Abstract
Loss of Mdm2 or Mdm4 leads to embryo lethal phenotypes that are p53-dependent. To determine whether Mdm2 and Mdm4 inhibit p53 function redundantly in a more restricted cell type, conditional alleles were crossed to a neuronal specific Cre transgene to delete Mdm2 and Mdm4 in the CNS. Mice lacking Mdm2 in the CNS developed hydranencephaly at embryonic day 12.5 due to apoptosis, whereas Mdm4 deletion showed a proencephaly phenotype at embryonic day 17.5 because of cell cycle arrest and apoptosis. The deletion of both genes, strikingly, contributed to an even earlier and more severe CNS phenotype. Additionally, Mdm2 and Mdm4 had a gene dosage effect, because loss of three of the four Mdm alleles also showed a more accelerated CNS phenotype than deletion of either gene alone. All phenotypes were rescued by deletion of p53. Thus, these in vivo data demonstrate the importance of Mdm4 independent of Mdm2 in inhibition of p53.
Keywords: apoptosis, cell cycle arrest, conditional alleles
The importance of the p53 tumor suppressor is highlighted by the observation that proliferating tumor cells have devised multiple mechanisms to disrupt its activity. Diminished p53 activity allows increased proliferation and inhibition of apoptosis, providing advantageous signals for tumor cell survival (1). By far the most common means of disrupting p53 is through mutation of the gene itself (2). Recently, however, several negative regulators of p53 have been discovered (3–6). Two of these, Mdm2 and Mdm4, are overexpressed in many tumors of diverse origin (7–9). Thus, increased levels of p53 inhibitors in tumor cells yield the same end result, loss of p53 function.
Mouse models have provided invaluable insight into the importance of Mdm2 and Mdm4 as negative regulators of p53 activity. Loss of either Mdm2 or Mdm4 leads to embryo lethal phenotypes that are completely rescued by concomitant deletion of p53, emphasizing the importance of these negative regulators for p53 activity (10–14). Loss of Mdm2 leads to cell death by a p53-dependent apoptotic mechanism, whereas deletion of Mdm4 leads to cell cycle arrest sometimes associated with apoptosis (12, 14, 15). Loss of cell viability in these examples occurs at different developmental stages, at implantation for Mdm2-null embryos, and at gastrulation for Mdm4-null embryos. The early embryo lethal phenotypes of Mdm2- and Mdm4-null embryos preclude analyses of the function of these proteins in a spatial and temporal specific manner.
Clearly, Mdm2 and Mdm4 are potent p53 inhibitors, but their relationship is more complex. Mdm2 is an ubiquitin ligase that catalyzes ubiquitination of p53 and itself (16–19). Mdm4 inhibits p53 by binding and masking the transcriptional activation domain, but it cannot ubiquitinate p53 (4, 20–22). However, both Mdm2 and Mdm4 bind the same p53 domain with similar affinities, implying a potential competition for inhibition of p53 activity (23). Additionally, interactions between Mdm2 and Mdm4 have been detected through their respective RING domains (24, 25). The dependency of Mdm2 and Mdm4 on each other for inhibition of p53 activity in vivo has not been examined. To address these questions, we have generated Mdm2 and Mdm4 conditional alleles (26, 27).
To examine the importance of Mdm2 and Mdm4 and their redundancy in a single cell type, we chose to delete both in the CNS for several reasons. First, upon γ radiation of embryos, the CNS shows a strong p53-dependent apoptotic response (28). Additionally, the CNS consists of a proliferating layer of cells that migrate as they differentiate. Proliferating cells are also present in different locations within the ventricular zone, depending on the stage of the cell cycle. Postmitotic cells exit the ventricular zone and migrate outward to form differentiated layers (29, 30). Thus, the role of Mdm2 and Mdm4 in proliferating and differentiated cells could be examined at the same time. Last, Mdm2 and Mdm4 are expressed in the early CNS (14, 31, 32). We therefore used the Nestin-Cre (Nes-Cre) transgenic mice that contain the Nestin enhancer and express Cre exclusively in the CNS (33) and asked whether Mdm2 and Mdm4 have a role in the inhibition of p53 activity in CNS development.
Results
Loss of Mdm2 or Mdm4 in the CNS Caused Neonatal Lethality.
The generation of conditional alleles for Mdm2 and Mdm4 indicates that Cre-mediated recombination results in loss-of-function alleles and phenotypes identical to the original deletions (26, 27). Both Mdm2 and Mdm4 conditional alleles were mated to the neuronal specific Nes-Cre transgenic mice that contain the Nestin enhancer and express Cre exclusively in the CNS (33). To examine the specificity of Cre expression in Nes-Cre transgenic mice, we also crossed Mdm2 conditional Nes-Cre mice to the ROSA26 reporter mice (34). Cre-specific recombination at the ROSA26 locus allows expression of β-galactosidase. Robust and specific LacZ staining in the CNS was observed beginning at embryonic day (E)10.5, consistent with published data (33). At later stages, E12.5 (Fig. 1A) and E14.5 (data not shown), LacZ staining remained specific to the CNS.
