Abstract
Cryptically colored prey species are often polymorphic, occurring in multiple distinctive pattern variants. Visual predators promote such phenotypic variation through apostatic selection, in which they attack more abundant prey types disproportionately often. In heterogeneous environments, disruptive selection to match the coloration of disparate habitat patches could also produce polymorphism, but how apostatic and disruptive selection interact in these circumstances is unknown. Here we report the first controlled selection experiment on the evolution of prey coloration on heterogeneous backgrounds, in which blue jays (Cyanocitta cristata) searched for digital moths on mixtures of dark and light patches at three different scales of heterogeneity. As predicted by ecological theory, coarse-grained backgrounds produced a functional dimorphism of specialists on the two patch types; fine-grained backgrounds produced generalists. The searching strategies of the jays also varied with the habitat configuration, however. Complex backgrounds with many moth-like features elicited a slow, serial search that depended heavily on selective attention. The result was increased apostatic selection, producing a broad range of moth phenotypes. Backgrounds with larger, more uniform patches allowed the birds to focus on the currently most rewarding patch type and to search entire patches rapidly in parallel. The result was less apostatic selection and lower phenotypic variability. The evolution of polymorphism in camouflaged prey depends on a complex interaction between habitat structure and predator cognition.
Keywords: apostatic selection, parallel vs. serial search, prey crypticity, selective attention, specialists vs. generalists
Color polymorphism is common among camouflaged prey species, such as stick insects (1), land snails (2), locusts (3), tree frogs (4), crab spiders (5), and water boatmen (6). Cryptic moths that rest on tree trunks during the day are frequently polymorphic, with some species occurring in up to nine distinctive forms (7–9). The evolution of color polymorphism is presumably driven, at least in part, by the searching behavior of visual predators. Predation can, however, influence prey coloration in a variety of different ways. Color patterns that closely match the background evolve by directional selection, but visual search for cryptic prey items is optimized when predators use searching images, focusing their attention on recently or commonly encountered prey types and effectively ignoring the alternatives (10–12). The use of searching images, in turn, results in frequency-dependent, apostatic selection, which promotes increased phenotypic variance and stabilizes existing polymorphisms (13–15).
Another selective influence is provided by the visual environment. For most prey species, the environment is heterogeneous in appearance, consisting of mosaics of patches that exhibit contrasting distributions of color or pattern (16). Because camouflage depends on achieving a sufficient resemblance to the background, these disparate patches effectively constitute ecological niches, distinctive regimes to which the appearance of the prey can be adapted. Under some circumstances, heterogeneous environments should promote disruptive selection, generating polymorphic specialists on each of the available patch types (17). Theory predicts that disruptive selection will vary with both the differences between niches and what Levins (18) has termed the “grain” of the habitat. Habitat grain is a function of the proportion of time individuals spend in regions characterized by contrasting adaptive regimes. When there is little overlap between niches, coarse-grained habitats, in which many individuals experience only a single adaptive regime, will select for ecological specialists. Fine-grained habitats, in which most individuals experience selection on all adaptive regimes, will select for generalists that are equally adapted to all alternatives (17–19).
Previous studies of the effect of spatial heterogeneity on color polymorphism have compared mortality rates on different backgrounds (1, 6, 20) or demonstrated that prey preferentially settle on backgrounds that match their coloration (21, 22). Other studies have quantified predator responses to fixed, artificial stimuli placed on a range of different backgrounds (23, 24). Controlled-selection experiments that manipulated habitat grain and tested the consequences for the evolution of phenotypic diversity are rarely encountered in the literature (25), however, and have never been conducted on predator–prey systems, presumably because of the difficulty of evoking the dynamic interplay between predator behavior and prey appearance under controlled conditions. To address this problem, we developed a “virtual ecology,” in which captive blue jays hunted for artificial, digital moths on computer displays.
Blue jays commonly prey on cryptically colored moths in the wild (7), and the results from laboratory emulations of this natural predator–prey system bear a strong functional resemblance to behavior observed in the field (26). Our previous work with digital moths has shown that blue jays searching for a set of fixed prey types show clear indications of hunting by searching image (11) and that the resulting frequency-dependent, apostatic selection serves to maintain stable prey polymorphism (14). When moth phenotypes are variable, evolving in response to predation pressure, the jays are much less likely to detect atypical cryptic moths, displaying apostatic selection even under conditions of high moth variability. Over successive generations, evolving moths show significantly greater phenotypic variance than unselected controls, indicating that apostatic selection encourages the evolution of phenotypic diversity (27). Here we report a new set of results from this system, comprising the first controlled selection experiment on the evolution of prey polymorphism in heterogeneous habitats.
