR-β and IL-1R1, which may synchronize PDGFR-β and IL-1R1 activation to produce synergistic effects at the levels of downstream signaling pathway and gene expression. The mechanisms underlying the synergistic effects of PDGF-BB/IL-1β remain to be determined. Sustained association between PDGFR-β and IL-1R1 induced by PDGF-BB/IL-1β may contribute to sustained phosphorylation of PDGFR-β, which subsequently induced sustained activations of Akt and p70S6K. This idea is supported by the report that receptor pairs such as hepatocyte growth factor receptor c-Met and epidermal growth factor receptor can associate on the cell membrane and that this association facilitates c-Met phosphorylation (11). Our finding that the sustained association between PDGFR-β and IL-1R1 was blocked by the specific inhibitor of PDGFR-β phosphorylation (i.e., AG1295) suggests that the sustained phosphorylation of PDGFR-β plays a role in the maintenance of its association with IL-1R1. Both PDGFR-β neutralizing antibody AF385 and IL-1R1-specific antagonist IL-1ra abrogated the PDGF-BB/IL-1β-induced signaling and phenotypic modulation, indicating that association between PDGFR-β and IL-1R1 is essential for PDGF-BB and IL-1β to produce synergistic effects.
The mechanisms underlying sustained phosphorylation of PDGFR-β and its association with IL-1R1 in response to PDGF-BB/IL-1β remain to be resolved. Growth-promoting effects of IL-1 on PDGF-BB-stimulated SMCs have recently been ascribed to the expression of PDGF-AA (12, 13). Our recent data that blockage of PDGFR-α, the sole PDGFR for PDGF-AA, has no effect on the synergism between IL-1β and PDGF-BB indicates that PDGF-AA does not play a role here (data not shown). One possible mechanism is through inhibition of regulating protein phosphotyrosine phosphatases (PTPs). A recent study by Chiarugi et al. (14) demonstrated that inhibition of PTPs upon PDGF-BB stimulation results in an increase in PDGFR-β phosphorylation and PI3K recruitment. They also showed that tyrosine phosphorylation of PDGFR-β is a long-lasting phenomenon; it reaches a maximal level 10 min after the receptor stimulation, and then declines but remains at an elevated level for up to at least 9 h. However, the long-lasting PDGFR-β phosphorylation was inhibited by the activation of PTPs. The inactivation of a redox-sensitive PTPs by IL-1β in cells has been reported (15). It is possible that costimulating PDGF-BB-treated cells with IL-1β inhibited the activation of regulating PTPs, thereby mediating the dynamic properties of PDGFR-β tyrosine phosphorylation and signaling.
The binding of SRF, a MADs box protein, to CArG boxes in the SMC promoters has been recognized to be critical in mediating transcription activation of contractile phenotypic marker genes (1). Our results from EMSA using the consensus oligonucleotides containing CArG box showed that SMCs cultured on polymerized collagen had higher levels of SRF-DNA binding activity than the cells cultured on monomeric collagen. PDGF-BB/IL-1β abolished this polymerized collagen-induced SRF-DNA-binding activity. AF385, IL-1ra, AG1295, wortmannin, LY294002, and rapamycin eliminated the PDGF-BB/IL-1β-mediated inhibition in SRF-DNA-binding activity, demonstrating the importance of sustained phosphorylation of PDGFR-β and its association with IL-1R1, as well as the PI3K/Akt/p70S6K pathway, in the inhibitory effect of PDGF-BB/IL-1β on SRF-DNA-binding activity in SMCs on polymerized collagen. These findings provide a mechanism that integrates signaling pathway from PDGFR-β/IL-1R1 receptor interaction through PI3K/Akt/p70S6K to the SRF-dependent gene transcription in SMCs during their phenotypic modulation from contractile to synthetic state.
In summary, our present study demonstrated that PDGF-BB and IL-1β exert synergistic effects on modulating the phenotype of SMCs cultured on polymerized collagen through the PDGFR-β/IL-1R1 receptor interaction and the PI3K/Akt/p70S6K pathway. Our findings provide a molecular basis for mechanisms contributing to function of smooth muscle in health and its disorders in the progression of diseases such as atherosclerosis, where mitogenic and inflammatory stimuli are concurrently generated.
Materials and Methods
Materials.
