Abstract
The RSC chromatin-remodeling complex completely disassembles a nucleosome in the presence of the histone chaperone Nap1 and ATP. Disassembly occurs in a stepwise manner, with the removal of H2A/H2B dimers, followed by the rest of the histones and the release of naked DNA. RSC and related chromatin-remodeling complexes may be responsible for the removal of promoter nucleosomes during transcriptional activation in vivo.
Keywords: Asf1, histones, Nap1, RSC, yeast
The remodeling of promoter chromatin is a prerequisite for transcription. Remodeling relieves repression by the nucleosome; it exposes promoter DNA for interaction with RNA polymerase and associated proteins. Remodeling has been thought to involve a “reconfiguration” rather than removal of the nucleosome (1). This view, until recently widely held, was based on two lines of evidence. First, promoter DNA becomes more accessible to nuclease attack after transcriptional activation. Second, histones remain associated with the DNA but in a highly modified state, as shown with the use of antibodies against acetylated, phosphorylated, methylated, and other forms of the N-terminal “tails.” Exposure of DNA was reconciled with the retention of histones by the hypothesis of an altered nucleosome, whose modified structure would be conducive to transcription.
This hypothesis has been challenged by a reexamination of promoter chromatin structure and a reinterpretation of the evidence. Quantitative measurements of DNA topology, nuclease digestion rate, and sedimentation profile were performed on the yeast PHO5 promoter. The results of these three very different types of analysis were in close quantitative agreement with one another (2), showing that nucleosomes are present on the activated promoter at levels 18–60% of those at the repressed promoter, and that activated promoter nucleosomes are indistinguishable in structure from repressed promoter nucleosomes (2). On this basis, it was proposed that transcriptional activation is accompanied by the continual removal and reformation of promoter nucleosomes. The modified histones detected on the activated promoter by chromatin immunoprecipitation (3) were interpreted as intermediates in the processes of removal and reformation (4).
Evidence has been presented for the removal of a nucleosome from the TATA box of a promoter by sliding of the histone octamer to an adjacent position on the DNA (5). The alternative is that nucleosomes are removed by dissociation of the octamer from the DNA. These possibilities could be distinguished for the PHO5 promoter by transcriptional activation on small chromatin circles (6). Nucleosomes were lost from the circles, demonstrating octamer dissociation. This result was obtained with a TATA box mutant, so it did not depend on replacement of the octamer by TATA-binding protein. Rather it reflects a natural mechanism for denuding promoter DNA.
What enzyme system(s) might be responsible for the removal of promoter nucleosomes? Genetic studies have implicated the SWI/SNF and closely related RSC complexes in promoter chromatin remodeling. Both complexes expose nucleosomal DNA to nuclease attack in an ATP-dependent manner in vitro (7, 8). The perturbation is transient, and the histone octamer remains bound to the DNA, except in the case of transfer to another DNA molecule (9, 10). Transfer to DNA can be revealed by the conversion of a radiolabeled fragment to nucleosomal form in the presence of a 100-fold excess of donor nucleosomes; the liberation of naked DNA from the donor is virtually undetectable. DNA, moreover, is of questionable significance as an acceptor for the octamer during transcriptional activation in vivo.
A more attractive candidate for an octamer acceptor was suggested by a recent report that Asf1 protein is required for the removal of PHO5 promoter nucleosomes and for the activation of PHO5 transcription (11). Asf1 is a histone chaperone that copurifies with H3 and H4 and that has been implicated in a variety of chromosome transactions, including replication, repair, and recombination (12–18). Asf1 is believed to deposit H3/H4 tetramers on newly replicated DNA, followed by the action of a second chaperone, such as Nap1, for the deposition of H2A/H2B dimers and the completion of nucleosome assembly.
We report here on the transfer of histone octamers from nucleosomes to protein acceptors by RSC and ATP in vitro. We find a remarkably efficient process of nucleosome disassembly, depending on the protein acceptor used. The results establish a principle whose relevance to chromatin remodeling in vivo remains to be determined.