Virtual Ecology
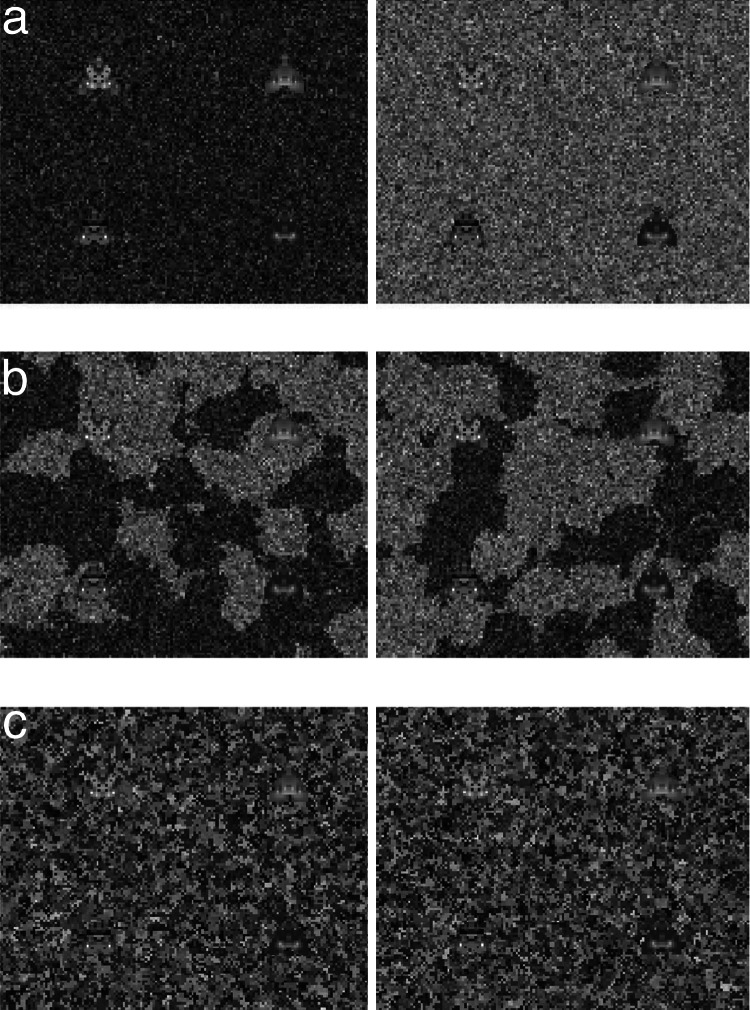
Digital moths were bilaterally symmetrical triangles with an often complex pattern of grayscale pixels on their wings (Fig. 1). Moth phenotypes were specified by virtual chromosomes through a developmental algorithm based on salient features of lepidopteran genetics (refs. 28 and 29; see Materials and Methods). Phenotypic traits were polygenic, in that the intensity of any given pixel was the result of additive interactions among a large number of loci. Moth images were displayed on a complex, granular background divided into two lateral fields. Half of the pixels in the display fields were drawn from each of two normal generating distributions, defining two visual niches that differed in mean pixel intensity. Depending on the experimental treatment, these light and dark patches were intermixed at different scales of heterogeneity. In the disjunct treatment, each background field was drawn from one of the two distributions, creating patches that were ≈15 times the size of a moth. In the mottled treatment, the two distributions were coarsely mixed across both fields, resulting in patches that were about the same size as a moth. In the speckled treatment, the two distributions were finely intermixed, resulting in patches that were ≈1/12th the size of a moth (Fig. 1).
Fig. 1.
Four digital moths shown on a sample of each of the three treatment backgrounds, in which the same dark and light pixel distributions are intermixed at progressively finer spatial scales. The moths in this figure evolved on the disjunct background and were among the most cryptic of the individuals in their population. Note that in the disjunct treatment (a), the moths are somewhat harder to detect on the patch that they most closely resemble but that all four can readily be located in a superficial scan. In the mottled (b) and speckled (c) treatments, the backgrounds incorporate high levels of noise at spatial frequencies comparable to the size of moths, and the moths are far more difficult to detect.
Three squads of six jays each were tested in three different operant chambers (see Materials and Methods). Each bird received a series of 160 predation trials per day. On half of the trials, one moth was placed in a randomly chosen position in one of the two fields of cryptic background. On the remaining, negative trials, only the background fields were shown. If the jay correctly detected a moth and pecked at it, it was rewarded with a food pellet; if the jay failed to find a moth, it pecked a central green disk, and the next trial was initiated almost immediately. These contingencies emulate natural foraging behavior and have been used with considerable success in previous studies (11, 14, 26, 27). For each trial, we recorded which moth was displayed, which patch type it was placed on, whether it was correctly detected, and how long the bird required to make a response.
An initial, monomorphic moth population was generated from a template that was roughly equally cryptic on all experimental backgrounds. Moth populations were held to a constant density of 200 individuals, a “soft selection” model, which should encourage the evolution of stable dimorphisms (19). In the course of each successive generation, each moth in the population was presented once to each of two jays. After all trials were completed, the accuracy and latency of the birds’ responses were entered into the selection algorithm (see Materials and Methods). Reproduction entailed choosing two chromosomes from the population at random and recombining them into a single progeny genome, which was then subjected to a mutation process that randomly inverted individual bits. Selection, recombination, and mutation steps were repeated until 200 progeny had been obtained. The parental population was then replaced with the new individuals, and the progeny were presented to jays in subsequent trials. Beginning each time with the same parental population, we produced three successive experimental lineages, continuing through the F100 generation, using each squad of jays. All squads were given all of the background treatments, in Latin-square order. Our design thus contrasted the selective effects of jay predation in three replicate lineages within each of three experimental regimens.
Results
Fitness Set Analysis.
