Abstract
Memory consolidation refers to a process by which newly learned information is made resistant to disruption. Traditionally, consolidation has been viewed as an event that occurs once in the life of a memory. However, considerable evidence now indicates that consolidated memories, when reactivated through retrieval, become labile (susceptible to disruption) again and undergo reconsolidation. Because memories are often interrelated in complex associative networks rather than stored in isolation, a key question is whether reactivation of one memory makes associated memories labile in a way that requires reconsolidation. We tested this in rats by creating interlinked associative memories using a second-order fear-conditioning task. We found that directly reactivated memories become labile, but indirectly reactivated (i.e., associated) memories do not. This suggests that memory reactivation produces content-limited rather than wholesale changes in a memory and its associations and explains why each time a memory is retrieved and updated, the entire associative structure of the memory is not grossly altered.
Keywords: consolidation, fear, memory, associative learning
Consolidation is the process through which temporary or short-term memory (STM) is converted into persistent or long-term memory (LTM) (1). Much research suggests that consolidation depends on protein synthesis (2–4). Traditionally, protein synthesis-dependent memory storage is viewed as occurring once, after which the memory is permanently stored and insensitive to disruption by protein synthesis inhibitors or other amnesia-inducing treatments (4). However, considerable evidence has emerged indicating that memory becomes newly dependent on protein synthesis after reactivation (retrieval), a process called reconsolidation (4–6). For example, we and others have used Pavlovian fear conditioning to study the consolidation and reconsolidation of associative memories (6–14). In fear conditioning, a neutral conditioned stimulus (CS), such as an auditory tone, is paired with an unconditioned stimulus (US), typically consisting of electric foot shock. After the pairing, the CS has the capacity to elicit conditioned fear responses, often measured in terms of immobility or freezing behavior (15). Blockade of protein synthesis in the lateral and basal nuclei of the amygdala (LBA), the presumed site of fear memory formation and storage (16), after training disrupts the consolidation of fear memories (17), and blockade after retrieval of consolidated memories disrupts their reconsolidation (6, 12).
In life, memories are typically not stored in isolation of other memories but instead are believed to be integrated into complex associative networks, such that activation of one element of an association leads to activation of related elements (18). A key question is whether reactivation of one component of an associative network renders all memories in the network labile and sensitive to disruption. Most studies of reconsolidation to date have either focused on simple associations in which stimuli are by design related to a single other stimulus (6, 7, 12, 14) or have used complex contextual situations (8–11, 13, 19) that, although composed of multiple associated stimuli, are not analyzable in terms of component associations and are typically treated as a single event (20, 21). By means of a variant of fear conditioning called second-order fear conditioning (SOFC), it is possible to create an associative network and thus test whether reactivation of one component of the memory leads to reconsolidation of other associations or only of the reactivated component.
SOFC begins with a standard fear-conditioning procedure in which a CS (CS1) is paired with a US. This is called first-order conditioning and results in the formation of an association (a first-order association) between CS1 and the US (22–24). If a second distinct CS (CS2) is then repeatedly paired with CS1 in the absence of the US, it can also acquire the ability to elicit conditioned responses (22–24). Thus, the acquisition by CS2 of the ability to control freezing depends on the existing CS1–US association. However, under certain circumstances (e.g., when the two CSs are of the same stimulus modality or are presented simultaneously during second-order training) (25–28), the CS1 and its relation to the US determines the ability of CS2 to elicit conditioned responses. When this occurs, it is believed that a CS2–CS1 (second-order) association is formed. The second- and first-order associations (CS2–CS1 and CS1–US, respectively) are then linked together in an associative network (CS2 → CS1 → US) (23–28). Such associative networks are traditionally viewed as consisting of internal representations of the stimuli and associations between the representations (23, 24).
Using a SOFC task that leads to the formation of a CS2 → CS1 → US associative network, we evaluated whether blockade of protein synthesis in the LBA after reactivation of one component of the associative memory renders the entire associative network vulnerable to disruption. Our results show that only the directly reactivated aspect of the memory becomes labile and suggest that reconsolidation after memory reactivation is limited to the active component of the associative memory.
Results
Experiment 1.