Results
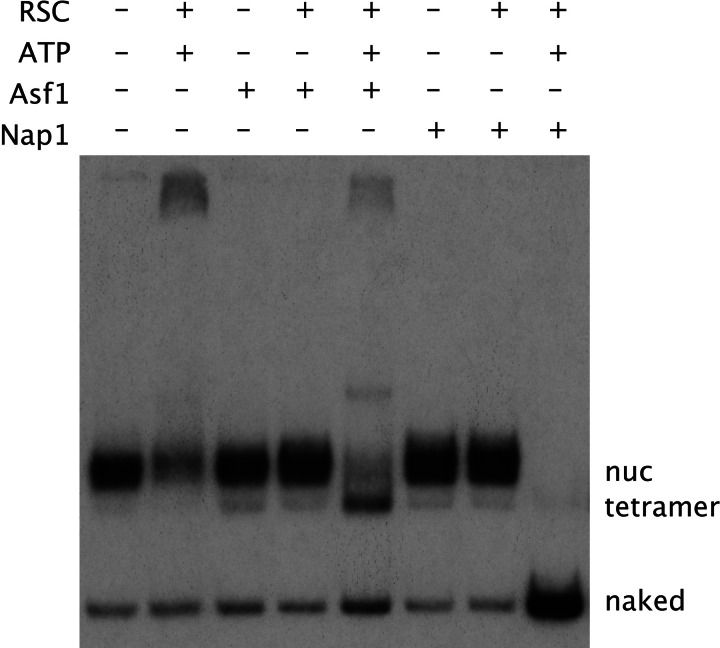
We investigated the possibility of RSC functioning in concert with Asf1 or Nap1 for nucleosome disassembly (Fig. 1). Nucleosomes were formed on a 160-bp DNA fragment containing the nucleosome-positioning sequence of the Xenopus laevis 5S rRNA gene. After treatment under various conditions and the addition of competitor DNA to remove any RSC, the nucleosomes were analyzed by gel electrophoresis. As reported (19), treatment with RSC and ATP converted many of the nucleosomes to aggregates that barely entered the gel; there was no increase in the level of naked DNA. Treatment with Asf1 alone or in the presence of RSC had no effect. Asf1 in the presence of RSC and ATP produced a band migrating faster than the nucleosome but again no significant change in the level of naked DNA. The faster-migrating band was identified as a nucleosome devoid of H2A/H2B dimers (termed a “tetramer” particle), on the basis of its conversion back to a nucleosome by the addition of excess dimers (Fig. 2) and comigration in a gel with a complex of DNA and the H3-H4 tetramer (not shown).
Fig. 1.
Nucleosome transactions catalyzed by RSC and ATP in the presence of histone chaperones: dimer depletion in the presence of Asf1 and disassembly in the presence of Nap1. Nucleosomes (3 ng) were treated for 2 h at 30°C with RSC (0.25 μg), ATP (0.5 mM), Asf1 (1.75 μg), and Nap1 (1.75 μg), as indicated. Competitor DNA (2 μg of an unrelated bacterial plasmid) was added for the removal of RSC, followed by incubation for another 5 min at 30°C, addition of 1.2 μl of 50% glycerol, and gel electrophoresis. An autoradiograph of the gel is shown. Bands due to nucleosomes, nucleosomes lacking H2A/H2B dimers (“tetramer”), and naked DNA are indicated.
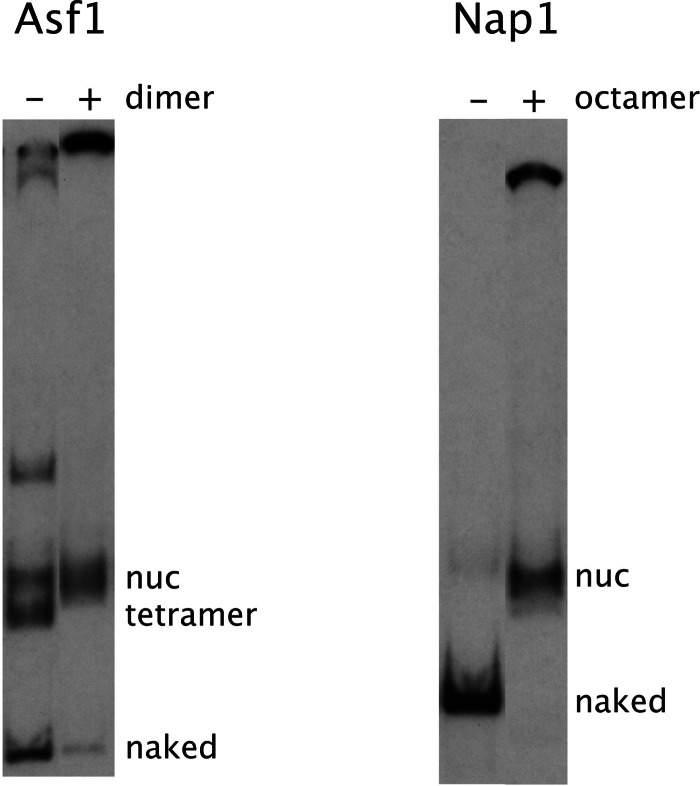
Fig. 2.