The difference between the means of the generating distributions was 2.5 times their common standard deviation (light, μ = 33.5; dark, μ = 12.8; δ = 8.1). For sufficiently coarse-grained habitats, this degree of separation should suffice to ensure a “concave” fitness set (17–19) in which the evolution of specialist phenotypes will be promoted. The speckled treatment constituted a fine-grained habitat, in that all individuals necessarily experienced a mixture of patch types. Because each moth was displayed to two different jays at random positions in the background fields, half of the moths in our disjunct and mottled treatments experienced selection on only one patch type, insuring that these populations were exposed to a relatively coarse-grained habitat. We would thus predict that the disjunct and mottled treatments should tend to select for dimorphic specialists on the two patch types, whereas the speckled treatment should produce monomorphic generalists.
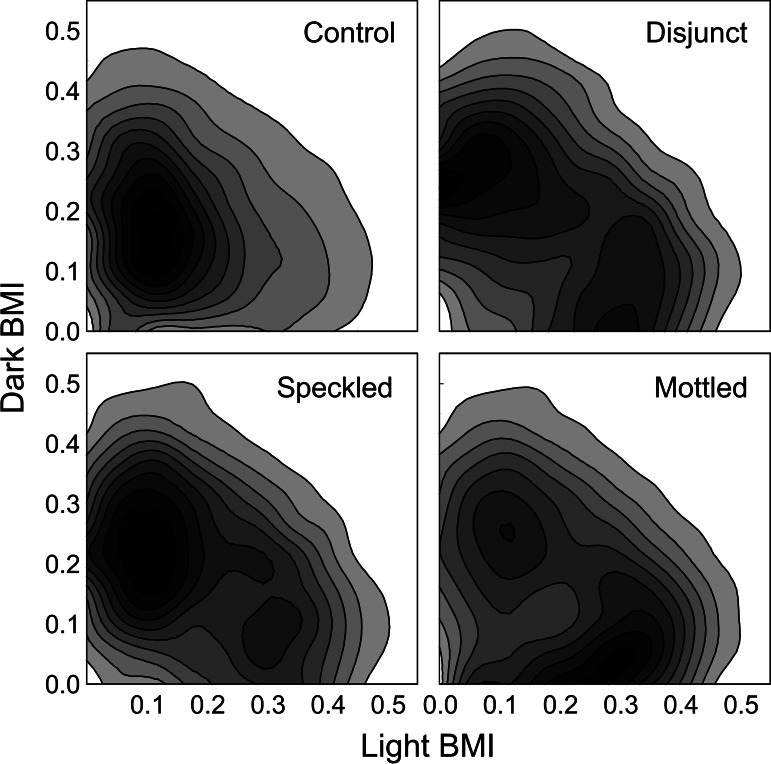
The degree of ecological specialization was displayed by plotting the location of each moth in the criterial populations in a niche space defined by its dark and light patch-level matching indices (see Materials and Methods) and contrasting the experimental results to a set of nonselected lineages in which the probability of being chosen to breed was uniform across the moth population, irrespective of phenotype (Fig. 2). To evaluate the treatment differences quantitatively, we partitioned the niche space radially into three regions of equal area: a central, generalist region that spanned the principal diagonal; a peripheral, specialist region of two equal-sized segments adjacent to the axes; and an intermediate region that was excluded from the analysis. Specialists, thus, were moths in which the matching index for one patch type was more than five times that for the other type; generalists were moths in which the matching index for either patch type was no more than twice that for the other.
Fig. 2.
Fitness sets in a niche space defined by dark and light matching indices, displayed as contour plots of the phenotypes of all moths in all lineages from the 50th through the 100th generations. Data resulting from selection on each of the three experimental backgrounds are contrasted with the results of a nonselective, control process. Note that both the disjunct and mottled treatments produced bimodal, concave fitness sets with peak densities of moths along the axes, dividing the population into dark and light specialists. The speckled treatment produced a mostly convex fitness set that was more cryptic than the controls but not significantly dimorphic.
The mottled and disjunct treatments both produced ≈25% fewer generalist moths than the control process [t(101) ≥ 2.7, P < 0.01] and more than twice as many specialists [t(101) ≥ 4.1, P < 0.0001]. The speckled treatment also produced fewer generalists and more specialists than controls, but the differences were not as strong [t(101) ≥ 1.83, P > 0.07]. Within experimental treatments, the mottled and disjunct backgrounds each produced fewer generalists than the speckled [F(1,72) ≥ 5.02, P < 0.03] and substantially more specialists [F(1,72) ≥ 7.49, P < 0.008]. There were, however, no significant differences between disjunct and mottled in mean numbers of moths in either category [F(1,72) ≤ 0.25, P > 0.6]. The results were, thus, in general accord with predictions from ecological theory: The disjunct and mottled treatments produced a division of the population into dark and light specialists, whereas the speckled treatment produced generalists with a single primary mode.
Phenotypic Variation.
