Abstract
IL-32 is a recently discovered cytokine that induces TNFα, IL-1β, IL-6, and chemokines. We investigated whether IL-32 is expressed in the synovia of patients with rheumatoid arthritis (RA) and studied associations with disease severity and the presence of other cytokines. Immunohistochemistry revealed that IL-32 is highly expressed in RA synovial tissue biopsies, whereas IL-32 was not observed in synovial tissues from patients with osteoarthritis. Moreover, in synovial biopsies from 29 RA patients with active disease, the level of IL-32 staining correlated with erythrocyte sedimentation rate, a marker of systemic inflammation (R = 0.63 and P < 0.0003). Synovial staining of IL-32 also correlated with indices of synovial inflammation (R = 0.80 and P < 0.0001) as well as synovial presence of TNFα (R = 0.68 and P < 0.004), IL-1β (R = 0.79 and P < 0.0001), and IL-18 (R = 0.82 and P < 0.001). IL-32 was a potent inducer of prostaglandin E2 release in mouse macrophages and human blood monocytes, an important property for inflammation. After the injection of human IL-32γ into the knee joints of naïve mice, joint swelling, with pronounced influx of inflammatory cells and cartilage damage, was observed. In TNFα-deficient mice, IL-32-driven joint swelling was absent and cell influx was markedly reduced, but loss of proteoglycan was unaffected, suggesting that IL-32 activity is, in part, TNFα-dependent. IL-32, strongly associated with TNFα, IL-1β, and IL-18, appears to play a role in human RA and may be a novel target in autoimmune diseases.
Keywords: autoimmune, inflammation, tumor necrosis factor
Rheumatoid arthritis (RA) is a systemic autoimmune inflammatory disease that predominantly affects multiple peripheral joints. Although the exact mechanisms that contribute to the disease pathogenesis are still largely unknown, it is generally well accepted that numerous inflammatory cells such as T cells, B cells, fibroblast-like synoviocytes, and antigen-presenting cells and their extensive production of proinflammatory mediators, such as TNFα and IL-1, are implicated. Histopathologic features of RA synovial tissue encompass infiltration by macrophages and T cells, synovial lining hyperplasia, neoangiogenesis, and pannus formation (1–5). A large body of evidence points toward an autoimmune component in RA. First, the recognition HLA-DR subtypes, which are associated with RA, indicate the involvement of antigen-presenting cells, such as dendritic cells and macrophages, as well as T cells (6, 7). Second, RA is associated with the production of autoantibodies such as the rheumatoid factor and antibodies against cyclic citrullinated peptide (8, 9). Moreover, studies suggest the involvement of both FcγR and Toll-like receptors in arthritis, which are of critical importance in autoimmunity (10–12).
IL-32 is a recently described cytokine produced by T lymphocytes, natural killer cells, epithelial cells, and blood monocytes (13, 14). Of particular importance, IL-32 is prominently induced by IFN-γ in epithelial cells and monocytes (13). Human recombinant IL-32 exhibits several properties typical of a proinflammatory cytokine (13, 14). For example, the cytokine induces other proinflammatory cytokines and chemokines such as TNFα, IL-1β, IL-6, and IL-8 by means of the activation of NF-κB and p38 mitogen-activated protein kinase (13, 14). An unexpected property of IL-32 is its ability to augment by 10-fold the production of IL-1β and IL-6 induced by muramyl dipeptides by means of the nucleotide oligomerization domains 1 and 2 (NOD1 and NOD2) through a caspase-1-dependent mechanism (14). A single mutation in NOD2 plays a role in a subgroup of patients with Crohn's inflammatory bowel disease. Together, studies suggest that IL-32 has an important role in inflammation, both during host defenses against microorganisms and in autoimmune diseases.
To determine the participation of IL-32 in the inflammatory processes of RA, we investigated the expression of IL-32 in RA synovial tissue from patients with unaffected joints and with moderate or severe arthritis. We examined whether the local expression of IL-32 is related to that of other proinflammatory cytokines. Moreover, we assessed potential arthritogenic capacity of IL-32 and determined whether IL-32-mediated inflammation depends on TNFα. We report here that the synovial expression of IL-32 is strongly correlated with that of TNFα and IL-1β, but also with the severity of microscopic and macroscopic joint inflammation. IL-32 itself induces joint inflammation with concomitant mild cartilage damage when injected intraarticularly (i.a.) in murine knee joints. Our findings support the role of IL-32 as a primary proinflammatory mediator in RA. The new cytokine IL-32 drives the local production of proinflammatory cytokines such as TNFα and therefore represents a potential target in RA.