Reversal of histone depletion by RSC and Asf1, and of nucleosome disassembly by RSC and Nap1. Asf1: After the 2-h incubation of a reaction identical to the fifth lane from the left in Fig. 1, 1.5 μg of yeast H2A/H2B dimer was added or not, as indicated. Incubation was extended for another 2 h at 30°C, competitor DNA was added, and gel electrophoresis was performed as in Fig. 1. Nap1: After the 2-h incubation of a reaction identical to the last lane in Fig. 1, 1 μg of yeast histone octamer was added or not, as indicated, and samples were processed as for Asf1.
As with Asf1, treatment of nucleosomes with Nap1 alone or in the presence of RSC was without effect (Fig. 1). In contrast with Asf1, however, Nap1 in the presence of RSC and ATP gave a dramatic result: all nucleosomes were converted to naked DNA. RSC presumably catalyzes an equilibrium between histones bound to nucleosomal DNA and to Nap1 and thus transferred all histones to Nap1, which was in 1,000-fold molar excess over the DNA (100-fold molar excess over the histones). The addition of octamers (2-fold molar excess of histones over Nap1) restored much of the DNA to nucleosomal form and the remainder to aggregates (Fig. 2).
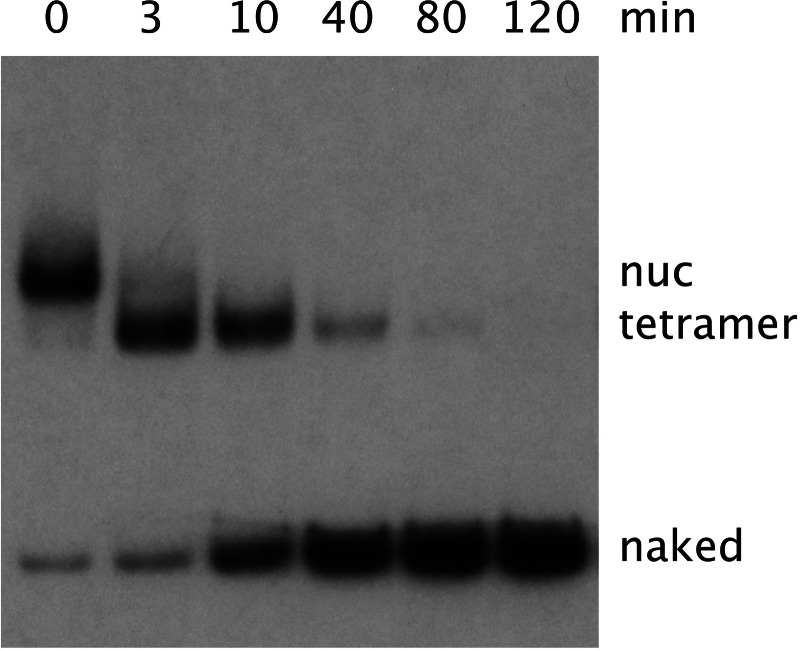
A time course of the RSC reaction revealed a stepwise mechanism (Fig. 3). All nucleosomes were reduced to “tetramers” before conversion to naked DNA. The half time for the overall reaction was ≈10 min, with RSC in excess and Nap1 at 2 μM. Nap1 was limiting, because the half time was inversely proportional to the Nap1 concentration (data not shown). The stepwise mechanism of the reaction was evidently a reversal of the natural nucleosome assembly process, with removal of H2A/H2B dimers followed by transfer of the H3/H4 tetramer.
Fig. 3.
Time course of nucleosome disassembly by RSC and Nap1. Reactions were identical to the last lane in Fig. 1, except that incubations were for the times indicated. Samples were processed as in Fig. 1.