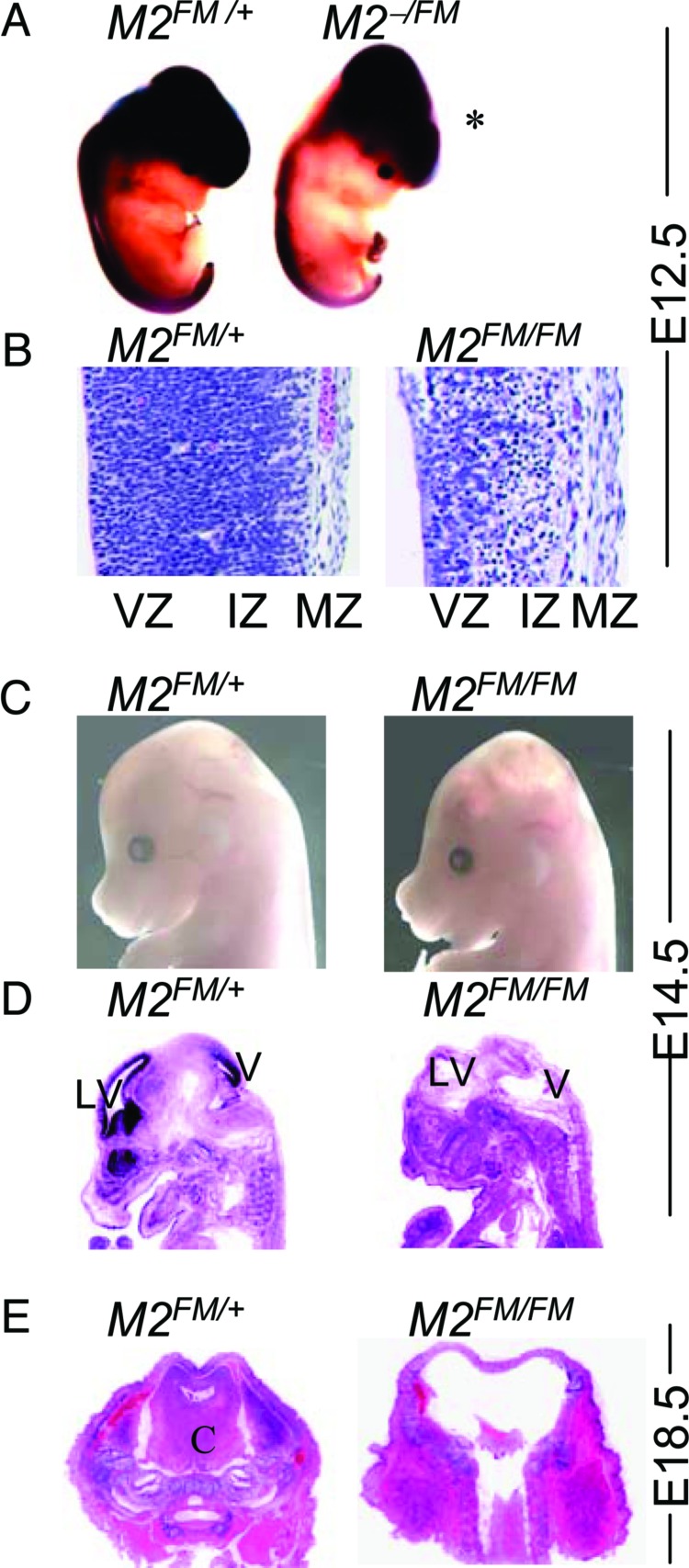
Fig. 1.
Hydranencephaly in mice lacking Mdm2 in the CNS. (A) Whole-mount LacZ staining of E12.5 embryos. (B) Hematoxylin/eosin (H&E)-stained sections of the forebrain at E12.5. (C) Embryos at E14.5. (D) Sagittal sections of embryos stained with H&E at E14.5. (E) Cross sections of E18.5 embryos. All embryos have the Nes-Cre transgene. The other alleles are labeled as follows: M2FM/+, Mdm2FM/+, M2FM/FM, Mdm2FM/FM, and M2−/FM, Mdm2−/FM; VZ, ventricular zone; IZ, intermediate zone; MZ, marginal zone; V, ventricle; LV, lateral ventricle; and C, cortex.
To determine whether Mdm2 is required during development of the CNS, we crossed Mdm2FM/FM mice (containing the Mdm2 conditional allele) to Mdm2FM/+ Nes-Cre mice. Among 44 mice born from this cross, no Mdm2FM/FM Nes-Cre mice were obtained at weaning (Table 1). However, on several occasions, pups with abnormal heads were born but died immediately thereafter. We therefore dissected embryos from the above cross. We observed a domed-head phenotype with decreased neuronal tissue throughout the brain compartment and especially in the hindbrain and spinal cord in Mdm2FM/FM Nes-Cre embryos (which sometimes also contained the ROSA26 locus) as early as E12.5 (Fig. 1A). From other crosses, we also generated Mdm2−/FM Nes-Cre mice so that recombination from a single Mdm2 allele would result in complete loss of Mdm2. These mice contained the same domed head phenotype and loss of neuronal cells, indicating the Cre-mediated recombination efficiently deleted both Mdm2 conditional alleles (data not shown). This phenotype was never observed in mice that had one wild-type Mdm2 allele and/or Nes-Cre. Sections through the embryonic brain revealed that the mutant embryos had much thinner cerebral cortexes at E12.5 (Fig. 1B). At this developmental stage, the cerebral cortex consists of ventricular and intermediate zones. Cells of the intermediate zone had lost their elongated morphology and appeared smaller and disorganized. The marginal zone was not affected in these embryos. At E14.5, all Mdm2FM/FM Nes-Cre mutant embryos clearly displayed an obvious domed head with excess cerebrospinal fluid in the brain, characterized as hydranencephaly (Fig. 1 C and D). Furthermore, the ventricular zone of CNS in these mutant brains was completely disordered (Fig. 1D). At E18.5, a normal Mendelian ratio of Mdm2FM/FM Nes-Cre embryos was still observed (Table 1), even though almost no neuronal tissue was left in the brain (Fig. 1E).
Table 1.
Deletion of Mdm2 (M2) in CNS causes neonatal lethality
| Age | Cross: M2FM/FM × M2FM/+ Nes-Cre |
|||||
|---|---|---|---|---|---|---|
| Phenotypes |
Genotypes* |
|||||
| Normal | Abnormal | M2FM/+ | M2FM/FM | M2FM/+ Nes-Cre | M2FM/FM Nes-Cre | |
| E12.5 | 36 | 5 | 11 | 10 | 10 | 10 |
| E14.5 | 12 | 4 | 4 | 5 | 3 | 4 |
| E18.5 | 24 | 10 | 9 | 7 | 8 | 10 |
| 3 weeks | 44 | 0 | 13 | 15 | 16 | 0 |
*All genotypes are expected at 25%.