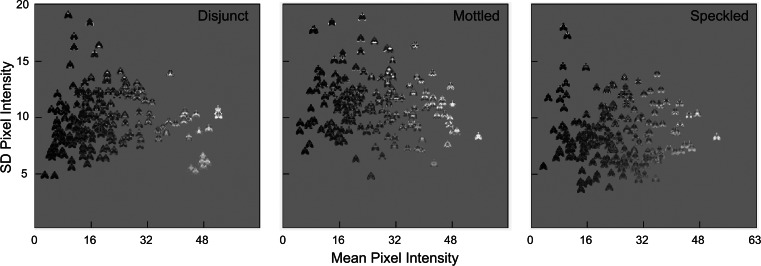
The magnitude of the dimorphism in niche space does not necessarily reflect the degree of phenotypic variability, however, because many different color patterns can produce the same level of background resemblance. A sense of the characteristic phenotypic differences among treatments is provided by displaying moths in a two-dimensional projection of phenotypic space, with the mean pixel color of each moth along the abscissa and the standard deviation of pixel color on the ordinate (Fig. 3). In these three typical populations, the speckled treatment produced a loose cluster of generalists intermediate between the peaks of the light and dark distributions. The disjunct treatment was strongly dimorphic along the abscissa, with a tight cluster of dark moths clearly separated from a cluster of light ones. The mottled treatment was comparable in variance to the disjunct along the abscissa, but the variance along the ordinate appeared to be much larger.
Fig. 3.
Distribution of moths in phenotypic space, from typical populations resulting from each of the three background treatments. The mean pixel color of each moth is plotted along the abscissa, and the standard deviation of pixel color is plotted on the ordinate. Thus, darker moths are to the left, lighter ones are to the right, more uniform moths are toward the bottom, and more diversely colored ones are at the top. The speckled population consists mainly of generalist moths that are intermediate in mean pixel color but that are relatively variable along the ordinate, reflecting high levels of apostatic selection. The disjunct population shows strong dimorphism along the mean color axis (due to disruptive selection for crypticity on disparate backgrounds) but less apostatic variation. Mottled moths exhibit the combined effects of both apostatic and disruptive selection.
To confirm the generality of these treatment differences, we calculated the variance in mean and standard deviation of pixel color within each criterial population in all lineages. Autocorrelation analysis indicated that there was no significant relationship between successive populations in these two variables at lags greater than four generations [DW(5) ≥ 1.47, P > 0.07], so we restricted our analysis to every fifth generation in each experimental lineage (30). Speckled treatment populations displayed significantly lower variance in mean pixel color than either disjunct or mottled [respective means 82.8, 109.8, and 113.7; F(1,90) ≥ 24.3, P < 0.0001], but there was no difference between the disjunct and mottled treatments [F(1,90) = 0.5, P > 0.4]. Along the standard deviation axis, in contrast, disjunct treatment populations displayed significantly lower variance than either speckled or mottled [respective means 9.21, 12.5, and 11.1; F(1,90) ≥ 10.7, P < 0.002], but there was no difference between the speckled and mottled treatments [F(1,90) = 0.01, P > 0.9]. The distinctions that are apparent in our three exemplar populations (Fig. 3), therefore, appear to be characteristic of the effects of the three treatments: Speckled backgrounds produced lower phenotypic variance along the mean color axis, as might be expected from the fitness set analysis. However, disjunct and mottled treatments, despite producing similar levels of functional dimorphism, had clearly distinguishable effects along the standard deviation axis, suggesting that these two treatments may have elicited different kinds of predatory search. We therefore undertook additional analyses to explore the source of the differences between the disjunct and mottled treatments.
Heterogeneity and Visual Search.
We tested for treatment effects on visual search using accuracy and response time measures for individual moths. To obtain consistent measures, we pooled moths from all criterial populations and sorted them sequentially by field-level matching index into groups of 100. The mean accuracy and mean log response time for correct detections were determined for each group, and the grouped results were subjected to linear regression analysis. Disjunct background moths were more readily detected than those from mottled backgrounds, even at the same level of background matching. The intercept and slope for disjunct were 0.84 and −0.17, respectively (r2 = 0.05), whereas the comparable values for mottled were 0.70 and −0.56 (r2 = 0.40). Analysis of covariance indicated a significant treatment difference in slope [F(1,1807) = 87.2, P < 0.001].
Detection accuracy was, thus, higher on disjunct backgrounds than on mottled ones, even at the same matching index levels. This difference may reflect treatment differences in the levels of noise at spatial frequencies comparable to the size of the moths. Because disjunct backgrounds include few such distracting components, many moths, irrespective of their matching indices, can readily be detected in a global scan for pattern anomalies, allowing an effectively parallel search of the display (see Fig. 1). On mottled backgrounds, the higher levels of noise at moderate spatial frequencies may have forced the birds to conduct a serial visual search, examining each part of the display in succession (31–33). These two searching mechanisms can most readily be distinguished in the relationship between accuracy and response latency. During a parallel search, the entire field is scanned at once, so accuracy is essentially independent of latency (34, 35). Serial searches, on the other hand, require a gradual accumulation of information until a decision criterion is reached (36). In serial tasks without an imposed time limit, easy stimuli are detected rapidly and accurately; more difficult ones are found both more slowly and more unreliably (37). Thus, serial searches will show a strong inverse relationship between accuracy and latency.