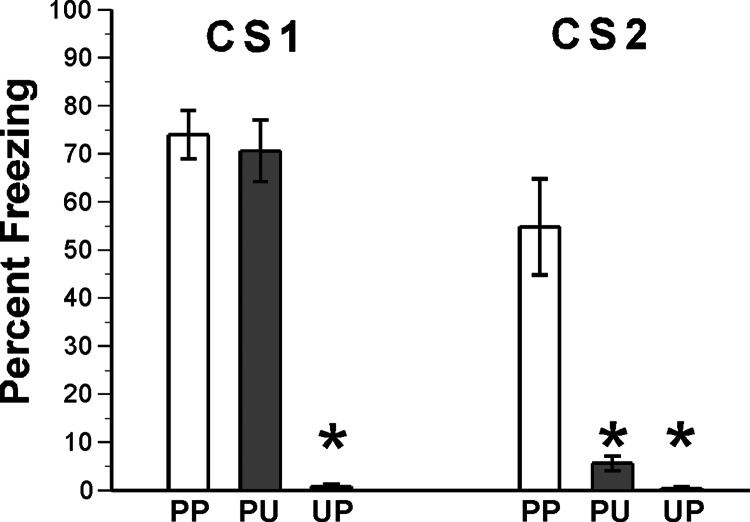
We first verified that, in our paradigm, SOFC was acquired as a series of associative relationships between the various cues. After 4 days of habituation, the rats were divided into three groups that underwent first-order conditioning on days 5 and 6 and then second-order conditioning on day 7 using paired (P) or unpaired (U) training: group PP (n = 6) received paired first-order and then paired second-order conditioning; group PU (n = 6) received paired first-order and unpaired second-order conditioning; group UP (n = 6) received unpaired first-order conditioning and then paired second-order conditioning. On day 8, memory was tested in the three groups (Fig. 1). Results were analyzed by ANOVA (statistics presented in the legend of Fig. 1). Animals that received pairing of CS1 and the US in first-order conditioning (groups PP and PU) showed equivalent high levels of freezing to CS1, but animals given unpaired presentations of CS1 and US during first-order conditioning (UP) did not freeze to CS1. A significant difference was found in freezing to CS1 between group UP and groups PP and PU. Similarly, only group PP, which received paired presentations of CS1 and the US during first-order conditioning and paired presentations of CS2 and CS1 during second-order conditioning, showed significant freezing to CS2. Responses in PP were significantly greater than in the other two groups, which did not differ (see Fig. 1 for statistics). Indeed, animals that had unpaired presentations in first- or second-order conditioning (UP and PU, respectively) showed minimal freezing to CS2.
Fig. 1.
First-order fear conditioning and SOFC each depend on associative learning. Rats received associative (paired, P) or nonassociative (unpaired, U) presentations of the CS and US during first- and second-order conditioning. Fear (freezing) responses were tested after completion of second-order conditioning. Results were analyzed by a two-factor ANOVA, with test CS (CS1, CS2) and training type (PP, PU, UP) as the factors (test CS was a repeated measure). The analysis revealed significant main effects of training type [F (2, 15) = 60.7; P < 0.000001] and test CS [F (1, 15) = 74.5; P < 0.000001] and a significant test CS × training type interaction: F (2, 15) = 34.0; P < 0.00001. Posthoc mean comparisons (Tukey’s honest significant difference text) showed that animals that received pairing of CS1 and the US in first-order conditioning (groups PP and PU) showed equivalent levels of freezing to CS1 (P = 0.99), but animals given unpaired presentations of CS1 and US during first-order conditioning (UP) did not freeze to CS1, indicated by a significant difference in freezing to CS1 between group UP and groups PP and PU (P < 0.001). Similarly, only group PP, which received paired presentations of CS1 and the US during first-order conditioning and paired presentations of CS2 and CS1 during second-order conditioning, showed significant freezing to CS2. Responses to CS2 were significantly greater in PP than in the other two groups (P < 0.001). These results indicate that both the first- and second-order paradigms depend on associative learning: first-order conditioning depends on the CS1–US association, and second-order conditioning depends on the CS2–CS1 association.
These results demonstrate that freezing to CS1 after first-order conditioning depends on the association of CS1 with the US, and freezing to CS2 after second-order conditioning depends on the association of CS2 with CS1. In other words, the ability of both the first- and the second-order CS to elicit freezing depends on associative learning. Next, we asked whether the ability of CS2 to elicit freezing after second-order conditioning depends on the first-order association between CS1 and the US, that is, on a CS2 → CS1 → US associative chain.
Experiment 2.
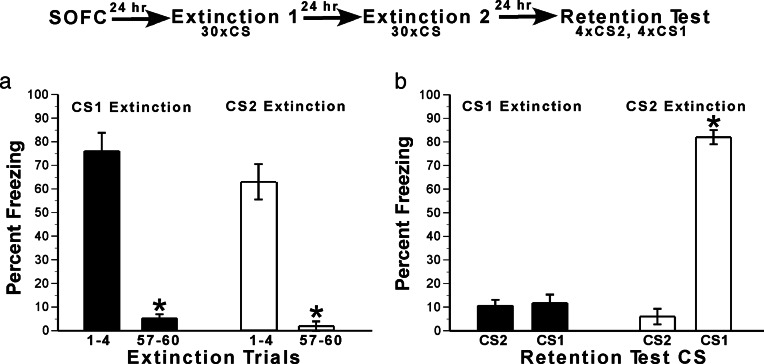
One way to assess whether responses controlled by CS2 require a CS2 → CS1 → US associative chain is by determining whether extinction of freezing to CS1 leads to concurrent decreases in responses elicited by CS2 (23, 24). To assess this, we examined the effects of giving extinction training with either CS2 or CS1 on responses elicited by the two stimuli. The day after SOFC, rats (n = 6) received 30 presentations of CS1 or CS2 on each of 2 consecutive days. The next day, a retention test was given in which the rats received four presentations of CS2 followed by four presentations of CS1.