Results
Clinical Features.
The clinical and demographic features of the patients with moderate or severe arthritis at the time of joint biopsies have been published (15, 16). All patients had active disease as defined by the inclusion criterion of disease activity scores >3.2. Not unexpectedly, patients with severe clinical knee joint arthritis tended to have a more active disease as reflected by higher values for disease activity score, erythrocyte sedimentation rate (ESR), and C-reactive protein.
IL-32 Is Highly Expressed in RA Synovial Tissue.
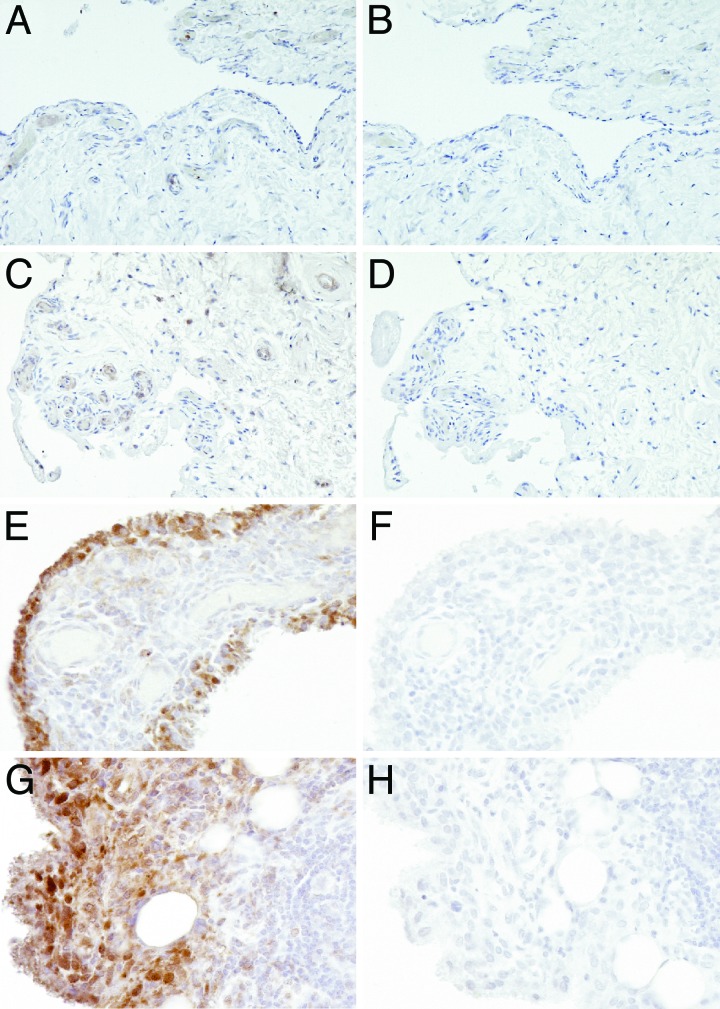
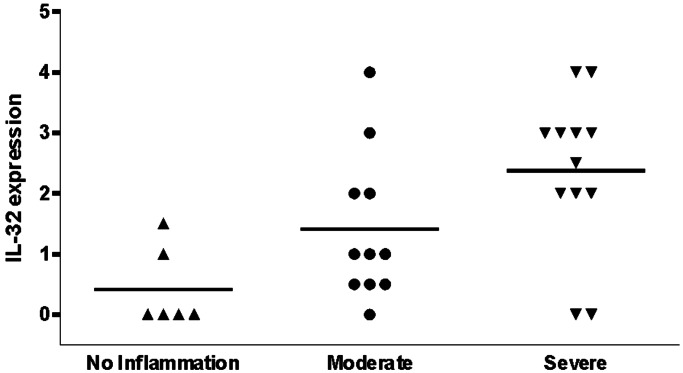
IL-32 staining was detectable in 83% (25 of the 29 patients) of the synovial biopsies, and, as shown in Fig. 1, the cytokine is distributed in lining, sublining, and endothelial cells. Intense staining was found predominantly in the lining layer of the synovium. The cells that were the most positive for IL-32 staining were macrophage-like cells. The percentage of RA patients with IL-32-positive biopsies was lower among the group showing little clinical arthritis than in those with moderate or severe visual knee inflammation (Fig. 2). Moreover, the semiquantitative scores for IL-32 in the lining were highly correlated with those for microscopic inflammation on hematoxylin and eosin (H&E) sections (R = 0.80 and P < 0.0001) and also with the acute phase reaction as measured by the ESR (R = 0.71 and P < 0.0001) as shown in Table 1.