When detection accuracy was plotted as a function of log response time, the regression line for the disjunct treatment was effectively parallel to the abscissa (slope = −0.05; r2 = 0.008), whereas the mottled regression showed a significant negative relationship (slope = −0.35, r2 = 0.24). Analysis of covariance confirmed the difference between treatment slopes [F(1,1807) = 64.2, P < 0.0001]. These results are consistent with the hypothesized difference between treatments in the search mechanism, and they provide one of the few clear demonstrations of a predicted distinction between serial and parallel searching in nonhuman subjects (38).
Heterogeneity and Apostatic Selection.
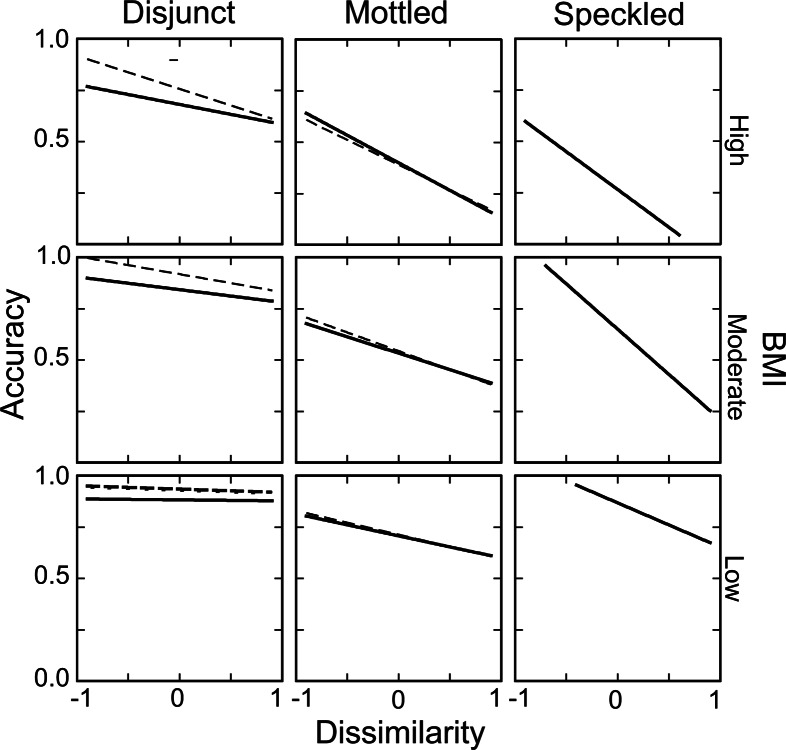
The effects of hunting by searching image are generally apparent only when the detection task is sufficiently difficult (39, 40). Only a serial search process is materially enhanced by selective attention to particular stimulus features. We might, therefore, expect that the differences between treatments in the mechanism of visual search would have effects on the use of searching images and the magnitude of apostatic selection. We sorted the pool of moths from each treatment by field-level matching index and aggregated them into groups of 100, this time determining for each group the detection accuracy and the average phenotypic disparity (as “taxonomic distance”; ref. 41) between the given moth and the last correctly detected one. The grouped results were separated into categories of low, medium, and high matching index and subjected to linear regression analysis, comparing slopes among matching index categories (27). For the disjunct and mottled treatments, there was an additional dimension: The previous and current moths could have been presented on the same patch type or on different patch types. Because of the demonstrated differential effects of heterogeneity on the mechanism of visual search, we particularly wished to test whether same vs. different patch type had an impact on the use of searching images.
Hunting by searching image entails that birds should be more accurate in detecting moths that are similar to others they had recently found. The criterion for searching image, thus, was a significant negative slope to the regression of accuracy on the phenotypic disparity between successive moths. We first analyzed for this effect in cases in which both moths occurred on the same patch type in all three background treatments (Fig. 4, solid lines). Regression slopes on patches of the same type were significantly negative across all matching index groupings [t(92) ≤ 7.49, P < 0.0001]. Slopes also decreased significantly as a function of matching index in all treatments [F(1,284) ≥ 4.13, P < 0.02], indicating that searching image effects were stronger for more difficult stimuli. The effect of matching index was strikingly stronger for mottled and speckled treatments than for disjunct [Fig. 4; F(1,867) ≥ 390.3, P < 0.0001]; there was no significant difference, in this regard, between mottled and speckled [F(1,867) = 0.39, P > 0.5]. We then analyzed for slope effects as a function of same vs. different patch type in the mottled and disjunct treatments (Fig. 4, dashed lines). There was no effect of patch type in the mottled treatment [F(1,568) = 2.48, P > 0.1], but there was a clear difference between patch type categories in the disjunct treatment, where successive moths shown on different patch types invariably displayed steeper slopes and higher intercepts than those shown on the same type [F(1,576) = 58.9, P < 0.0001].
Fig. 4.
Detection accuracy in blocks of 100 trials as a function of the matching index of the target moth and the dissimilarity between the target and the last previous correctly detected moth. Matching index increases from the bottom to the top, dividing the range of indices within each treatment into percentile groupings: low (0–33rd), medium (34–66th), and high (67–100th). Regression lines indicate the relationship between accuracy and dissimilarity within matching index groupings. Solid lines show results from trials in which both the target moth and the previous one occurred on the same patch type; dashed lines indicate results from moths occurring on different patch types.