To assess the effectiveness of extinction training, responses to the CS during the first four CS presentations during extinction training (trials 1–4) were compared with responses from the last four CS presentations (trials 57–60) (Fig. 2a). Results were analyzed by ANOVA (see Fig. 2 legend for statistics). At the beginning of extinction training (trials 1–4), high levels of freezing were seen to both CS1 and CS2. By the end of extinction training, responding to both stimuli was minimal. Thus, extinction training with CS1 led to extinction of responses elicited by CS1, and extinction training with CS2 led to extinction of responses elicited by CS2 during the extinction session.
Fig. 2.
SOFC results in the formation of an associative network in which responding to CS2 depends on the first-order (CS1–US) association. After completion of first- and second-order conditioning, rats were exposed to CS1 or CS2 30 times on each of 2 consecutive days. Freezing to the CSs was examined during extinction training (a) and in a retention test (b) performed on the day after completion of extinction training. (a) Freezing during extinction training. Average freezing responses elicited by CS1 during the last four trials of CS1 extinction training (trials 57–60) were lower than responses elicited during the first four trials (trials 1–4). Similarly, average freezing responses elicited by CS2 during the last four trials of CS2 extinction training (trials 57–60) were lower than responses elicited during the first four trials (trials 1–4). The data were analyzed by a two-factor ANOVA, with extinction CS (CS1, CS2) and extinction training phase (first four trials, last four trials) as the factors (extinction training phase was a repeated measure). The ANOVA revealed a main effect of extinction training phase, due to the decrease in freezing between the beginning and end of extinction: F (1, 10) = 158.8; P < 0.000001. There was no main effect of stimulus type (P = 0.13), nor was there an interaction between stimulus type and extinction training phase (P = 0.37). Thus, extinction training with CS1 led to extinction of responses elicited by CS1, and extinction training with CS2 led to extinction of responses elicited by CS2 during the extinction session. (b) Freezing during the retention test. One day after the completion of extinction training, the rats received a LTM retention test in which they were exposed to CS2 and then CS1. The data were analyzed with a two-factor ANOVA, with extinction CS (CS1, CS2) and test CS (CS1, CS2) as the factors (test CS was a repeated measure). CS1 extinction training led to low levels of freezing to both CS2 and CS1, whereas CS2 extinction led to low levels of freezing to CS2 but not CS1. ANOVA revealed significant main effects of extinction CS [F (1, 10) = 114.3; P < 0.0001] and test CS [F (1, 10) = 213.8; P < 0.00001], and an extinction CS × test CS interaction [F (1, 10) = 202; P < 0.000001]. Post-hoc Tukey’s honest significant difference tests revealed that rats given extinction training with CS1 showed similarly low levels of freezing when tested with CS2 or CS1 (P = 0.99) (b), and that rats given extinction training with CS2 also had low levels of freezing to CS2 that did not differ from the low level of responses to CS1 (P = 0.52) and CS2 (P = 0.72) after extinction training with CS1. In contrast, freezing levels to CS1 were significantly higher than freezing to CS2 after CS2 extinction (P < 0.001) and significantly higher than both CS1 (P < 0.001) and CS2 (P < 0.001) after CS1 extinction (b). These results demonstrate that, in our protocol, extinction of freezing responses to first-order stimulus (CS1) leads to concurrent impairment of responding to CS2, but extinction of the second-order stimulus (CS2) impairs only freezing to CS2. Freezing to CS2 thus depends on the first-order association. This suggests that our SOFC protocol results in the formation of an associative network involving CS2 → CS1 → US. In this network, the first-order association (CS1–US) is activated directly by presenting CS1 and indirectly by presenting CS2.
The next day, all rats received a memory-retention test during which four trials of CS2 and then CS1 were given (Fig. 2b). Results were analyzed with a two-factor ANOVA (statistics presented in the legend of Fig. 2b). After extinction training with CS1, low levels of freezing were seen to CS2 and CS1 during the retention test. In contrast, after extinction training with CS2, freezing was low to CS2 but high to CS1. These results demonstrate that in our protocol, extinction of freezing responses to the first-order stimulus (CS1) leads to concurrent impairment of responding to CS2, but extinction of the second-order stimulus (CS2) impairs only freezing to CS2.
The results from this extinction study validate that the SOFC paradigm establishes a CS2 → CS1 → US associative network (23, 24). In this network, the CS1–US relation constitutes the primary (first-order) association and the CS2–CS1 relation the secondary (second-order) association. The ability of CS2 to elicit freezing depends on the primary association between CS1 and the US, as well as on secondary association between CS2 and CS1; that is, CS2 responses depend on the whole CS2 → CS1 → US associative network. The key point, though, is that the first-order association between CS1 and the US, although involved in responding to both CS1 and CS2, is activated in different ways. The first-order association is activated directly by presenting CS1 and indirectly by presenting CS2 (via the second-order association).
Experiment 3.