Fig. 1.
IL-32 expression in RA synovial tissue biopsies. (A) Synovial tissue sample from a healthy individual. (C) RA synovial tissue, not inflamed. (E) RA synovial tissue, moderately inflamed. (G) RA synovial tissue, severely inflamed. (B, D, F, and H) Control staining was performed with nonrelevant isotope control antibody. H&E staining was performed as counterstaining. (Original magnification ×400.)
Fig. 2.
Expression of synovial IL-32 in relation to macroscopic knee scores of patients with RA. On macroscopic scoring, the knee joints that underwent percutaneous biopsing were categorized as noninflamed, moderately inflamed, and severely inflamed. Staining for IL-32 was semiquantitatively scored on a five-point scale (range 0–4) at ×200 magnification; a score of 0 represented no or minimal staining, a score of 1 indicated 10–20% positive cells, a score of 2 indicated 30–40% positive cells, a score of 3 indicated 50–60% positive cells, and a score of 4 represented staining of >60% of the cells. Data were derived from 29 RA patients with active disease.
Table 1.
IL-32 correlations with inflammation and cytokines
| Parameters | R value | P value |
|---|---|---|
| Inflammation | 0.80 | <0.0001 |
| ESR | 0.63 | <0.0003 |
| TNFα | 0.68 | <0.004 |
| IL-1β | 0.079 | <0.0001 |
| IL-18 | 0.082 | <0.0001 |
Synovial lining expression of IL-32 was correlated with microscopic inflammation of the biopsies. Serum levels of ESR were measured in 29 RA patients with active disease and correlated with IL-32 expression in synovial tissue. Correlations are expressed by using Spearman's rank correlation coefficient.
Expression of IL-32 Strongly Associates with TNFα, IL-1β, and IL-18 Protein Levels in RA Synovial Tissue.
We assessed whether the synovial expression of IL-32 was related to the expression of other cytokines. IL-32 was expressed in 83% of the RA patients and variably present in the synovial lining, sublining layer, and endothelial cells, whereas TNFα was detectable in only 50% of the RA patients. In contrast, IL-1β staining was observed in most synovial biopsies (90% of the RA patients), whereas IL-18 was detectable in 79% of the RA synovial tissue samples (15). The levels of IL-32 and TNFα expression in the same biopsies were strongly correlated (R = 0.68 and P < 0.004 for lining). However, a greater association was found for IL-32 presence in the lining layers with the expression of IL-1β and IL-18 in the same biopsies (R = 0.79 and P < 0.0001 for IL-1β; R = 0.82 and P < 0.0001 for IL-18). These data are shown in Table 1.
Recombinant IL-32 Induces the Release of Proinflammatory Mediators by Mouse Macrophages and Human Peripheral Blood Mononuclear Cells (PBMC).