What caused the patch type difference in the disjunct treatment? It seems likely that when the two entire fields were drawn from different pixel distributions at least some of the jays developed a transitory preference for one or the other patch type based on their recent history of reward (12, 42). The resulting bias in their searching effort would necessarily reduce their accuracy in detecting moths on the less preferred patch. If this hypothesis is correct, we would expect that jays in the disjunct treatment would show greater asymmetry in their detection performance than those in the mottled, showing significantly higher accuracy toward one patch type than toward the other. We analyzed the accuracy of each individual bird’s responses in the criterial populations under disjunct and mottled treatments, separating moths that were shown on the dark patch type from those on the light. The distribution of asymmetry across birds was highly nonnormal, so we compared the two experimental treatments using a Wilcoxon two-sample test. Birds responded significantly more asymmetrically to the disjunct than to the mottled displays (W+ = 517, P < 0.02).
Discussion
When many moths in each population experienced only a single patch type [“coarse-grained” habitats, in Levins’ (18) sense] disruptive selection produced dimorphic populations of ecological specialists; when all moths experienced both patch types (“fine-grained” habitats) we obtained monomorphic generalists. These results offer a striking confirmation of theoretical models of the effects of habitat grain on fitness tradeoffs, one of the few selection experiments in which manipulation of habitat grain alone has sufficed to both promote and inhibit the evolution of niche diversity (25). The results also support the common assessment that color polymorphism in cryptic prey species is, at least in part, a consequence of disruptive selection.
The scale of heterogeneity exerted additional selective effects, however, which were independent of habitat grain and mediated by differences in how predators searched for and detected prey items. High phenotypic variance in the speckled and mottled treatments appeared to be due to background features that were comparable in spatial frequency to those shown by the moths. These treatments required a slower, serial search process in which selective attention played a major role in enhancing detection. The result was increased apostatic selection, producing a broad range of moth phenotypes orthogonal to the primary dimension that distinguished patch types. Phenotypic variance was reduced in the disjunct treatment, where the separation of the background into large, coherent patches allowed the jays to maintain a high rate of detection by focusing on the currently most rewarding patch type and searching entire fields in parallel.
The extent of disruptive selection, which determines consistency and distinctiveness in the array of prey phenotypes, thus appears to be substantially affected by cognitive processes in the predator. Disjunct and mottled treatments produced equivalent numbers of ecological specialists, but apostatic selection on the mottled backgrounds increased phenotypic variance, resulting in less coherent clusters of moth phenotypes. The effects of the two selective factors in the mottled treatment appear to have operated orthogonally: Much of the increased variance due to apostatic selection was channeled into portions of phenotypic space that did not disturb the functional correspondence to the background distributions. Similar processes may be involved in the generation and maintenance of polymorphism in species, such as locusts (21) or land snails (2), that are found in a range of different patch types but that occur in multiple forms even within individual patches.
Although spatial heterogeneity can promote ecological diversity, our results clearly show that heterogeneity alone does not necessarily produce a classical, discrete polymorphism with a limited number of highly distinctive forms (43). It is possible that selection for discrete polymorphism may not readily be maintained in the absence of active habitat selection by the prey, that disruptive selection may function mainly in association with a bias toward choosing an appropriate resting substrate. This idea has been discussed extensively in the theoretical literature, where habitat selection has been shown to select for stable polymorphism and even sympatric speciation over a broad range of parameters (44–46).
Materials and Methods
Predators and Apparatus.
Blue jays were captured in the field as nestlings and hand-reared in the laboratory. They were housed in individual cages and maintained at 85–90% of their free-feeding weight on a controlled diet of turkey starter and cockatiel pellets. A total of 27 jays participated in the experiment, nine of them for only one of the experimental treatments. All but six of them were experienced birds, having taken part in previous operant experiments involving searching for virtual moths. Images were displayed on flat-screen monitors framed with infrared touch screens to record peck responses. Rewards were customized Noyes pellets dispensed into a food cup centered below the monitor. Naïve jays were first habituated to the apparatus and were shaped to peck at small filled circles on a uniform gray background. They were then trained on the standard stimulus display: two 9.5- × 13-cm fields of background separated by a 6-cm-wide region that contained the “advance” key, a green 2.7-cm disk. Jays were trained on a variety of fixed-phenotype moths, first on flat gray fields and then on cryptically colored ones, and were taught to peck the advance key in the absence of a moth. They were subsequently given extensive experience with the parental population under nonevolving conditions. When each bird was able to detect 80% of the parental moths at least 2 days in a row, selection experiments were initiated. Training naïve jays to a level appropriate for experimental work generally required ≈6–8 months.
Genetic Algorithm.
Moth phenotypes were developed from specifications in a virtual haploid chromosome, a string of 117 bytes. The wing pattern was encoded in 18 loci, each consisting of five bytes that defined elliptical patches of specific location, orientation, shape, and intensity. Each pixel value in the phenotype was determined by the additive result of multiple overlapping patches. The chromosome was divided into nine linkage groups, each consisting of two patch loci and a regulatory locus that included genes for brightness, contrast, and recombination probability. Once the primary pattern was decoded from the patch loci, the developmental algorithm calculated the mean values of the brightness and contrast genes and modified the final image accordingly.