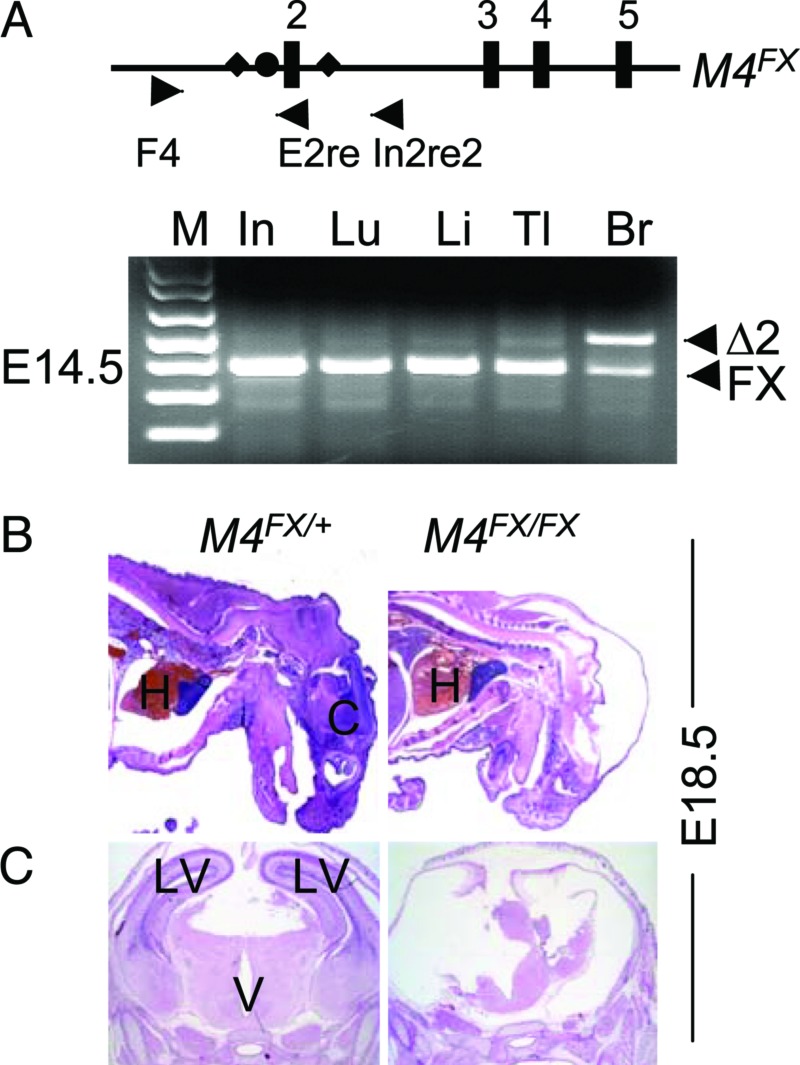
The same strategy was used to generate Mdm4-specific deletion in CNS. No Mdm4FX/FX Nes-Cre mice were obtained at weaning among 40 mice from a cross between Mdm4FX/FX and Mdm4FX/+ Nes-Cre mice (Table 2). When the embryos were examined from this cross, the Mdm4FX/FX Nes-Cre mice appeared to be normal at E14.5 (data not shown), even though PCR using DNA prepared from the whole embryo head (including some nonneuronal tissue) showed most of the conditional allele recombined at this stage (Fig. 2A). Embryos examined at later stages showed that deletion of Mdm4 did not cause obvious defects in the mutant embryos until E17.5 (Table 2). At this stage, the mutant embryos exhibited flat heads caused by the presence of a large cavity in the brain, characteristic of a proencephaly phenotype (data not shown). This phenotype was even more pronounced at E18.5 (Fig. 2 B and C). Thus, results from deletion of Mdm2 and Mdm4 alleles in the CNS clearly demonstrated that Mdm2 and Mdm4 were absolutely essential during the development of the CNS. Additionally, loss of the Mdm2 allele caused an earlier and more severe phenotype than loss of Mdm4.
Table 2.
Deletion of Mdm4 (M4) in CNS causes neonatal lethality
| Age | Cross: M4FX/FX × M4FX/+ Nes-Cre |
|||||
|---|---|---|---|---|---|---|
| Phenotypes |
Genotypes* |
|||||
| Normal | Abnormal | M4FX/+ | M4FX/FX | M4FX/+ Nes-Cre | M4FX/FX Nes-Cre | |
| E14.5 | 11 | 0 | 3 | 3 | 2 | 3 |
| E17.5 | 16 | 2 | 6 | 5 | 5 | 2 |
| E18.5 | 18 | 5 | 7 | 5 | 6 | 5 |
| 3 weeks | 40 | 0 | 10 | 15 | 15 | 0 |
*All genotypes are expected at 25%.
Fig. 2.
Proencephaly in mice lacking Mdm4 in the CNS. (A) Specific recombination of the Mdm4FX allele in E14.5 Mdm4FX/FX Nes-Cre embryos. Numbers label Mdm4 exons (boxes). The closed circle is an frt site, and diamonds are loxP sites. The triangles are PCR primers used for detection of the recombination event. F4 and E2re amplify the Mdm4 conditional allele. F4 and In2re2 amplify the Mdm4 allele (Δ2) after recombination. Hematoxylin/eosin-stained sagittal (B) and coronal (C) sections of embryos at E18.5. All embryos have the Nes-Cre transgene. M4FX/+, Mdm4FX/+, M4FX/FX, and Mdm4FX/FX; M, 1-kb-plus marker; In, intestine; Lu, Lung; Li, Liver; Tl, Tail; Br, Brain; H, Heart; C, cortex; V, ventricle; and LV, lateral ventricle.
Loss of Mdm2 or Mdm4 Induces Aberrant Apoptosis and Cell Proliferation.
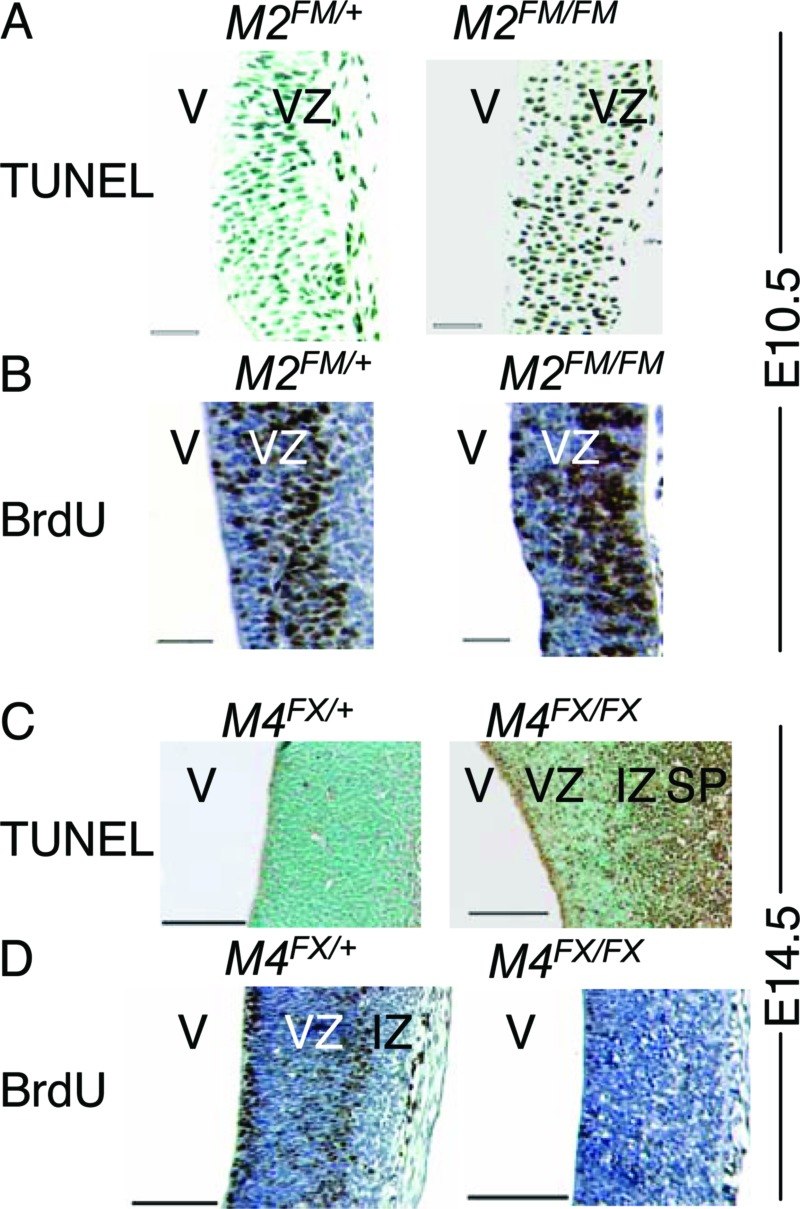
Loss of Mdm2 induces p53-dependent apoptosis at E3.5, whereas loss of Mdm4 causes p53-dependent cell cycle arrest at E7.5 (12, 15). A different allele of Mdm4 showed a delayed phenotype, with onset of cell cycle arrest and apoptosis in the neuroepithelium at E10.5 (14). We therefore, examined embryos for the mechanism of cell loss. Because Nes-Cre transgenic mice showed robust Cre recombinase activity as early as E10.5 (33), we examined whether loss of Mdm2 caused neuronal progenitor cells to undergo apoptosis or cell cycle arrest in sections of E10.5 embryos. Loss of Mdm2 induced massive apoptosis in the ventricular zone (Fig. 3A). By contrast, cell proliferation as measured by BrdUrd incorporation was the same between the mutant and the control mice (Fig. 3B). The only difference was that at this developmental stage, the location of BrdUrd-positive cells varied between mutant and control embryos. These data indicated that the hydranencephaly phenotype caused by loss of Mdm2 was due to increased apoptosis, consistent with previous observations in pluripotent cells from Mdm2-null embryos (15).