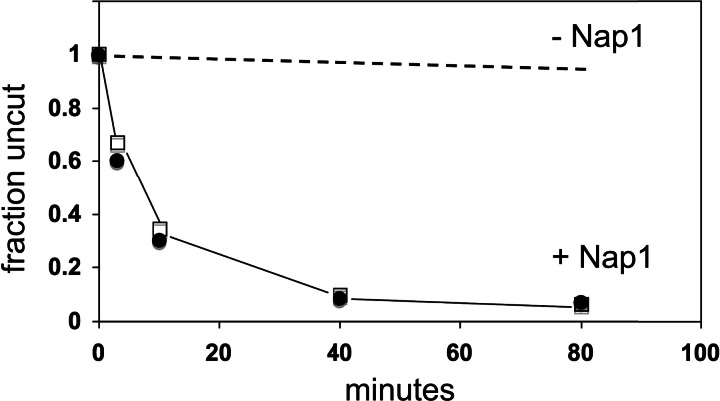
It may be asked whether RSC and Nap1 disassemble the nucleosome or merely destabilize it, perhaps through the formation of ternary complex, which unfolds during gel electrophoresis. We therefore assessed exposure to restriction enzyme digestion as an independent measure of unfolding. AluI and Aat II sites located 11 and 26 bp from the dyad of the nucleosome are cleaved slowly, even in the presence of RSC and ATP (Fig. 4, dashed line, 4% digestion after 80 min). Upon addition of Nap1, however, cleavage was rapid and complete (Fig. 4, solid line). The half time for cleavage was 8 min, essentially the same as that for the appearance of naked DNA in gel electrophoresis (≈10 min, Fig. 3). We conclude that RSC and Nap1 completely disassemble the nucleosome.
Fig. 4.
Exposure of restriction enzyme sites near the dyad of the nucleosome by RSC and Nap1. Reactions were identical to the last lane in Fig. 1, except that incubations were for the times indicated. After incubation, addition of competitor DNA, and further incubation for 5 min, AluI (20 units, open squares) or Aat II (20 units, filled circles) was added, and incubation was extended for another 30 min. Samples were processed by proteinase K digestion, phenol extraction, and gel electrophoresis. Bands due to uncut DNA were quantitated by PhosphorImager analysis. Results obtained by the same procedure except with the omission of Nap1 (36) are shown for comparison (dashed line).
A number of further controls served to reinforce this conclusion. Neither Asf1 nor NAP1 exhibited any activity in nucleosome perturbation or histone octamer transfer in the absence of RSC, even upon prolonged incubation in the presence of ATP. The termination of RSC reactions by the addition of competitor DNA was not a source of artifact, because competitor could be omitted without effect on the rates of restriction enzyme digestion. Finally, RSC was used in ≈10 -fold molar excess over nucleosomes in the octamer transfer reactions performed here. This was done for reasons of convenience and had no bearing on the results. The concentration of RSC could be reduced 100-fold or more without effect on the rate or extent of the octamer transfer observed.
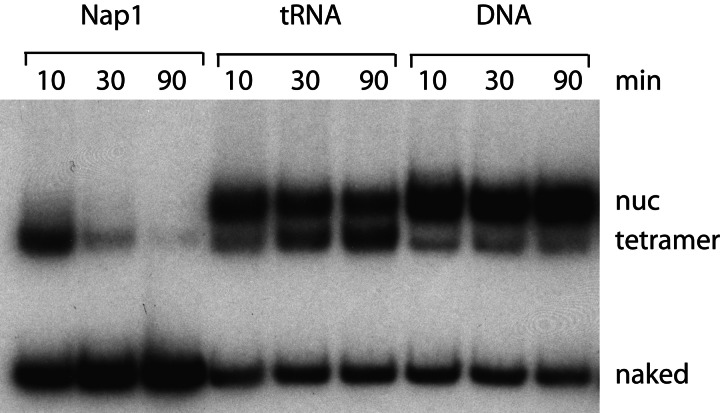
Finally, the distinctive nature of histone octamer transfer to Nap1 was emphasized by comparison with DNA and RNA as octamer acceptors. Whereas transfer to Nap1 was nearly complete in 30 min, there was no appreciable transfer to DNA or RNA at 5-fold higher concentrations than Nap1 in 90 min (Fig. 5). A slow transfer of H2A/H2B dimers to RNA was observed, but without the appearance of naked DNA. Previous evidence for octamer transfer to DNA was obtained with the use of a radiolabeled acceptor (9), and the rate and extent of transfer were orders of magnitude less than reported here for transfer to Nap1. Various negatively charged polypeptides, such as polyglutamic acid, long known to serve as a histone chaperone in vitro, also failed to support detectable octamer transfer under the conditions used here (data not shown).