Reproduction entailed choosing two different chromosomes at random from the population of 200 moths and recombining them into a single offspring genome. Moths that had been overlooked by both jays during predation trials had 2.6 times the probability of being chosen as the average singly detected moth and 4.3 times the probability of the average doubly detected moth. The sets of singly and doubly detected moths were ranked in inverse order of the time the predators took to find them, and the highest-ranked individual had a 25% higher probability of being chosen than the lowest-ranked (47). To enable maintenance of integrated pattern features, recombination took place only between linkage groups, and the crossover probability was determined by the combined values of the recombination regulators above and below the exchange point. Each offspring genome was subsequently subjected to a mutation process that randomly inverted individual bits with a probability of 0.003 (47).
Analytical Techniques.
Objective measures of the resemblance between moths and backgrounds were obtained by using distributional correspondence indices (16, 48, 49). First, the joint bivariate distribution of pixel colors and the sizes of contiguous regions of a single color were extracted empirically from dark, light, mottled, and speckled backgrounds. Each moth was evaluated for the mean probability of occurrence of the color regions on its wings, given expectations based on the empirical background distributions. This value was then converted into a matching index, varying between 0 and 1, that was specific to the particular comparison distribution. Previous studies have shown that matching indices provide a reasonable measure of the difficulty of the detection task, accounting for 30–40% of the variance in accuracy and response time (26). To approximate the effective crypticity of the moth under experimental conditions, we used an index derived from the pixel distribution across entire fields (a “field-level” index). In the mottled and speckled treatments, therefore, this matching index was accumulated across patch boundaries. For analysis of fitness tradeoffs, the index had to be explicitly separated from the patch configuration, so in this case we used only pixel distributions from within dark or light patches (a “patch-level” index).
To determine the appropriate sample for analysis, the mean field-level matching index and a measure of phenotypic variance (27) were calculated for each population. These measures generally appeared to reach a plateau after ≈50 generations of selection, although there were significant subsequent fluctuations. To obtain reliable estimates of experimental differences, we limited our analyses to generations from F50 to F100 (the “criterial” populations). Differences among experimental treatments in the distribution of moths in phenotypic or niche space were tested with treatment × squad ANOVAs, considering squad as a random effect. Significant main and interaction effects of squad were found in several analyses, apparently reflecting coincidental differences between groups in the vectors of their respective lineages through evolutionary time. Because these effects showed no informative consistencies across treatments, they were not discussed. Because much of the significance of the results derived from contrasts between pairs of treatments, the statistics reported are mainly planned comparisons within the treatment main effects.
Acknowledgments
We thank J. Endler for his crucial initial suggestions on experimental design and K. Goto and J. Christensen for comments and criticisms on the manuscript. This research was supported in part by Grant IOB 0234441 from the National Science Foundation and Grant MH68426 from the National Institutes of Mental Health.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Sandoval C. P. Biol. J. Linn. Soc. 1994;52:341–356. [Google Scholar]
- 2.Cook L. M. Philos. Trans. R. Soc. London B. 1998;353:1577–1593. [Google Scholar]
- 3.Dearn J. M. In: Biology of Grasshoppers. Chapman R. F., Joern A., editors. New York: Wiley; 1990. pp. 517–549. [Google Scholar]
- 4.Wente W. H., Phillips J. B. Am. Nat. 2003;162:461–473. doi: 10.1086/378253. [DOI] [PubMed] [Google Scholar]
- 5.Théry M., Casas J. Nature. 2002;415:133. doi: 10.1038/415133a. [DOI] [PubMed] [Google Scholar]
- 6.Popham E. J. Proc. Zool. Soc. London Ser. A.; 1941. pp. 135–172. [Google Scholar]
- 7.Sargent T. D. Legion of Night: The Underwing Moths. Amherst, MA: Univ. of Massachusetts Press; 1976. [Google Scholar]
- 8.Kettlewell H. B. D. The Evolution of Melanism. Oxford: Clarendon; 1973. [Google Scholar]