Fig. 3.
Apoptosis and proliferation in mice lacking Mdm2 or Mdm4 in the forebrain. TUNEL (A and C) and BrdUrd assays (B and D) in the CNS from E10.5 embryonic brains (A and B) from control Mdm2FM/+ Nes-Cre (M2FM/+) and mutant Mdm2FM/FM Nes-Cre (M2FM/FM) mice. E14.5 embryonic brains (C and D) from control Mdm4FX/+ Nes-Cre (M4FX/+) and mutant Mdm4FX/FX Nes-Cre (M4FX/FX) mice. VZ, ventricular zone; IZ, intermediate zone; SP, subplate; and V, ventricle. (Scale bars, 50 μm for A and B; 100 μm for C and D.)
Because deletion of Mdm4 in the CNS had a phenotype at E17.5, but Mdm4 loss was clearly apparent at E14.5, sections from E14.5 embryos were assayed for apoptosis and cell proliferation. Apoptosis was clearly increased in the mutant CNS (Fig. 3C). Additionally, when DNA synthesis was measured, loss of Mdm4 dramatically decreased BrdUrd staining in the ventricular zone (Fig. 3D). These data suggested that Mdm4 loss in the CNS actually induced both apoptosis and cell cycle arrest.
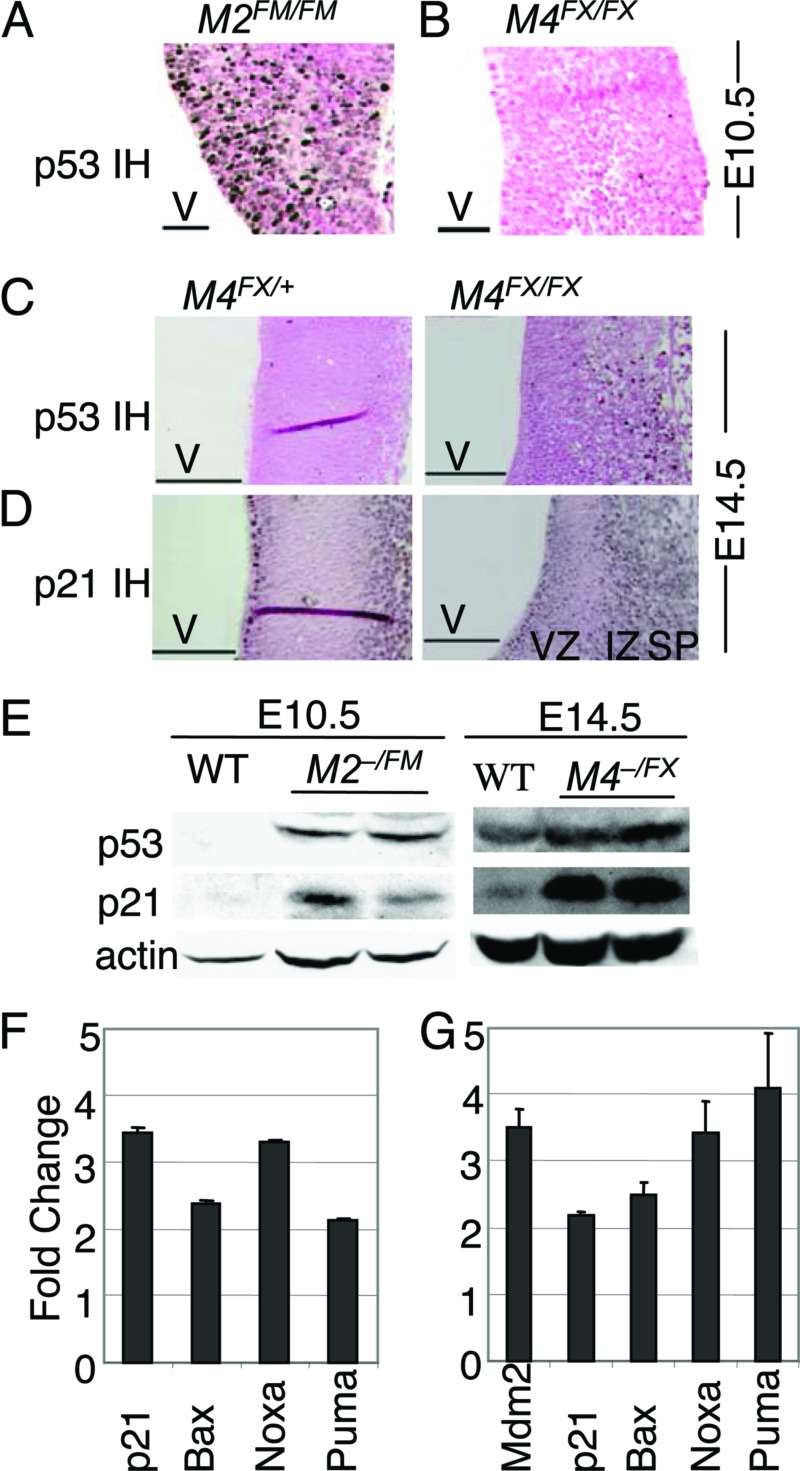
Mdm2 mediates p53 degradation through its E3 ubiquitin ligase activity (17). Although Mdm4 does not degrade p53, it interacts with Mdm2 to modulate p53 activity (24, 35). Therefore, to determine whether loss of Mdm2 or Mdm4 in the CNS caused elevation of p53 protein levels, immunohistochemistry was performed on brain sections from embryos. At E10.5, clear p53 staining was detected in Mdm2- but not Mdm4-null cells (Fig. 4A and B). Wild-type controls also do not stain with p53 antibodies (data not shown). However, when p53 staining was performed on E14.5 embryos, Mdm4-null cells also clearly had high levels of p53 (Fig. 4C). Mdm2-null embryos could not be analyzed at this developmental stage because of loss of most neuronal tissue. To further probe the functionality of increased p53 in cells lacking Mdm4, sections of embryos were immunostained with an antibody for p21, a p53 target. Again, clearly p21 levels were higher in the ventricular and intermediate zones although some background was visible in normal embryos (Fig. 4D). Western blot analyses of embryos confirmed the increase in p53 and p21 in Mdm2- and Mdm4-null CNS at E10.5 and E14.5, respectively (Fig. 4E). The increase in p53 levels in Mdm2-null embryos could not be quantitated because of the lack of detectable p53 in wild-type embryos at this stage. However, at E14.5, Mdm4-null embryos showed a 3- and 10-fold increase in p53 and p21 levels, respectively. Real-time PCR, performed with five different p53 targets, showed that p53 targets were also expressed at higher levels in Mdm2- and Mdm4-null CNS at these developmental stages (Fig. 4 F and G).
Fig. 4.
Loss of Mdm2 and Mdm4 caused increased p53 protein levels. p53 immunohistochemical staining of E10.5 (A and B) and E14.5 (C) sections of the forebrain. (D) p21 immunohistochemical staining of E14.5 embryonic brains. (E) Western blot analysis of embryo extracts. Real-time RT-PCR of Mdm2FM/FM Nes-Cre (F) and Mdm4FX/FX Nes-Cre (G) samples. V, ventricle; VZ, ventricular zone; IZ, intermediate zone; and SP, subplate. (Scale bars, 50 μm for A and B; 100 μm for C and D.)