Fig. 5.
RSC transfers histone octamers to Nap1 but not to DNA or RNA. Reactions were identical to the last lane in Fig. 1, except that incubations were for the times indicated and with RNA (total yeast tRNA, 1.65 μg) or DNA (pUC19 plasmid, 1.65 μg) instead of Nap1, where indicated.
Discussion
Our findings establish the principle of histone octamer transfer by a SWI/SNF-related chromatin-remodeling complex to a histone chaperone protein. This activity is rapid and efficient and, if targeted to promoter nucleosomes, for example by histone modification, could account for their removal upon transcriptional activation in vivo. Such a mechanism would transform our view of remodeling by SWI/SNF family members. Previous reports have emphasized the perturbation of nucleosome structure by these complexes without loss of the histone octamer. Such perturbation fit well with the idea of reconfiguration rather than removal of promoter nucleosomes upon transcriptional activation (1). Our findings, however, suggest that the perturbation may reflect an intermediate in the disassembly of nucleosomes and not a final state of remodeled chromatin. Progress beyond the intermediate may require a histone chaperone, such as Nap1. This change in view of SWI/SNF-related chromatin-remodeling complexes would accord with the reinterpretation of evidence regarding histone modification and transcriptional activation mentioned above. According to this reinterpretation, posttranslationally modified nucleosomes represent intermediates rather than final forms of transcribed promoter chromatin. Taken together, histone modification, octamer transfer by a remodeling complex, and the consequent removal of promoter nucleosomes may constitute a complete picture, in outline, of the transcriptional activation process.
It may be argued that the role of Nap1 pertains to the H2A/H2B dimer and not the H3/H4 tetramer, inasmuch as Nap1 is isolated as a complex with the dimer. Indeed, Nap1 may serve to chaperone the dimer during nucleosome assembly in vivo. But Nap1 is commonly used to chaperone all four histones for the purpose of nucleosome assembly in vitro. Interaction of Nap1 with the H3/H4 tetramer has been directly demonstrated (20).
The stepwise mechanism of nucleosome disassembly by RSC and Nap1 can be understood in terms of the unraveling of nucleosomal DNA from the ends by RSC. Unraveling of ≈40 bp from one end, well within the capacity of RSC (21, 22), will expose an H2A/H2B dimer for transfer to Nap1. Transfer of both dimers will result in the observed “tetramer” intermediate. A stepwise mechanism is consistent with previous studies of Nap1–nucleosome interaction. Transfer of an H2A/H2B dimer to Nap1 has been observed in the presence of the ISWI chromatin-remodeling complex and p300 (23). Replacement of a histone octamer by a DNA-binding transcription factor, facilitated by Nap1, is blocked by crosslinking the octamer (24). Finally, the displacement of histones from DNA by transcription (25), especially H2A and H2B, is enhanced by Nap1 (26).
In view of the known role of Asf1 as an H3/H4 chaperone, and our observation of H2A/H2B transfer to Asf1 by RSC, it is surprising that Asf1 fails to support nucleosome disassembly by RSC. Asf1 may compete less effectively with DNA for binding the H3/H4 tetramer than does Nap1, or only Nap1 may be capable of cooperating with RSC, through coupling of chaperone and chromatin-remodeling activities or direct interaction. Indeed interaction of Asf1 with Brahma, the Drosophila counterpart of the SWI/SNF complex, has been demonstrated (27), raising the possibility of nucleosome disassembly in the presence of Asf1 catalyzed by SWI/SNF complex rather than RSC.
The molecules and mechanisms under discussion here are widely conserved. The removal of promoter nucleosomes upon transcription activation is not limited to PHO5 in yeast but is evidently true for most, if not all, RNA polymerase II promoters in yeast and for RNA polymerase II promoters in mammalian cells. Both Asf1 and Nap1 have homologues in higher organisms (28, 29), and counterparts of RSC and SWI/SNF complex have been identified in human cells and Drosophila as well.
The outstanding question is whether the principle uncovered here is applicable to chromatin remodeling in vivo. Deletion of Nap1 or degradation of Asf1 compromises the expression of hundreds of yeast genes (30, 31). The SWI/SNF complex is required under some conditions for the transcriptional activation of PHO5 (32). SWI/SNF can catalyze the replacement of a histone octamer by a DNA-binding protein within a nucleosomal array in vitro (33), but it remains to be determined whether SWI/SNF or RSC can target a particular promoter nucleosome and remove the octamer, by transfer to Nap1, Asf1, or any other chaperone protein, in vivo.