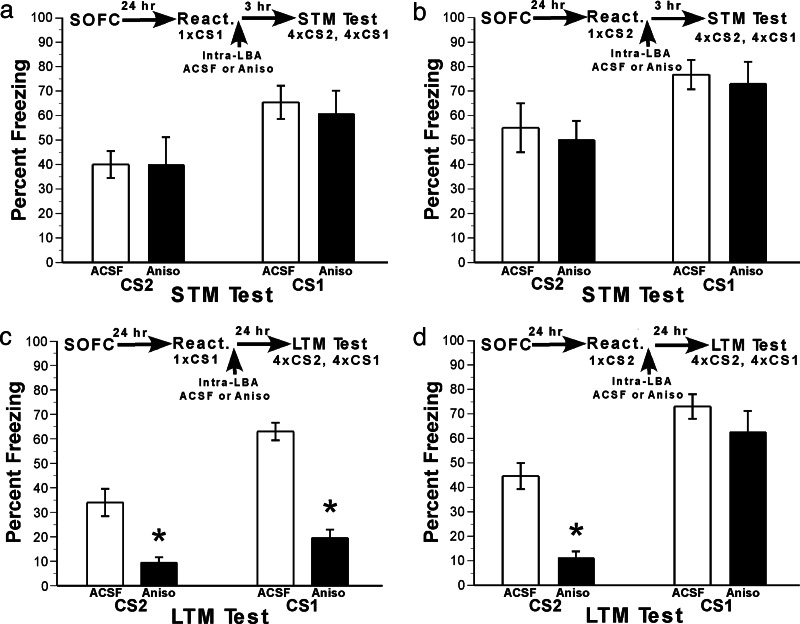
We next used our SOFC protocol in conjunction with infusions of anisomycin (ANISO) into the LBA. This was done to achieve two goals. The first was to determine whether SOFC, like first-order conditioning, undergoes protein synthesis-dependent reconsolidation in LBA. The second, and more important, goal was to determine whether protein synthesis-dependent reconsolidation of the first-order memory occurs in LBA when it is reactivated directly (by presentation of the first-order CS, CS1) as well as indirectly (by presentation of the second-order CS, CS2). These goals were achieved by assessing whether reactivation of CS2 in the presence of the protein synthesis inhibitor ANISO affects LTM elicited by CS2 and CS1, and whether reactivation of CS1 in the presence of ANISO affects LTM elicited by both CSs.
To conclude that pharmacological manipulation disrupts memory consolidation, it must be shown that immediate posttraining administration of the drug leaves STM (usually 1–4 h) intact but affects LTM several hours later (29). By analogy, postretrieval disruption of reconsolidation should leave the postreactivation STM intact and affect only the postreactivation LTM (6).
To assess whether blockade of protein synthesis in LBA leaves postreactivation STM intact, cannulated rats were exposed to either CS1 or CS2 for a single reactivation trial 1 day after SOFC. Freezing responses during the reactivation trial did not differ in the groups that were exposed to CS1 or CS2 (F < 1). Immediately thereafter, they received intraLBA infusions of either ANISO or artificial cerebrospinal fluid (aCSF). During the postreactivation STM test, which was given 3 h after the infusions, responses elicited by CS2 and CS1 were tested. The data were analyzed by a three-factor ANOVA with reactivation CS × drug × test CS as the factors (statistics are presented in the legend of Fig. 3). As shown in Fig. 3a, after CS1 reactivation, postreactivation STM elicited by CS2 or CS1 was similar in animals given aCSF (n = 6) or ANISO (n = 8) infusions. Fig. 3b shows that after CS2 reactivation, STM elicited by CS2 or CS1 was similar in animals given aCSF (n = 6) or ANISO (n = 8) infusions. Thus, postreactivation STM was unaffected by intraLBA ANISO regardless of the stimulus presented during reactivation or during the STM test.
Fig. 3.
The effects of protein synthesis inhibition in the LBA on postreactivation LTM depend on whether reactivation involved CS1 or CS2. Rats underwent SOFC and then were exposed to either CS1 or CS2, followed immediately by intraLBA infusion of ANISO or aCSF. Three hours later, postreactivation STM was tested. A separate group of rats underwent the same procedure, except that postreactivation LTM was tested. The two sets of data were analyzed with separate three-factor ANOVAs, with reactivation CS (CS1, CS2), drug (aCSF, ANISO), and retention test CS (CS1, CS2) as the factors (the retention test CS was a repeated-measures factor). (a and b) Protein synthesis inhibition in the LBA has no effect on postreactivation STM. Rats that received ANISO or aCSF infusions after exposure to CS1 (a) or CS2 (b) showed comparable levels of freezing to both CS1 and CS2 during the postreactivation STM test. The main effect of neither reactivation CS (P = 0.13) nor drug (P = 0.67) nor the interaction (P ≥ 0.7) was significant. There was a main effect of retention test CS [F (1, 24) = 45.7; P < 0.00001], indicating overall higher freezing to CS1 than CS2, which is commonly observed in studies of second-order conditioning (24–29). (c and d) Protein synthesis inhibition in LBA affects postreactivation LTM but in different ways, depending on which stimulus was reactivated. In contrast to the postreactivation STM test (a and b), in the postreactivation LTM test (c and d), there was a significant drug × reactivation CS × retention test CS interaction [F (1, 25) = 16.5; P < 0.001], indicating that the pattern of ANISO effects on fear memory for CS1 and CS2 depended on whether the reactivation stimulus was CS1 (c) or CS2 (d). The first-order interaction between drug and retention test CS was significant for CS1 reactivation [F (1, 13) = 9.0, P < 0.05] and for CS2 reactivation [F (1, 12) = 7.65; P < 0.05]. Post-hoc Tukey’s honest significant difference test mean comparisons revealed that after reactivation with CS1, ANISO had a significant effect on responses in the postreactivation LTM test elicited by CS2 (P < 0.001) and CS1 (P < 0.045), but after reactivation with CS2, ANISO affected responses elicited by CS2 (P < 0.05) and not CS1 (P = 0.71). These findings indicate that for the first-order associative memory (CS1–US) to undergo protein synthesis-dependent reconsolidation, it must be directly reactivated by CS1. Indirect reactivation of the first-order association by CS2 via the CS2 → CS1 → US associative network, fails to induce reconsolidation.