Lethal Phenotypes from Loss of Mdm2 and Mdm4 in the CNS Are p53-Dependent.
Because Mdm2 and Mdm4 negatively regulate p53, and deletion of Mdm2 and Mdm4 in the CNS clearly showed elevated p53 activity, we asked whether the phenotypes due to loss of Mdm2 and Mdm4 in the CNS were p53-dependent. We therefore generated Mdm2FM/FM Nes-Cre p53−/− mice. Remarkably, the Mdm2FM/FM Nes-Cre p53−/− mice were born at the correct Mendelian ratio and had no obvious defects (Table 3). The same strategy was used to generate Mdm4FX/FX Nes-Cre p53−/− mice. These mice were also alive without apparent defects (Table 4). These data demonstrated that the neuronal defects induced by deletion of Mdm2 or Mdm4 were p53-dependent and indicated that Mdm2 and Mdm4 are essential negative regulators of p53 in CNS development.
Table 3.
Deletion of p53 rescues the lethality of Mdm2 (M2) loss
| Cross: M2+/FM p53+/−
Nes-Cre × M2FM/FM p53−/− genotypes* |
||||
|---|---|---|---|---|
| M2FM/+ | M2FM/FM | M2FM/+ Nes-Cre | M2FM/FM Nes-Cre | |
| p53+/− | 5 | 6 | 5 | 0 |
| p53−/− | 7 | 5 | 5 | 4 |
*All genotypes are expected at the same ratio.
Table 4.
Deletion of p53 rescues the lethality of Mdm4 (M4) loss
| Cross: M4+/FX p53+/− Nes-Cre × M4FX/FX p53−/− genotypes* |
||||
|---|---|---|---|---|
| M4FX/+ | M4FX/FX | M4FX/+ Nes-Cre | M4FX/FX Nes-Cre | |
| p53+/− | 11 | 15 | 16 | 0 |
| p53−/− | 14 | 11 | 9 | 6 |
*All genotypes are expected at the same ratio.
Synergistic Inhibition of p53 by Mdm2 and Mdm4.
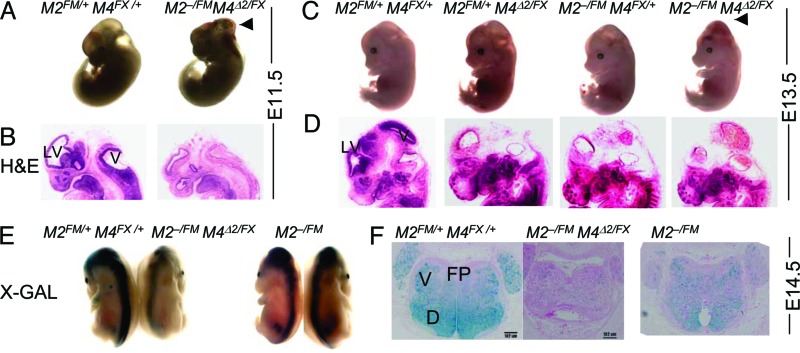
The relationship between Mdm2 and Mdm4 is complex. Mdm2 loss results in increased p53 stability (36, 37), but Mdm2 also binds Mdm4 and decreases stability of Mdm4 (38, 39). Mdm4 overexpression stabilizes p53 by inhibiting Mdm2-mediated degradation of p53 (20, 22). Modulation of p53 protein levels by Mdm4 could occur through Mdm2. However, whether Mdm2 and Mdm4 cooperate or independently regulate p53 is unknown. A requirement for both proteins in a single cell type has not been examined. Therefore, we generated mice null for both Mdm2 and Mdm4 in the CNS by crossing Mdm2+/− Mdm4Δ2/+Nes-Cre mice to Mdm2FM/FM Mdm4FX/FX mice. In this cross, 50% of the mice will have one conditional and one null allele for Mdm2 and Mdm4. Strikingly, mice lacking Mdm2 and Mdm4 in the CNS showed gross abnormalities at E11.5 (Fig. 5A and B), which was even earlier than the phenotype seen with loss of Mdm2 at E12.5 (Fig. 1 A and B). In addition, hemorrhaging in the CNS was visible at E11.5, a phenotype not observed in cells lacking either Mdm2 or Mdm4 (data not shown). At E13.5, embryos lacking Mdm2 and Mdm4 clearly showed a domed-head phenotype with obvious hemorrhaging in the brain (Fig. 5 C and D). These embryos died at E15.5, as measured by absence of movement and pale color, in contrast to loss of Mdm2 or Mdm4 alone, which yielded live pups at birth. When crossed to the ROSA26 reporter strain, Mdm2FM/− Mdm4FX/Δ2 Nes-Cre mice had fewer LacZ-positive cells in the hindbrain and spine than the Mdm2FM/− mice, indicating that loss of Mdm2 and Mdm4 caused cell depletion earlier than Mdm2 loss alone (Fig. 5 E and F).