Rabbit polyclonal antibodies against phospho-Akt (Ser-473), Akt, phospho-p70S6K1 (Thr-389), and p70S6K1 were purchased from Cell Signaling Technology. Polyclonal antibodies against phospho-PDGFR-β (Tyr-751), PDGFR-β, IL-1R1, and SRF were purchased from Santa Cruz Biotechnology. IL-1ra, AF385, and AG1295 were purchased from R & D Systems. Recombinant adenoviruses encoding control green fluorescence protein (Ad-GFP), hemagglutinin (HA)-tagged dominant-negative Akt (Ad-DN-Akt), or constitutively active myristoylated Akt (Ad-Myr-Akt) driven by the CMV promoter was prepared as described (17). Unless otherwise stated, all other antibodies and chemicals of reagent grade were obtained from Sigma.
Cell Cultures.
Human aortic SMCs were obtained commercially (Clonetics, Palo Alto, CA) and maintained in F12K medium (GIBCO) supplemented with 10% FBS (GIBCO). Cells with passages 4–6 were used.
Collagen Matrices.
Collagen gels (0.1%) were prepared by mixing 4 mg/ml rat tail type I collagen (Fisher Scientific; 25%), 0.1 M NaOH (5%), 2× F12K medium (40%), FBS (10%), and complete medium (F12K with 10% FBS; 20%). The mixture (0.15 ml/cm2) was allowed to polymerize for at least 1 h at 37°C. To coat with monomeric collagen, dishes were soaked in 0.5% acetic acid for 20 min at 60°C, rinsed with distilled water, incubated with 0.1 mg/ml collagen solution in 0.1 M acetic acid for at least 3 h at room temperature, and then washed and stored in F12K. SMCs were cultured on the surface of collagen preparations (polymerized and monomeric collagens) as described (2). The culture medium was then exchanged with a medium that was identical to the previous medium except that it contained only 2% FBS. Cells were incubated for the designated times before treatment with the reagents.
Immunoprecipitation.
For immunoprecipitation from the membrane fraction, the cells were washed twice with ice-cold PBS containing 0.1 μM sodium orthovanadate and resuspended in TNE buffer (10 mM Tris·HCl/150 mM NaCl/1 mM EDTA). Samples were centrifuged for 15 min at 80,000 × g. The pellets were resuspended in TNE buffer with 1% Nonidet P-40 and sonicated for 30 s. After centrifugation at 80,000 × g for 90 min, the supernatants (1 mg of protein per ml) were incubated with the designated antibody for 2 h at 4°C with gentle shaking. The immune complex was then incubated with protein A/G agarose for 1 h and collected by centrifugation. This agarose-bound immunoprecipitates were washed and incubated with boiling sample buffer containing 62 mM Tris·HCl (pH 6.7), 1.25% (wt/vol) SDS, 10% (vol/vol) glycerol, 3.75% (vol/vol) mercaptoethanol, and 0.05% (wt/vol) bromophenol blue. The samples were then subjected to SDS/PAGE and Western blotting.
Western Blot Analysis.
SMCs were lysed with a buffer containing 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, and a protease inhibitor mixture (PMSF, aprotinin, and sodium orthovanadate). Total cell lysates (100 μg of protein) were separated by SDS/PAGE (12% running, 4% stacking) and transferred onto a poly(vinylidene difluoride) membrane (Immobilon P, 0.45-μm pore size). The membrane was then incubated with the designated antibodies. Immunodetection was performed by using the Western-Light chemiluminescent detection system (Applied Biosystems, Foster City, CA).
Adenoviral Infection.
SMCs were plated in Petri dishes and infected with Ad-GFP, Ad-DN-Akt, or Ad-Myr-Akt at a multiplicity of infection of 1:50 for 24 h in F12K with 10% FBS. The cells were washed, replated on the gels, and cultured in a medium that was identical to the previous medium except that it contained only 2% FBS. The cells were further incubated for 48 h before treatment with the reagents.
EMSA.
SMCs were collected by scraping in PBS, and nuclear proteins were extracted by centrifugation in cold buffer as described (16). Double-stranded consensus oligonucleotides (5′-GACAGTCCTATTATGGGAAACC-3′) containing CArG box were synthesized and end-labeled with [γ-32P]ATP. The extracted nuclear proteins (10 μg) were incubated with 0.1 ng of 32P-labeled DNA for 15 min at room temperature in 25 μl of binding buffer containing 1 μg of poly(dI-dC). The mixtures were electrophoresed on 5% nondenaturing polyacrylamide gels. The gels were dried and imaged by autoradiography.