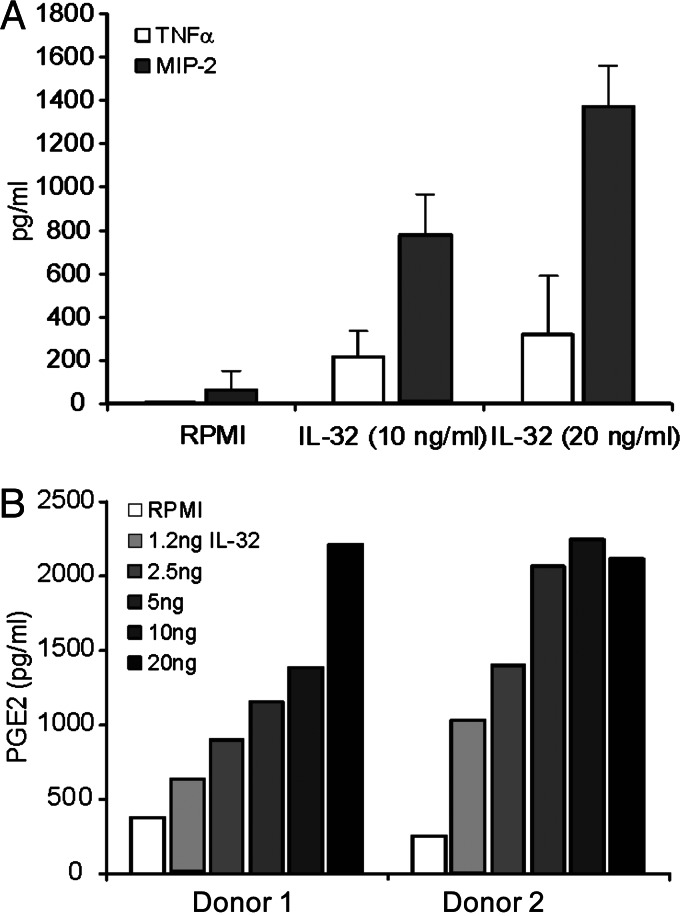
When peritoneal macrophages collected from C3H/HeJ mice were cultured in vitro, human IL-32 induced the production of the TNFα and IL-1β, as well as that of the chemokine macrophage inflammatory protein 2 (MIP-2) (Fig. 3A). In addition, IL-32 stimulated the production of prostaglandin E2 (PGE2) by human PBMC, an important mediator of cartilage and bone destruction in RA (Fig. 3B).
Fig. 3.
Production of proinflammatory mediators by IL-32. (A) TNFα and MIP-2 production of peritoneal macrophages isolated from LPS-resistant mice (C3H/HeJ mice). Murine TNFα and MIP-2 were measured after 24-h IL-32 exposure by electrochemiluminescence (13). Data are the mean ± SD levels of three identical experiments. (B) PGE2 production of human PBMC stimulated with IL-32. PBMC isolated from two healthy donors were exposed for 24 h to IL-32 in a dose ranging from 1.25 ng/ml to 20 ng/ml. PGE2 concentrations were measured by enhanced electrochemiluminescence with acetylcholinesterase-conjugated tracer used for quantification. The sensitivity of the assay was 25 pg/ml.
Local Injection of Human IL-32 Induces Severe Joint Inflammation.
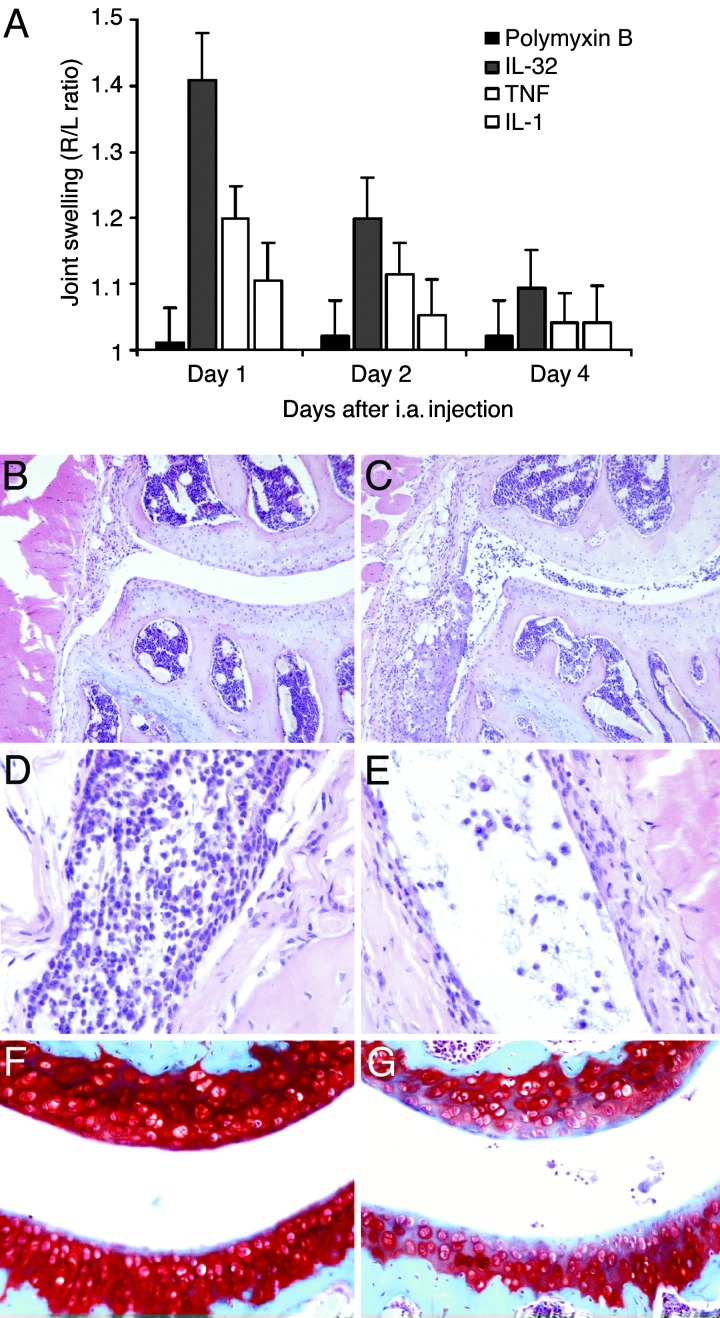
It was not known whether human IL-32 directly induces joint inflammation as an indication of its role in the arthritic processes of RA. Therefore, we injected recombinant human IL-32γ locally in knee joints of naïve C57/Bl6 mice. The inflammatory response of IL-32γ was compared with that of injections of either TNFα or IL-1β. Fig. 4A shows that a single injection of 100 ng of IL-32γ resulted in moderate joint swelling at day 1 (right-to-left knee ratio of 1.41 ± 0.09) and decline at day 4. Interestingly, IL-32γ was more potent in its ability to induce joint swelling than was TNFα (right-to-left ratio of 1.24 ± 0.06) at day 1. In contrast, injection of IL-1β did not result in detectable joint swelling, which is consistent with previous studies (17). Histological analysis of injected knee joints revealed that IL-32 