Fig. 5.
Loss of Mdm2 and Mdm4 in the CNS caused an earlier and more severe phenotype than loss of either gene alone. Embryos and hematoxylin/eosin sections at E11.5 (A and B) and E13.5 (C and D) Arrows indicate hemorrhaging in the brain. Embryos at E14.5 were stained for LacZ and shown as whole embryos (E) and cross sections (F). M2, Mdm2; M4, Mdm4; D, dorsal; V, ventral; and FP, floor plate.
These crosses also produced mice with different combinations of Mdm2 and Mdm4 alleles in a Nes-Cre background. Mice lacking Mdm2 coupled with loss of one Mdm4 allele displayed ventricular defects and hemorrhaging in the CNS at E13.5, phenotypes that were more dramatic than Mdm2 loss alone (Fig. 5 C–E). Additionally, loss of Mdm4 with loss of one Mdm2 allele also had a more severe phenotype than loss of Mdm4 alone (Fig. 5 C–E). The severity of phenotypes was gradated with double null>Mdm2 null Mdm4 heterozygous>Mdm4 null Mdm2 heterozygous>Mdm2 null>Mdm4 null. These data suggest a gene dosage effect of Mdm2 and Mdm4 on inhibition of p53 activity. These data indicate that loss of both Mdm2 and Mdm4 contributed to a more severe phenotype and imply that Mdm4 together with Mdm2 contributed to inhibition of p53 in the same cell type. These observations strongly support the synergistic nature of Mdm2 and Mdm4 in the inhibition of p53 activity in the development of the CNS.
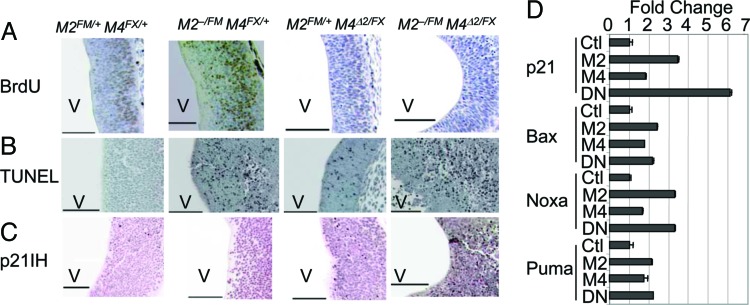
The proliferation defects distinguish Mdm2- from Mdm4-null cells in this and other systems (12, 14, 15, 36). Because Mdm4 loss but not Mdm2 loss showed a clear defect in proliferation, we examined embryo sections for cell cycle arrest. Remarkably, although cells lacking Mdm2 did not show a proliferation defect, cells lacking both Mdm2 and Mdm4 showed a clear cell cycle arrest phenotype similar to loss of Mdm4 alone (Fig. 6A). In addition, cells in embryos lacking both Mdm2 and Mdm4 still exhibited apoptosis similar to that of loss of Mdm2 (Fig. 6B). These data suggest Mdm4 has a unique function in inhibition of p53-dependent cell cycle arrest, and that it contributes to the overall inhibition of p53 activity independent of Mdm2. We also performed immunostaining of p21 in E10.5 embryonic sections and observed that only Mdm2FM/− Mdm4FX/Δ2 Nes-Cre mice had clear p21 expression in the midbrain at this time point, suggesting that loss of Mdm2 and Mdm4 activated p53 transcriptional activity even better than loss of Mdm2 or Mdm4 alone (Fig. 6C). This observation was supported by real-time RT-PCR of mice lacking Mdm2, Mdm4, or both genes in the CNS (Fig. 6D). Although increases in all p53 targets analyzed were clear, p21 was the only target that increased additively in mice lacking Mdm2 and Mdm4 in the CNS. These data strongly support the cooperative nature of Mdm2 and Mdm4 function in inhibition of p53 activity during the development of the CNS. Last, but important, the embryo lethal phenotype of Mdm2FM/− Mdm4FX/Δ2 Nes-Cre mice was completely rescued by deletion of p53.
Fig. 6.
Apoptosis and cell cycle arrest in embryos lacking Mdm2 and Mdm4 in the CNS. BrdUrd (A), TUNEL (B), and p21 immunohistochemical staining (C) of CNS sections of E10.5 embryos. (D) Real-time RT-PCR was performed by using RNA from heads of E10.5 wild-type (Ctl), Mdm2FM/FM Nes-Cre (M2), Mdm4FX/FX Nes-Cre (M4), and Mdm2FM/FM Mdm4FX/FX Nes-Cre (DN) embryos. V, ventricle. (Scale bars, 100 μm.)
Discussion
This study makes several observations. The first is that Mdm2 and Mdm4 were required for proper development of the CNS. Loss of Mdm2 yielded a dramatic phenotype with embryos exhibiting hydranencephaly as early as E12.5, whereas embryos lacking Mdm4 showed the first signs of gross defects at E17.5. Second, a synergistic effect was visible upon deletion of both Mdm2 and Mdm4. These data provide clear genetic support for independent function of Mdm4. Third, all defects are p53-dependent, affirming the important role of these negative regulators on p53 function. Importantly, these embryos were not subject to DNA-damaging agents and thus highlight the role of p53 in normal cell cycling.