Having shown that postreactivation STM of CS1 and CS2 is unaffected by protein synthesis blockade after reactivation of either CS1 or CS2, we next examined the effects of the same treatment on postreactivation LTM. Another set of cannulated rats were exposed either to CS1 or to CS2 for a single reactivation trial 1 day after SOFC (different rats were used in the STM and LTM retention tests to eliminate the possibility that extinction occurring as a result of CS presentations during the STM test would affect performance in the LTM test). Freezing responses during the reactivation trial did not differ in the groups that were exposed to CS1 or CS2 (F < 1). Immediately thereafter, they received intraLBA infusions of either ANISO or aCSF. The next day, responses elicited by CS2 and then CS1 during a postreactivation LTM retention test were assessed. The data were analyzed by a three-factor ANOVA with reactivation CS × drug × test CS as the factors (statistics presented in the legend of Fig. 3). In contrast to postreactivation STM, postreactivation LTM was differentially affected by ANISO infusions in LBA depending on the stimulus used for reactivation and testing. Fig. 3c shows that after CS1 reactivation, postreactivation LTM elicited by both stimuli was impaired in animals given ANISO (n = 8) compared with aCSF (n = 7). Fig. 3d shows that after CS2 reactivation, postreactivation LTM elicited by CS2, but not CS1, was impaired in animals given ANISO (n = 8) compared with aCSF (n = 6).
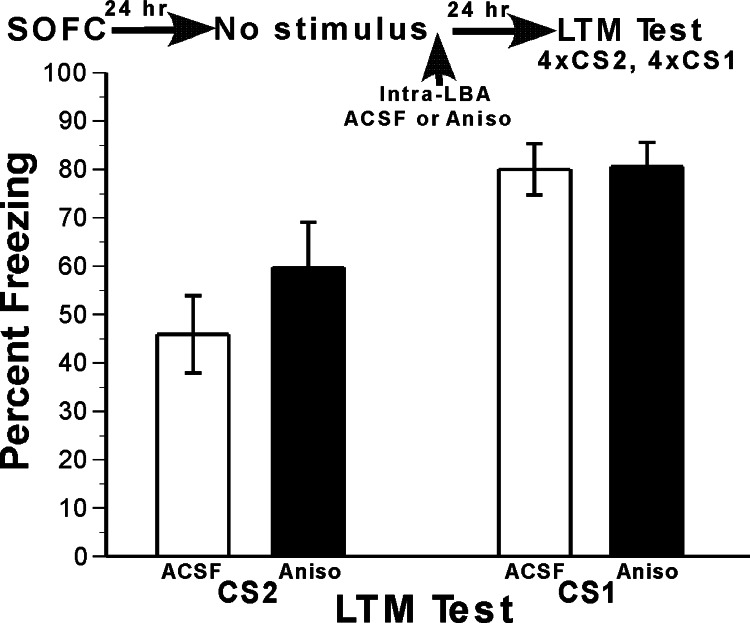
An essential control in reconsolidation studies involves the administration of the drug treatment but without the CS during the reactivation session. As shown in Fig. 4, groups receiving aCSF (n = 6) and ANISO (n = 6) responded similarly in response to CS2 and CS1 when no CS was presented before the drug treatment (see legend of Fig. 4 for statistical analysis). This control experiment demonstrates that reactivation of the memory by an exposure to the CS is necessary for the ANISO to impair the memory. In addition, the absence of an effect when the CS was omitted shows that the SOFC memories are consolidated by this time point.
Fig. 4.
Protein synthesis inhibition in LBA does not affect fear memory in the absence of memory reactivation. To confirm that the SOFC memories are consolidated at the time of reactivation, it is necessary to show that protein synthesis inhibition in the absence of memory reactivation has no effect. Rats underwent SOFC and then were given infusions of ANISO or aCSF into LBA. The next day, they received a LTM retention test in which CS2 and then CS1 were presented. Groups given ANISO and aCSF did not differ. Results were analyzed by two-factor ANOVA, with drug (aCSF, ANISO) and retention test CS (CS1, CS1) as the factors (retention test CS was a repeated measure). The ANOVA indicated that in the absence of stimulus exposure, there was neither a main effect of drug (P = 0.78) nor a CS × drug interaction (P = 0.46). There was a main effect of CS [F (1, 10) = 22.8; P < 0.001], indicating freezing was higher to CS1 than to CS2, a common occurrence in second-order conditioning studies (24–29). This experiment demonstrates that reactivation of the memory by an exposure to the CS is necessary for protein synthesis blockade to impair the memory.