resulted in a severe influx of inflammatory cells into the joint space as well as into synovial tissue at day 1 (Fig. 4 C and D). Importantly, joint inflammation was still present 4 days after a single IL-32 injection, although the number of inflammatory cells in the synovial lining was limited (Fig. 4E). Shortly after local injection of IL-32 (days 1 and 2), the infiltrating cells were predominantly neutrophils, whereas in later stages monocyte/macrophage-like cells were present (Fig. 4E). Interestingly, IL-32 did not induce marked loss of proteoglycan shortly after i.a. application (days 1 and 2). However, at day 4 a depletion of the cartilage layer was noted when compared with control knee joint (Fig. 4F and G). As controls, recombinant IL-32γ was injected in two LPS-resistant mouse strains (C3H/HeJ and Toll-like receptor 4-deficient), and identical results were seen as in WT mice (data not shown).
Fig. 4.
Joint inflammation provoked by local IL-32γ injection. (A) Joint swelling after i.a. injection of 100 ng of recombinant IL-32γ into the right knee joints of C57/Bl6 mice, determined by the 99mTc-uptake method. IL-32 was compared with 100 ng of either murine TNFα or murine IL-1β. Polymyxin B (7 ng) was injected as control. (B) Histopathology at day 2 after i.a. injection of polymyxin B (7 ng). H&E staining was performed. (Original magnification, ×100.) (C) Joint inflammation after i.a. injection of 100 ng of IL-32γ. H&E staining was performed. (Original magnification, ×100.) (D) Severe cell influx in joint cavity and synovial tissue. H&E staining was performed. (Original magnification, ×200.) (E) Monocyte/macrophage-like cells at day 4 after IL-32γ injection. H&E staining was performed. (Original magnification, ×400.) (F) No cartilage matrix proteoglycan loss at day 4 after polymyxin B injection, visualized by Safranin O staining. (Original magnification, ×200.) (G) Depletion of cartilage proteoglycans at day 4 after IL-32γ exposure.
IL-32-Mediated Joint Inflammation Is, in Part, TNFα-Dependent.