Supplementary Material
Acknowledgments
This work was supported by National Health Research Institutes (Taiwan) Grant ME-094-PP-06 (to J.-J.C.); National Science Council (Taiwan) Grants 94-3112-B-400-005 and 94-2321-B-400-002 (to J.J.C.); and National Heart, Lung, and Blood Institute Grants HL064385 and HL080518 (to S.C.).
Abbreviations
- SMC
smooth muscle cell
- SMα
smooth muscle α
- SM-MHC
smooth muscle myosin heavy chain
- PDGF
platelet-derived growth factor
- PDGFR
PDGF receptor
- PI3K
phosphatidylinositol 3-kinase
- IL-1R
IL-1 receptor
- SRF
serum response factor.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Owens G. K., Kumar M. S., Wamhoff B. R. Physiol. Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 2.Koyama H., Raines E. W., Bornfeldt K. E., Roberts J. M., Ross R. Cell. 1996;87:1069–1078. doi: 10.1016/s0092-8674(00)81801-2. [DOI] [PubMed] [Google Scholar]
- 3.Raines E. W., Koyama H., Carragher N. O. Ann. N.Y. Acad. Sci. 2000;902:39–51. doi: 10.1111/j.1749-6632.2000.tb06299.x. [DOI] [PubMed] [Google Scholar]
- 4.Fredriksson L., Li H., Eriksson U. Cytokine Growth Factor Rev. 2004;15:197–204. doi: 10.1016/j.cytogfr.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Libby P., Warner S. J., Friedman G. B. J. Clin. Invest. 1988;81:487–498. doi: 10.1172/JCI113346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornwell T. L., Arnold E., Boerth N. J., Lincoln T. M. Am. J. Physiol. 1994;267:C1405–C1413. doi: 10.1152/ajpcell.1994.267.5.C1405. [DOI] [PubMed] [Google Scholar]
- 7.Bourcier T., Dockter M., Hassid A. J. Cell Physiol. 1995;164:644–657. doi: 10.1002/jcp.1041640323. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez B., Garrido E., Garcia-Sanz J. A., Carrera A. C. J. Biol. Chem. 2003;278:26466–26473. doi: 10.1074/jbc.M300663200. [DOI] [PubMed] [Google Scholar]
- 9.Panayotou G., Bax B., Gout I., Federwisch M., Wroblowski B., Dhand R., Fry M. J., Blundell T. L., Wollmer A., Waterfield M. D. EMBO J. 1992;11:4261–4272. doi: 10.1002/j.1460-2075.1992.tb05524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waters C. M., Connell M. C., Pyne S., Pyne N. J. Cell Signal. 2005;17:263–277. doi: 10.1016/j.cellsig.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Jo M., Stolz D. B., Esplen J. E., Dorko K., Michalopoulos G. K., Strom S. C. J. Biol. Chem. 2000;275:8806–8811. doi: 10.1074/jbc.275.12.8806. [DOI] [PubMed] [Google Scholar]
- 12.Raines E. W., Dower S. K., Ross R. Science. 1989;243:393–396. doi: 10.1126/science.2783498. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda U., Ikeda M., Oohara T., Kano S., Yaginuma T. Atherosclerosis. 1990;84:183–188. doi: 10.1016/0021-9150(90)90089-2. [DOI] [PubMed] [Google Scholar]
- 14.Chiarugi P., Cirri P., Taddei M. L., Talini D., Doria L., Fiaschi T., Buricchi F., Giannoni E., Camici G., Raugei G., Ramponi G. J. Cell Sci. 2002;115:2219–2232. doi: 10.1242/jcs.115.10.2219. [DOI] [PubMed] [Google Scholar]
- 15.Guy G. R., Cairns J., Ng S. B., Tan Y. H. J. Biol. Chem. 1993;268:2141–2148. [PubMed] [Google Scholar]
- 16.Chiu J.-J., Chen L.-J., Chang S.-F., Lee P.-L., Lee C.-I., Tsai M.-C., Lee D.-Y., Hsieh H.-P., Usami S., Chien S. Arterioscler. Thromb. Vasc. Biol. 2005;25:963–969. doi: 10.1161/01.ATV.0000159703.43374.19. [DOI] [PubMed] [Google Scholar]
- 17.Andjelkovic M., Alessi D. R., Meier R., Fernandez A., Lamb N. J., Frech M., Cron P., Cohen P., Lucocq J. M., Hemmings B. A. J. Biol. Chem. 1997;272:31515–31524. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.