- 9.Barnes W., McDunnough J. H. Mem. Am. Mus. Nat. Hist. 1918;3 part 1. [Google Scholar]
- 10.Langley C. M. J. Exp. Psychol. Anim. Behav. Proc.; 1996. pp. 152–163. [DOI] [PubMed] [Google Scholar]
- 11.Bond A. B., Kamil A. C. Anim. Learn. Behav. 1999;27:461–471. [Google Scholar]
- 12.Shettleworth S. J. Cognition, Evolution, and Behaviour. Oxford: Oxford Univ. Press; 1998. pp. 382–397. [Google Scholar]
- 13.Allen J. A. Philos. Trans. R. Soc. London B. 1988;319:485–503. doi: 10.1098/rstb.1988.0061. [DOI] [PubMed] [Google Scholar]
- 14.Bond A. B., Kamil A. C. Nature. 1998;395:594–596. [Google Scholar]
- 15.Ruxton G. D., Sherratt T. N., Speed M. P. Avoiding Attack: The Evolutionary Ecology of Crypsis, Warning Signals, and Mimicry. Oxford: Oxford Univ. Press; 2004. [Google Scholar]
- 16.Endler J. A. Biol. J. Linn. Soc. 1990;41:315–352. [Google Scholar]
- 17.Merilaita S., Tuomi J., Jormalainen V. Biol. J. Linn. Soc. 1999;67:151–161. [Google Scholar]
- 18.Levins R. Evolution in Changing Environments. Princeton: Princeton Univ. Press; 1968. [Google Scholar]
- 19.Kisdi E. Evol. Ecol. Res. 2001;3:721–727. [Google Scholar]
- 20.Cain A. J., Sheppard P. M. Genetics. 1954;39:89–116. doi: 10.1093/genetics/39.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cabral Eterovick P., Eugênio Côrtes Figueira J., Vasconcellos-Neto J. Biol. J. Linn. Soc. 1997;61:485–499. [Google Scholar]
- 22.Sargent T. In: Foraging Behavior: Ecological, Ethological, and Psychological Approaches. Kamil A., Sargent T., editors. New York: Garland; 1981. pp. 259–284. [Google Scholar]
- 23.Merilaita S., Lyytinen A., Mappes J. Proc. R. Soc. London Ser. B.; 2001. pp. 1925–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cook L. M., Kenyon G. Heredity. 1991;66:67–73. [Google Scholar]
- 25.Kassen R. J. Evol. Biol. 2002;15:173–190. [Google Scholar]
- 26.Kamil A. C., Bond A. B. In: Model Systems in Behavioral Ecology. Dugatkin L. A., editor. Princeton: Princeton Univ. Press; 2001. pp. 288–310. [Google Scholar]
- 27.Bond A. B., Kamil A. C. Nature. 2002;415:609–613. doi: 10.1038/415609a. [DOI] [PubMed] [Google Scholar]
- 28.Nijhout H. F. The Development and Evolution of Butterfly Wing Patterns. Washington, DC: Smithsonian Institution; 1991. [Google Scholar]
- 29.Beldade P., Brakefield P. M. Genetics. 2002;3:442–452. doi: 10.1038/nrg818. [DOI] [PubMed] [Google Scholar]
- 30.Neter J., Kutner M. H., Nachtsheim C. J., Wasserman W. Applied Linear Regression Models (Irwin, Chicago) 3rd Ed. 1996. [Google Scholar]
- 31.Merilaita S. Evolution. 2003;57:1248–1254. doi: 10.1111/j.0014-3820.2003.tb00333.x. [DOI] [PubMed] [Google Scholar]
- 32.Wolfe J. M. Vision Res. 1994;34:1187–1195. doi: 10.1016/0042-6989(94)90300-x. [DOI] [PubMed] [Google Scholar]
- 33.Wolfe J. M., Oliva A., Horowitz T. S., Butcher S. J., Bompas A. Vision Res. 2002;42:2985–3004. doi: 10.1016/s0042-6989(02)00388-7. [DOI] [PubMed] [Google Scholar]
- 34.Cohen E., Ruppin E. Percept. Psychophys. 1999;61:1449–1461. doi: 10.3758/bf03206193. [DOI] [PubMed] [Google Scholar]
- 35.Nakayama K., Joseph J. S. In: The Attentive Brain. Parasuraman R., editor. Cambridge, MA: MIT Press; 1998. pp. 279–298. [Google Scholar]
- 36.Ratcliff R. A. Psychon. Bull. Rev. 2002;9:278–291. doi: 10.3758/bf03196283. [DOI] [PubMed] [Google Scholar]
- 37.Reddi B. A. J., Asrress K. N., Carpenter R. H. S. J. Neurophysiol. 2003;90:3538–3546. doi: 10.1152/jn.00689.2002. [DOI] [PubMed] [Google Scholar]
- 38.Cook R. G., Cavoto K. K., Cavoto B. R. Anim. Learn. Behav. 1996;24:150–167. [Google Scholar]
- 39.Bond A. B. J. Exp. Psychol. Anim. Behav. Proc.; 1983. pp. 292–306. [PubMed] [Google Scholar]
- 40.Bond A. B., Riley D. A. Ethology. 1991;87:203–224. [Google Scholar]
- 41.Sneath P. H. A., Sokal R. R. Numerical Taxonomy. San Francisco: Freeman; 1973. [Google Scholar]
- 42.Staddon J. E. R. In: Limits to Action: The Allocation of Individual Behaviour. Staddon J. E. R, editor. New York: Academic; 1980. pp. 101–141. [Google Scholar]
- 43.Ford E. B. Ecological Genetics. 4th Ed. Chapman & Hall, London: 1975. [Google Scholar]
- 44.Maynard Smith J. Nature. 1962;195:60–62. [Google Scholar]
- 45.García-Dorado A. Theor. Popul. Biol. 1987;32:66–75. doi: 10.1006/tpbi.1993.1024. [DOI] [PubMed] [Google Scholar]
- 46.Johnson P. A., Hoppensteadt F. C., Smith J. J., Bush G. L. Evol. Ecol. 1996;10:187–205. [Google Scholar]
- 47.Bäck T. Evolutionary Algorithms in Theory and Practice. New York: Oxford Univ. Press; 1996. [Google Scholar]
- 48.Endler J. A. Biol. J. Linn. Soc. 1984;22:187–231. [Google Scholar]
- 49.Endler J. A. Evol. Biol. 1978;11:319–364. [Google Scholar]