In summary, these findings demonstrate that protein synthesis inhibition after exposure to a single CS1 impairs responses to both CS1 and CS2, or both first- and second-order memories, whereas the same treatment after exposure to a single CS2 disrupts only responding to CS2 and leaves freezing to CS1 intact. Further, exposure to the CS is required for ANISO to block reconsolidation of SOFC, just as it is for first-order conditioning.
Discussion
The objective of this study was to address the question of whether reactivation of one component of a restricted associative memory network leads to the activation and subsequent reconsolidation of associated elements. We tested this possibility in rats using a SOFC task. We first asked whether SOFC in our paradigm constitutes an associative network. Then, in a series of experiments, we selectively reactivated particular components of this network and infused the protein-synthesis inhibitor ANISO into the LBA.
When extinction of CS1 responding does not affect responding to CS2, it is generally assumed that the ability of CS2 to elicit conditioned responses is independent of the first-order fear memory (23, 24). Conversely, if responding to CS2 decreases with CS1 extinction (25–28), the stimuli are believed to constitute an associative chain CS2 → CS1 → US. Thus, that in our protocol CS2’s ability to produce freezing was compromised by extinction of freezing to CS1 (Fig. 2) is evidence for a CS2 → CS1 → US associative network linking the cues. Although responding to both CS1 and CS2 requires activation of the first-order association between CS1 and the US, in the case of CS1-elicited responses, the first-order association is directly activated, whereas in the case of CS2-elicited responses, the first-order association is indirectly activated via the second-order association.
Freezing elicited by CS2 was impaired after reactivation of either CS2 or CS1 in conjunction with infusion of ANISO into LBA. In contrast, freezing to CS1 was impaired by reactivation of CS1, but not CS2, in conjunction with ANISO infusion in LBA. This difference most likely reflects unique sensitivities of directly and indirectly reactivated memories to undergo reconsolidation. The intact postreactivation STM and impaired postreactivation LTM for CS1 after exposure to CS1 are likely because reconsolidation of the first-order memory was interfered with. The simplest interpretation of the CS2 freezing deficit after exposure to CS1 is that freezing to CS2 requires an intact CS1 → US memory and, as just noted, this memory was impaired. In contrast, the impaired freezing to CS2 but not CS1 after exposure to CS2 most likely reflects interference with the blockade of second-order memory reconsolidation. The intact CS1 freezing suggests that the first-order memory is resistant to undergoing reconsolidation under these conditions. Our interpretation of these various effects is that the SOFC paradigm produced a CS2 → CS1 → US associative chain and that, although exposure to CS2 reactivated the first-order memory, it did not trigger reconsolidation of the first-order memory in LBA. In other words, sensitivity of the first-order memory to disruption by blockade of protein synthesis in the amygdala was completely different when activated directly by presenting CS1 and when activated indirectly by presenting CS2. We therefore conclude that, for fear memories to undergo reconsolidation in the amygdala, they must be directly reactivated.
The question remains why a directly, as opposed to an indirectly, reactivated memory undergoes reconsolidation and requires protein synthesis. Because indirectly activated memories by nature involve weaker associations, they may not produce sufficient stimulation to the memory network to engage signaling pathways that lead to protein synthesis, as opposed to less-persistent forms of plasticity mediated by posttranslational modifications of proteins.
It is very unlikely that the memory deficits we observed were caused by nonspecific effects that grossly impaired the function of the amygdala, because the memory was intact during the postreactivation STM test. That freezing impairments were observed only during a postreactivation LTM test demonstrates that drug manipulations specifically affected reconsolidation processes (6, 8, 12). It is also unlikely that the observed deficits reflected a disruption of reconsolidation of contextual memories triggered by stimuli in the conditioning box (8, 11, 13), because the rats expressed minimal freezing to the conditioning context itself (see Fig. 5 and Supporting Text, which are published as supporting information on the PNAS web site). Finally, it is also unlikely that CS2-elicited freezing is mediated by a CS2–US memory and thus a CS2 → US association, because our data clearly demonstrate that freezing to CS2 critically depends upon an intact CS1 → US memory representation, as shown by the fact that it was affected by extinction of freezing to CS1 (Fig. 2) as well as by the impairment of CS2 freezing when reconsolidation of CS1 → US memory was blocked (Fig. 3c).
In summary, we have demonstrated here that higher-order memories, which are similar to memories that occur in everyday life, undergo reconsolidation. Further, we have shown that one of the requirements for a consolidated first-order memory to return to a labile state and be subject to protein synthesis-dependent reconsolidation in the amygdala is that it must be directly reactivated by the first-order conditioned cue. In other words, the disruption of reconsolidation and by inference the updating of memory during retrieval are restricted to those aspects of the memory network that are specifically and directly activated. Such a boundary condition may explain why vast networks of interrelated associative memories (18) are not radically altered each time a single isolated memory is reactivated. It may, therefore, be possible to use the disruption of reconsolidation to reduce the fear-arousing aspects of emotional memory in posttraumatic stress disorder without running the risk of radically altering personality by producing widespread changes in the associative structure of memory. Such studies are currently in progress.