Materials and Methods
Nucleosomes were prepared with the use of32P-labeled 160-bp DNA and rat liver histones, as described (34). RSC, Asf1, and Nap1 were prepared as described (15, 34, 35). Reactions contained 15 mM Hepes, pH 8.0, 3 mM MgCl2, 17.5 mM potassium acetate, and 75 μg/ml BSA, in a total volume of 15 μl.
Electrophoresis was in a 3.2% polyacrylamide gel in 10 mM Tris·Cl, pH 7.5/1 mM EDTA for nucleosomes, and in a 7% polyacrylamide gel in TBE buffer for phenol-extracted DNA.
Acknowledgments
This research was supported by National Institutes of Health Grant GM36659 (to R.D.K.).
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Paranjape S. M., Kamakaka R. T., Kadonaga J. T. Annu. Rev. Biochem. 1994;63:265–297. doi: 10.1146/annurev.bi.63.070194.001405. [DOI] [PubMed] [Google Scholar]
- 2.Boeger H., Griesenbeck J., Strattan J. S., Kornberg R. D. Mol. Cell. 2003;11:1587–1598. doi: 10.1016/s1097-2765(03)00231-4. [DOI] [PubMed] [Google Scholar]
- 3.Reinke H., Horz W. Mol. Cell. 2003;11:1599–1607. doi: 10.1016/s1097-2765(03)00186-2. [DOI] [PubMed] [Google Scholar]
- 4.Boeger H., Bushnell D. A., Davis R., Griesenbeck J., Lorch Y., Strattan J. S., Westover K. D., Kornberg R. D. FEBS Lett. 2005;579:899–903. doi: 10.1016/j.febslet.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 5.Lomvardas S., Thanos D. Cell. 2001;106:685–696. doi: 10.1016/s0092-8674(01)00490-1. [DOI] [PubMed] [Google Scholar]
- 6.Boeger H., Griesenbeck J., Strattan J. S., Kornberg R. D. Mol. Cell. 2004;14:667–673. doi: 10.1016/j.molcel.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Cote J., Quinn J., Workman J. L., Peterson C. L. Science. 1994;265:53–60. doi: 10.1126/science.8016655. [DOI] [PubMed] [Google Scholar]
- 8.Cairns B. R., Lorch Y., Li Y., Zhang M., Lacomis L., Erdjument-Bromage H., Tempst P., Du J., Laurent B., Kornberg R. D. Cell. 1996;87:1249–1260. doi: 10.1016/s0092-8674(00)81820-6. [DOI] [PubMed] [Google Scholar]
- 9.Lorch Y., Zhang M., Kornberg R. D. Cell. 1999;96:389–392. doi: 10.1016/s0092-8674(00)80551-6. [DOI] [PubMed] [Google Scholar]
- 10.Phelan M. L., Schnitzler G. R., Kingston R. E. Mol. Cell. Biol. 2000;20:6380–6389. doi: 10.1128/mcb.20.17.6380-6389.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adkins M. W., Howar S. R., Tyler J. K. Mol. Cell. 2004;14:657–666. doi: 10.1016/j.molcel.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 12.Tyler J. K., Adams C. R., Chen S. R., Kobayashi R., Kamakaka R. T., Kadonaga J. T. Nature. 1999;402:555–560. doi: 10.1038/990147. [DOI] [PubMed] [Google Scholar]
- 13.Adams C. R., Kamakaka R. T. Curr. Opin. Genet. Dev. 1999;9:185–190. doi: 10.1016/S0959-437X(99)80028-8. [DOI] [PubMed] [Google Scholar]
- 14.Mello J. A., Almouzni G. Curr. Opin. Genet. Dev. 2001;11:136–141. doi: 10.1016/s0959-437x(00)00170-2. [DOI] [PubMed] [Google Scholar]
- 15.Sharp J. A., Fouts E. T., Krawitz D. C., Kaufman P. D. Curr. Biol. 2001;11:463–473. doi: 10.1016/s0960-9822(01)00140-3. [DOI] [PubMed] [Google Scholar]