Additionally, it is clear that the loss of Mdm2 results in a p53-dependent apoptotic phenotype in the developing CNS. Embryonic stem cells and mouse embryo fibroblasts also die by initiating apoptosis in the absence of Mdm2 (15, 36). Thus, no Mdm2-null cell is viable unless p53 is absent. In contrast, loss of Mdm4 yields a cell cycle arrest phenotype that is in some, but not all, cases accompanied by apoptosis (12, 14). This cell cycle arrest phenotype is not seen in Mdm2−/− cells regardless of developmental stage and as thus distinguishes Mdm2- from Mdm4-null cells. Possibly Mdm2 and Mdm4 bind different p53 molecules that are posttranslationally modified to regulate apoptosis or cell cycle arrest. The data clearly show a role for Mdm4 in inhibition of p53, complementing that of Mdm2. In line with this thought, loss of one additional Mdm2 allele in an Mdm4-null background showed a more severe phenotype than loss of Mdm4 alone, and vice versa, deletion of Mdm2 and one Mdm4 allele also showed a more severe phenotype than Mdm2-null cells alone. These data indicate that Mdm2 and Mdm4 have a gene dosage effect for inhibition of p53 activity, emphasizing that both have important roles in regulating p53. That both apoptosis and cell cycle arrest functions of p53 are important in tumor suppression has already been determined by analysis of a p53 mutant that differentiates between these activities (40).
Two additional negative inhibitors of p53 have been cloned, Pirh2 and Cop1 (5, 6). The importance of these regulators in embryogenesis is unknown, although studies in cells suggest they may cooperate with Mdm2 in ubiquitinating p53. Mdm2, Pirh2, and Cop1 encode E3 ubiquitin ligases that ultimately result in p53 degradation, whereas Mdm4 does not. Thus, Mdm4 may fine tune the transcriptional activity of p53. Differences in the mechanisms of p53 inactivation imply exquisite control at all levels. The presence of so many p53 inhibitors emphasizes the importance of monitoring and dampening p53 function, because its activity is so lethal to the cell.
Materials and Methods
Mice.
Nes-Cre transgene were purchased from The Jackson Laboratory. The Mdm2 and Mdm4 conditional alleles (labeled Mdm2FM and Mdm4FX, respectively) have been described (26, 27). Mdm4Δ2 represents the recombined Mdm4FX allele, which is due to loss of exon 2, the first coding exon. The Mdm2- and Mdm4-null alleles have been published (10, 27).
Embryo Analysis.
Mouse embryos at different developmental stages were stained for LacZ expression (41). For immunohistochemistry, embryos were fixed overnight in 10% buffered formalin. Immunohistochemistry for p53 or p21 was performed by using the CM5 antibody (Novocastra, Newcastle upon Tyne, U.K.) at a 1:200 dilution or Ab-9 (NeoMarkers, Fremont, CA) at a 1:80 dilution for 2 h at 37°C. ABC and DAB kits from Vector Laboratories were used for subsequent detection. For Western blots, CM5 (Novocastra) and C-19 antibodies (Santa Cruz Biotechnology) were used to detect p53 and p21, respectively. Total RNA was extracted from embryonic brains by using TRIzol reagent (Invitrogen). The first-strand cDNA synthesis kit (Amersham Pharmacia Bioscience) was used for reverse-transcriptase reactions. Real-time PCR was performed according to the manufacturer’s specifications (Applied Biosystems) with previously reported primers (42, 43).
Apoptosis and Proliferation Assays.
Apoptosis was assayed in paraffin-embedded sections by using the DNA Fragmentation Detection Kit (Oncogene Research Products, Cambridge, MA). For cell proliferation assays, pregnant females were injected i.p. with 100 μg/g body mass of BrdUrd (Zymed Laboratories) and killed 2 h later. Paraffin embedded sections were analyzed by using the Zymed BrdUrd staining kit.
Acknowledgments
We thank J. Parant and J. Grier for helpful discussions and Sara Amra for careful preparation of sections. This research was supported by National Institutes of Health Grants CA47296 (to G. Lozano) and CA16672 (to M. D. Anderson Cancer Center).
Abbreviations
- En
embryonic day n
- Nes-Cre
Nestin-Cre.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Vousden K. H., Prives C. Cell. 2005;120:7–10. doi: 10.1016/j.cell.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 2.Hollstein M., Hergenhahn M., Yang Q., Bartsch H., Wang Z. Q., Hainaut P. Mutat. Res. 1999;431:199–209. doi: 10.1016/s0027-5107(99)00162-1. [DOI] [PubMed] [Google Scholar]
- 3.Momand J., Zambetti G. P., Olson D. C., George D., Levine A. J. Cell. 1992;69:1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- 4.Shvarts A., Steegenga W. T., Riteco N., van Laar T., Dekker P., Bazuine M., van Ham R. C., van der Houven van Oordt W., Hateboer G., et al. EMBO J. 1996;15:5349–5357. [PMC free article] [PubMed] [Google Scholar]
- 5.Leng R. P., Lin Y., Ma W., Wu H., Lemmers B., Chung S., Parant J. M., Lozano G., Hakem R., Benchimol S. Cell. 2003;112:779–791. doi: 10.1016/s0092-8674(03)00193-4. [DOI] [PubMed] [Google Scholar]
- 6.Dornan D., Wertz I., Shimizu H., Arnott D., Frantz G. D., Dowd P., O’Rourke K., Koeppen H., Dixit V. M. Nature. 2004;429:86–92. doi: 10.1038/nature02514. [DOI] [PubMed] [Google Scholar]
- 7.Momand J., Wu H. H., Dasgupta G. Gene. 2000;242:15–29. doi: 10.1016/s0378-1119(99)00487-4. [DOI] [PubMed] [Google Scholar]
- 8.Evans S. C., Viswanathan M., Grier J. D., Narayana M., El-Naggar A. K., Lozano G. Oncogene. 2001;20:4041–4049. doi: 10.1038/sj.onc.1204533. [DOI] [PubMed] [Google Scholar]
- 9.Danovi D., Meulmeester E., Pasini D., Migliorini D., Capra M., Frenk R., de Graaf P., Francoz S., Gasparini P., Gobbi A., et al. Mol. Cell. Biol. 2004;24:5835–5843. doi: 10.1128/MCB.24.13.5835-5843.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montes de Oca Luna R., Wagner D. S., Lozano G. Nature. 1995;378:203–206. doi: 10.1038/378203a0. [DOI] [PubMed] [Google Scholar]