Materials and Methods
Subjects.
Male Sprague–Dawley rats (250–300, Hilltop Labs, Philadelphia) were housed individually in plastic Nalge cages and maintained on a 12/12-h light/dark cycle. Food and water were provided ad libitum.
Surgery, histology, and drug infusions were described in previous studies (6, 12) (see Supporting Text). All procedures were in accordance with the National Institutes of Health guidelines for the care and use of experimental animals and were approved by the New York University Animal Care and Use Committee.
Apparatus and Stimuli.
All procedures took place in a custom-made conditioning chamber (height × width × length: 28 × 26 × 29 cm). The walls were constructed of stainless-steel bars, and the floor was a standard conditioning chamber grid floor used for delivering foot shock (Model E10–10, Coulbourn Instruments, Lehigh Valley, PA). The conditioning chamber was enclosed within a ventilated and temperature-regulated acoustic isolation box lined with anechoic panels. A diffuse light illuminated the chamber during the experiment. Behavior was recorded by using a microvideo camera.
Stimulus delivery was controlled by an A/D converter (Cambridge Electronics Design, Cambridge, U.K., 1401+) controlled by matlab (Mathworks, Natick, MA) software. Two conditioned stimuli were used, CS1 and CS2. CS1 was a 20-s series of acoustic tone pips (1 kHz, 50-ms duration, 1-ms ramp, intensity 20 dB higher than the background noise, delivered at 1 Hz), and CS2 was a 20-s series of frequency modulation tone sweeps (12.5-kHz carrier frequency, 50-Hz modulation frequency, 2.5-kHz modulation depth, 250-ms duration, 1-ms ramp, intensity 20 dB higher than background noise, delivered at 1 Hz). The US was a 0.5-s (1.5-mA) electric foot shock.
Behavioral Procedures.
Habituation.
Rats were placed in the conditioning chamber for 30 min on 4 consecutive days. The purpose of these sessions was to minimize the development of conditioning to the context once the US was introduced (see Supporting Text). On day 4, rats additionally received three preexposures of CS1 followed by three preexposures of CS2. The intertrial interval (ITI) was variable (157 s on average).
First-order conditioning.
On days 5 and 6, first-order conditioning took place. The rats were placed in the chamber. After a 120-s acclimation, four CS1–US pairings were given on each day. The US was delivered immediately after the end of each CS (ITI = 130 s on average). Nonassociative controls (23) received nonoverlapping (explicitly unpaired) presentations of four CS1s and four USs.
Second-order conditioning.
In studies involving second-order conditioning, the rats were placed in the chamber on day 7. After 120 s, they received four trials in which CS2 was paired with CS1, with CS1 occurring immediately after CS2 (ITI = 130 s on average). Nonassociative controls (23) received nonoverlapping (unpaired) presentations of four CS2s and four CS1s.
Memory-retention tests.
Rats were placed in the chamber and after 120 s, the CS was presented on the same delivery schedule as during conditioning (ITI = 130 s on average). To measure STM, the retention test was given 3 h after reactivation (see below). To measure LTM, the retention test was given ≈24 h after reactivation. The STM or LTM retention test involved presentation of both CS1 and CS2; the four CS2 trials were presented first, and then after 180 s, the four CS1 trials were presented. Freezing during the CS presentation was videotaped and scored off-line by an observer blind to the experimental conditions. An average of the four scores for each CS for each rat was used for the statistical analysis.
Extinction.
In Experiment 2, the effects of extinction of one CS on responding to the other CS was tested. The rats first underwent first- and second-order conditioning. Then, 24 h after completion of SOFC, they received 30 exposures to either CS1 or CS2 (ITI = 72.5 s on average) on each of 2 consecutive days for a total of 60 extinction trials. Freezing was measured during the first and last four trials of extinction (trials 1–4 and 57–60). The next day, a retention test was given in which four trials of CS2 followed by four trials of CS1. Freezing was measured during this test.
Memory reactivation.
In Experiment 3, a memory reactivation session occurred 24 h after completion of SOFC. The rats were acclimated to the chamber for 120 s. A single CS (either CS1 or CS2) was then presented. Freezing in response to the CS was measured and used to equate performance for groups that were to receive either drug or vehicle. Immediately thereafter, the rats received an infusion of drug or vehicle in LBA (see above).
Statistical Analysis.
Data were analyzed by using two- and three-factor ANOVA with CS test as a within-subject factor and all other variables as between-subjects factors. Significant effects were analyzed with a single interaction and a posthoc Tukey’s honest significant difference test where appropriate. The software used was statistica, Ver. 7 (StatSoft, Tulsa, OK). Statistical results are presented in the figure legends.