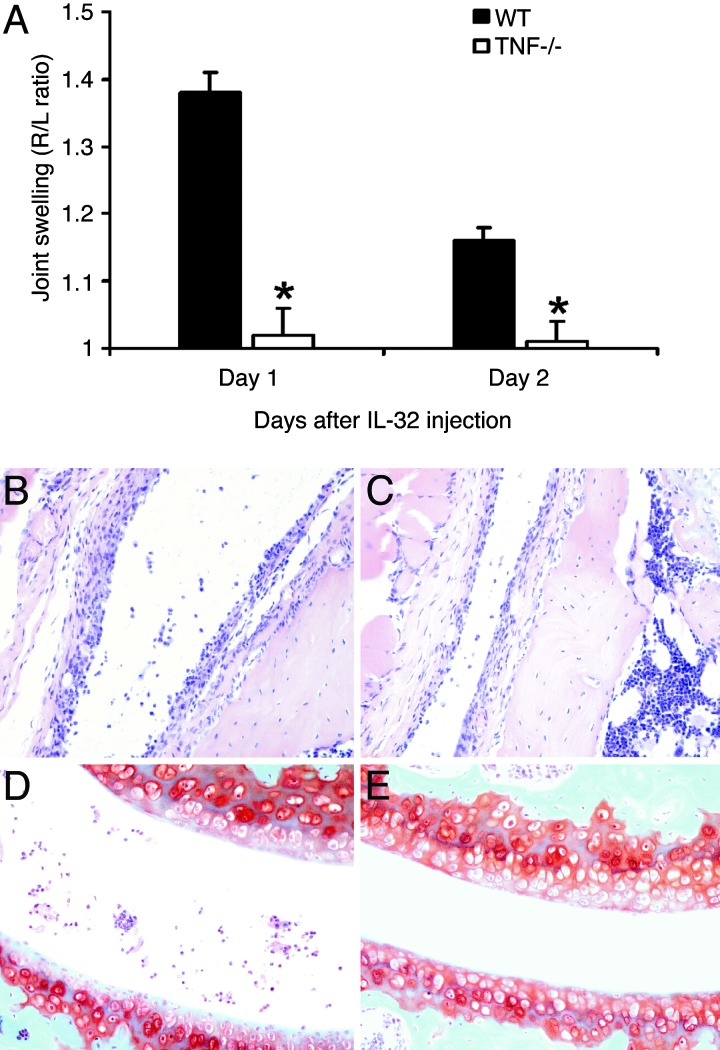
Because it is demonstrated that IL-32 induces TNFα in several cells types in vitro, we investigated the extent of TNFα dependency in vivo. Therefore, we injected IL-32γ directly into knee joints of TNFα gene knockout mice and analyzed joint swelling and histopathology. Fig. 5A shows that IL-32-induced joint swelling was completely TNFα-dependent, because no joint swelling was detectable on day 1 or day 2. Histological examination revealed that TNFα is partially involved in IL-32-driven influx of inflammatory cells into the joint. Fig. 5 B and C illustrates the TNFα dependency of IL-32γ-induced influx of inflammatory cells. Reduced numbers of cells were noted in the synovial tissue or joint cavity of TNFα-deficient mice (Fig. 5C). In addition, IL-32-mediated proteoglycan loss of the cartilage at day 4 was slightly reduced in TNFα-deficient mice when compared with WT mice (Fig. 5 D and E).
Fig. 5.
IL-32-driven joint inflammation in TNFα-deficient mice. (A) Joint swelling was determined at days 1 and 2 after i.a. injection of 100 ng of IL-32γ into WT or TNFα-deficient (TNF−/−) mice. As control, 6 μl of polymyxin B (7 ng) was injected in the left knee joint (data not shown). The data represent five mice per group. ∗, P < 0.001, Mann–Whitney U test, compared with WT mice. (B) Synovitis at day 2 after i.a. injection of 100 ng of IL-32γ into the knee of a WT mouse. H&E staining was performed. (Original magnification, ×200.) (C) Cell influx in the synovial lining of same dose of IL-32 in TNFα-deficient mice. (D and E) Proteoglycan loss in patella and femur cartilage layers in both WT and TNFα-deficient mice. Safranin O staining was performed. (Original magnification, ×200.) See Fig. 4F for normal cartilage proteoglycan staining.
Discussion
In the present study we demonstrate that the proinflammatory cytokine IL-32 has arthritogenic effects in mice, and it correlates with the severity of inflammation in synovial biopsies of RA patients. This study directly demonstrates the role of IL-32 in a human disease and identifies IL-32 as a potential therapeutic target in RA.