- 16.Groth A., Ray-Gallet D., Quivy J. P., Lukas J., Bartek J., Almouzni G. Mol. Cell. 2005;17:301–311. doi: 10.1016/j.molcel.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 17.Emili A., Schieltz D. M., Yates J. R., III, Hartwell L. H. Mol. Cell. 2001;7:13–20. doi: 10.1016/s1097-2765(01)00150-2. [DOI] [PubMed] [Google Scholar]
- 18.Prado F., Cortes-Ledesma F., Aguilera A. EMBO Rep. 2004;5:497–502. doi: 10.1038/sj.embor.7400128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lorch Y., Cairns B. R., Zhang M., Kornberg R. D. Cell. 1998;94:29–34. doi: 10.1016/s0092-8674(00)81218-0. [DOI] [PubMed] [Google Scholar]
- 20.McBryant S. J., Park Y. J., Abernathy S. M., Laybourn P. J., Nyborg J. K., Luger K. J. Biol. Chem. 2003;278:44574–44583. doi: 10.1074/jbc.M305636200. [DOI] [PubMed] [Google Scholar]
- 21.Lorch Y., Zhang M., Kornberg R. D. Mol. Cell. 2001;7:89–95. doi: 10.1016/s1097-2765(01)00157-5. [DOI] [PubMed] [Google Scholar]
- 22.Kassabov S. R., Zhang B., Persinger J., Bartholomew B. Mol. Cell. 2003;11:391–403. doi: 10.1016/s1097-2765(03)00039-x. [DOI] [PubMed] [Google Scholar]
- 23.Ito T., Ikehara T., Nakagawa T., Kraus W. L., Muramatsu M. Genes Dev. 2000;14:1899–1907. [PMC free article] [PubMed] [Google Scholar]
- 24.Walter P. P., Owen-Hughes T. A., Cote J., Workman J. L. Mol. Cell. Biol. 1995;15:6178–6187. doi: 10.1128/mcb.15.11.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lorch Y., LaPointe J. W., Kornberg R. D. Cell. 1987;49:203–210. doi: 10.1016/0092-8674(87)90561-7. [DOI] [PubMed] [Google Scholar]
- 26.Levchenko V., Jackson B., Jackson V. Biochemistry. 2005;44:5357–5372. doi: 10.1021/bi047786o. [DOI] [PubMed] [Google Scholar]
- 27.Moshkin Y. M., Armstrong J. A., Maeda R. K., Tamkun J. W., Verrijzer P., Kennison J. A., Karch F. Genes Dev. 2002;16:2621–2626. doi: 10.1101/gad.231202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujii-Nakata T., Ishimi Y., Okuda A., Kikuchi A. J. Biol. Chem. 1992;267:20980–20986. [PubMed] [Google Scholar]
- 29.Tagami H., Ray-Gallet D., Almouzni G., Nakatani Y. Cell. 2004;116:51–61. doi: 10.1016/s0092-8674(03)01064-x. [DOI] [PubMed] [Google Scholar]
- 30.Ohkuni K., Shirahige K., Kikuchi A. Biochem. Biophys. Res. Commun. 2003;306:5–9. doi: 10.1016/s0006-291x(03)00907-0. [DOI] [PubMed] [Google Scholar]
- 31.Zabaronick S. R., Tyler J. K. Mol. Cell. Biol. 2005;25:652–660. doi: 10.1128/MCB.25.2.652-660.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neef D. W., Kladde M. P. Mol. Cell. Biol. 2003;23:3788–3797. doi: 10.1128/MCB.23.11.3788-3797.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Owen-Hughes T., Utley R. T., Coté J., Peterson C. L., Workman J. L. Science. 1996;273:513–516. doi: 10.1126/science.273.5274.513. [DOI] [PubMed] [Google Scholar]
- 34.Lorch Y., Kornberg R. D. Methods Enzymol. 2003;377:316–322. doi: 10.1016/S0076-6879(03)77019-0. [DOI] [PubMed] [Google Scholar]
- 35.Wittmeyer J., Saha A., Cairns B. Methods Enzymol. 2004;377:322–343. doi: 10.1016/S0076-6879(03)77020-7. [DOI] [PubMed] [Google Scholar]
- 36.Lorch Y., Davis B., Kornberg R. D. Proc. Natl. Acad. Sci. USA. 2005;102:1329–1332. doi: 10.1073/pnas.0409413102. [DOI] [PMC free article] [PubMed] [Google Scholar]