Supplementary Material
Acknowledgments
We thank Dr. D. E. Bush for help with the statistical analysis, P. Lau for technical assistance, and Dr. B. L. Brown for helpful discussions. This research was supported by grants to J.E.L. (Public Health Service, National Institutes of Health Grants R37 MH38774, R01 MH46516, P50 MH58911, and K05 MH067048) and to J.E.L and K.N. (Volkswagen-Stiftung Grant I/79 894 and Human Frontier Science Program Grant RGP0094/2001-B). K.N. is also funded by Canadian Institutes of Health Research, National Sciences and Engineering Research Council, the EJLB Foundation, and the A.P. Sloan Foundation. V.D. is funded by Centre National de la Recherche Scientifique–National Science Foundation Grant 17089. J.D. was a Fulbright Fellow when this project was initiated.
Abbreviations
- aCSF
artificial cerebrospinal fluid
- ANISO
anisomycin
- CS
conditioned stimulus
- ITI
intertrial interval
- LBA
lateral and basal nuclei of the amygdala
- LTM
long-term memory
- SOFC
second-order fear conditioning
- STM
short-term memory
- US
unconditioned stimulus.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.McGaugh J. L. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 2.Davis H. P., Squire L. R. Psychol. Bull. 1984;96:518–559. [PubMed] [Google Scholar]
- 3.Goelet P., Castellucci V. F., Schacher S., Kandel E. R. Nature. 1986;322:419–422. doi: 10.1038/322419a0. [DOI] [PubMed] [Google Scholar]
- 4.Dudai Y. Annu. Rev. Psychol. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- 5.Sara S. J. Learn. Mem. 2000;7:73–84. doi: 10.1101/lm.7.2.73. [DOI] [PubMed] [Google Scholar]
- 6.Nader K., Schafe G. E., LeDoux J. E. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 7.Misanin J. R., Miller R. R., Lewis D. J. Science. 1968;160:554–555. doi: 10.1126/science.160.3827.554. [DOI] [PubMed] [Google Scholar]
- 8.Dębiec J., LeDoux J. E., Nader K. Neuron. 2002;36:527–538. doi: 10.1016/s0896-6273(02)01001-2. [DOI] [PubMed] [Google Scholar]
- 9.Eisenberg M., Kobilo T., Berman D. E., Dudai Y. Science. 2003;301:1102–1104. doi: 10.1126/science.1086881. [DOI] [PubMed] [Google Scholar]
- 10.Pedreira M. E., Maldonado H. Neuron. 2003;38:863–869. doi: 10.1016/s0896-6273(03)00352-0. [DOI] [PubMed] [Google Scholar]
- 11.Lee J. L., Everitt B. J., Thomas K. L. Science. 2004;304:839–843. doi: 10.1126/science.1095760. [DOI] [PubMed] [Google Scholar]
- 12.Duvarci S., Nader K. J. Neurosci. 2004;24:9269–9275. doi: 10.1523/JNEUROSCI.2971-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki A., Josselyn S. A., Frankland P. W., Masushige S., Silva A. J., Kida S. J. Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dębiec J., Ledoux J. E. Neuroscience. 2004;129:267–272. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 15.Blanchard R. J., Blanchard D. C. J. Comp. Physiol. Psychol. 1969;67:370–375. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- 16.Fanselow M. S., LeDoux J. E. Neuron. 1999;23:229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- 17.Schafe G. E., LeDoux J. E. J. Neurosci. 2000;20:RC96. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson J. R., Bower G. H. Human Associative Memory. New York: Wiley; 1974. [Google Scholar]
- 19.Przybyslawski J., Sara S. J. Behav. Brain Res. 1997;84:241–246. doi: 10.1016/s0166-4328(96)00153-2. [DOI] [PubMed] [Google Scholar]
- 20.Anagnostaras S. G., Gale G. D., Fanselow M. S. Hippocampus. 2001;11:8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 21.Rudy J. W., O’Reilly R. C. Cognit. Affect. Behav. Neurosci. 2001;1:66–82. doi: 10.3758/cabn.1.1.66. [DOI] [PubMed] [Google Scholar]
- 22.Pavlov I. P. Conditioned Reflexes: An Investigation of the Physiological Activity of the Cerebral Cortex. London: Oxford Univ. Press; 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rescorla R. A. Pavlovian Second-Order Conditioning: Studies in Associative Learning. Hillsdale, NJ: Erlbaum; 1980. [Google Scholar]
- 24.Gewirtz J. C., Davis M. Learn. Mem. 2000;7:257–266. doi: 10.1101/lm.35200. [DOI] [PubMed] [Google Scholar]
- 25.Leyland C. M. Q. J. Exp. Psychol. 1977;29:607–619. [Google Scholar]
- 26.Rashotte M. E., Griffin R. W., Sisk C. L. Anim. Learn. Behav. 1977;5:25–38. [Google Scholar]
- 27.Rescorla R. A. J. Exp. Psychol. Anim. Behav. Process. 1979;5:79–95. doi: 10.1037//0097-7403.5.1.79. [DOI] [PubMed] [Google Scholar]
- 28.Rescorla R. A. J. Exp. Psychol. Anim. Behav. Process. 1982;8:23–32. [PubMed] [Google Scholar]
- 29.Rodrigues S. M., Schafe G. E., LeDoux J. E. Neuron. 2004;44:75–91. doi: 10.1016/j.neuron.2004.09.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.