- 11.Jones S. N., Roe A. E., Donehower L. A., Bradley A. Nature. 1995;378:206–208. doi: 10.1038/378206a0. [DOI] [PubMed] [Google Scholar]
- 12.Parant J., Chavez-Reyes A., Little N. A., Yan W., Reinke V., Jochemsen A. G., Lozano G. Nat. Genet. 2001;29:92–95. doi: 10.1038/ng714. [DOI] [PubMed] [Google Scholar]
- 13.Finch R. A., Donoviel D. B., Potter D., Shi M., Fan A., Freed D. D., Wang C. Y., Zambrowicz B. P., Ramirez-Solis R., Sands A. T., et al. Cancer Res. 2002;62:3221–3225. [PubMed] [Google Scholar]
- 14.Migliorini D., Denchi E. L., Danovi D., Jochemsen A., Capillo M., Gobbi A., Helin K., Pelicci P. G., Marine J. C. Mol. Cell. Biol. 2002;22:5527–5538. doi: 10.1128/MCB.22.15.5527-5538.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chavez-Reyes A., Parant J. M., Amelse L. L., de Oca Luna R. M., Korsmeyer S. J., Lozano G. Cancer Res. 2003;63:8664–8669. [PubMed] [Google Scholar]
- 16.Haupt Y., Maya R., Kazaz A., Oren M. Nature. 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 17.Honda R., Tanaka H., Yasuda H. FEBS Lett. 1997;420:25–27. doi: 10.1016/s0014-5793(97)01480-4. [DOI] [PubMed] [Google Scholar]
- 18.Kubbutat M. H., Jones S. N., Vousden K. H. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 19.Fang S., Jensen J. P., Ludwig R. L., Vousden K. H., Weissman A. M. J. Biol. Chem. 2000;275:8945–8951. doi: 10.1074/jbc.275.12.8945. [DOI] [PubMed] [Google Scholar]
- 20.Jackson M. W., Berberich S. J. Mol. Cell. Biol. 2000;20:1001–1007. doi: 10.1128/mcb.20.3.1001-1007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Little N. A., Jochemsen A. G. Oncogene. 2001;20:4576–4580. doi: 10.1038/sj.onc.1204615. [DOI] [PubMed] [Google Scholar]
- 22.Stad R., Little N. A., Xirodimas D. P., Frenk R., van der Eb A. J., Lane D. P., Saville M. K., Jochemsen A. G. EMBO Rep. 2001;2:1029–1034. doi: 10.1093/embo-reports/kve227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bottger V., Bottger A., Garcia-Echeverria C., Ramos Y. F., van der Eb A. J., Jochemsen A. G., Lane D. P. Oncogene. 1999;18:189–199. doi: 10.1038/sj.onc.1202281. [DOI] [PubMed] [Google Scholar]
- 24.Sharp D. A., Kratowicz S. A., Sank M. J., George D. L. J. Biol. Chem. 1999;274:38189–38196. doi: 10.1074/jbc.274.53.38189. [DOI] [PubMed] [Google Scholar]
- 25.Tanimura S., Ohtsuka S., Mitsui K., Shirouzu K., Yoshimura A., Ohtsubo M. FEBS Lett. 1999;447:5–9. doi: 10.1016/s0014-5793(99)00254-9. [DOI] [PubMed] [Google Scholar]
- 26.Grier J. D., Yan W., Lozano G. Genesis. 2002;32:145–147. doi: 10.1002/gene.10066. [DOI] [PubMed] [Google Scholar]
- 27.Grier J. D., Xiong S., Elizondo-Fraire A. C., Parant J. M., Lozano G. Mol. Cell. Biol. 2006;26:192–198. doi: 10.1128/MCB.26.1.192-198.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herzog K. H., Chong M. J., Kapsetaki M., Morgan J. I., McKinnon P. J. Science. 1998;280:1089–1091. doi: 10.1126/science.280.5366.1089. [DOI] [PubMed] [Google Scholar]
- 29.O’Rourke N. A., Sullivan D. P., Kaznowski C. E., Jacobs A. A., McConnell S. K. Development (Cambridge, U.K.) 1995;121:2165–2176. doi: 10.1242/dev.121.7.2165. [DOI] [PubMed] [Google Scholar]
- 30.Noctor S. C., Martinez-Cerdeno V., Ivic L., Kriegstein A. R. Nat. Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- 31.de Oca Luna R. M., Tabor A. D., Eberspaecher H., Hulboy D. L., Worth L. L., Colman M. S., Finlay C. A., Lozano G. Genomics. 1996;33:352–357. doi: 10.1006/geno.1996.0210. [DOI] [PubMed] [Google Scholar]
- 32.Leveillard T., Gorry P., Niederreither K., Wasylyk B. Mech. Dev. 1998;74:189–193. doi: 10.1016/s0925-4773(98)00074-4. [DOI] [PubMed] [Google Scholar]
- 33.Graus-Porta D., Blaess S., Senften M., Littlewood-Evans A., Damsky C., Huang Z., Orban P., Klein R., Schittny J. C., Muller U. Neuron. 2001;31:367–379. doi: 10.1016/s0896-6273(01)00374-9. [DOI] [PubMed] [Google Scholar]
- 34.Soriano P. Nat. Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 35.Stad R., Ramos Y. F., Little N., Grivell S., Attema J., van Der Eb A. J., Jochemsen A. G. J. Biol. Chem. 2000;275:28039–28044. doi: 10.1074/jbc.M003496200. [DOI] [PubMed] [Google Scholar]
- 36.de Rozieres S., Maya R., Oren M., Lozano G. Oncogene. 2000;19:1691–1697. doi: 10.1038/sj.onc.1203468. [DOI] [PubMed] [Google Scholar]
- 37.Linares L. K., Hengstermann A., Ciechanover A., Muller S., Scheffner M. Proc. Natl. Acad. Sci. USA. 2003;100:12009–12014. doi: 10.1073/pnas.2030930100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Graaf P., Little N. A., Ramos Y. F., Meulmeester E., Letteboer S. J., Jochemsen A. G. J. Biol. Chem. 2003;278:38315–38324. doi: 10.1074/jbc.M213034200. [DOI] [PubMed] [Google Scholar]
- 39.Pan Y., Chen J. Mol. Cell. Biol. 2003;23:5113–5121. doi: 10.1128/MCB.23.15.5113-5121.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu G., Parant J. M., Lang G., Chau P., Chavez-Reyes A., El-Naggar A. K., Multani A., Chang S., Lozano G. Nat. Genet. 2004;36:63–68. doi: 10.1038/ng1282. [DOI] [PubMed] [Google Scholar]
- 41.Carroll P. M., Tsirka S. E., Richards W. G., Frohman M. A., Strickland S. Development (Cambridge, U.K.) 1994;120:3173–3183. doi: 10.1242/dev.120.11.3173. [DOI] [PubMed] [Google Scholar]
- 42.Bruins W., Zwart E., Attardi L. D., Iwakuma T., Hoogervorst E. M., Beems R. B., Miranda B., van Oostrom C. T., van den Berg J., van den Aardweg G. J., et al. Mol. Cell. Biol. 2004;24:8884–8894. doi: 10.1128/MCB.24.20.8884-8894.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu W. S., Heinrichs S., Xu D., Garrison S. P., Zambetti G. P., Adams J. M., Look A. T. Cell. 2005;123:641–653. doi: 10.1016/j.cell.2005.09.029. [DOI] [PubMed] [Google Scholar]