IL-32 was initially identified as a transcript expressed after stimulation by IL-2 or IFNγ (reviewed in ref. 13), but the gene product was subsequently shown to possess the property of a proinflammatory cytokine inducing other cytokines such as TNFα, IL-1β, IL-6, and chemokines (13). These properties suggested that IL-32 might play an important role in the amplification of inflammatory reactions. Indeed, we have described an additional but unexpected proinflammatory effect of IL-32, namely the augmentation of cytokine production by muramyl peptides (14). Muramyl peptides are present in all bacteria. In fact, the production of IL-6 by muramyl peptides depends on active IL-1β release, which, in turn, requires caspase-1. This property of IL-32 to amplify the proinflammatory signals induced by the intracellular pattern recognition receptor NOD2 has particular importance because a mutation in NOD2 is known to be involved in the pathogenesis of Crohn's disease (14). These and the present data suggest that IL-32 is an important proinflammatory mediator possibly involved in the pathogenesis of several autoimmune diseases. This hypothesis is supported by the data observation that IL-32 is expressed in the synovial tissue from 83% of the RA patients tested.
The importance of IL-32 for the induction of inflammation in the RA-affected joints is sustained by the observation that IL-32 expression was correlated with markers of inflammation such as ESR. In addition, IL-32 expression strongly correlated with the expression of other proinflammatory cytokines (TNFα, IL-1β, and IL-18) in the synovial biopsies. It is generally accepted that TNFα and IL-1β are the master cytokines in the process of chronic joint inflammation (18, 19). Arthritis could be elicited by local injection of recombinant cytokines into the joint (17), and chronic, erosive arthritis develops spontaneously in mice overexpressing human TNFα (20, 21) or lacking the natural inhibitor of IL-1, namely the IL-1 receptor antagonist (22, 23). In addition, IL-18 has been described by us and other authors to be an important proinflammatory mediator in RA (15, 24–26). Because IL-32 was strongly correlated with the expression of these cytokines in the synovial biopsies and because IL-32 induces these other cytokines in vitro, we conclude that IL-32 may participate in the positive-feedback mechanisms inducing inflammation.
An important aspect of this study deals with the possible mechanisms through which IL-32 induces inflammation in the joint. One important aspect is represented by the induction of other proinflammatory cytokines (TNFα, IL-1, and IL-18), as detailed above. The effects of these cytokines on cell recruitment and activation, cartilage destruction, and bone destruction (21, 22) could in turn explain part of the effects of IL-32. Indeed, when TNFα-deficient mice were injected with recombinant human IL-32γ, the joint swelling induced by a single i.a. injection of IL-32 was fully absent, demonstrating that this particular proinflammatory effect of IL-32 is due to intermediary production of TNFα. It was previously demonstrated that TNFα is the pivotal cytokine that drives joint swelling in acute joint inflammation (27, 28). Interestingly, the influx of proinflammatory cells was partly TNF-dependent. The IL-32-driven cell influx might be a result of the direct effect of IL-32 and IL-32-induced TNFα production. Both IL-32 and TNFα are potent inducers of MIP-2 and IL-8 in macrophages (Fig. 3A and refs. 13 and 14). The latter cytokines attract inflammatory cells to sites of inflammation (29, 30).
In addition, IL-32 could exert direct effects on the inflammatory reaction in the joint. Injection of IL-32 induced a marked recruitment of inflammatory cells in the joints, which is a crucial event in the inflammatory reaction. Moreover, a substantial body of evidence exists that PGE2 exerts pathological roles in the pathogenesis of RA, especially through its effects on cartilage and bone destruction (31, 32). Low concentrations of IL-32 induced production of PGE2 from human monocytes and mouse macrophages, which is an additional likely mechanism through which IL-32 exerts its arthritogenic effects. Consistent with these in vitro data is the observation that IL-32 induced depletion of proteoglycans from the cartilage layer 4 days after the i.a. injection (Fig. 4G). Interestingly, cartilage proteoglycan depletion was not TNFα-dependent, because only a minor reduction of proteoglycan loss was noted in TNFα-deficient mice after IL-32 injection. Of note, because the murine homolog of IL-32 has yet to been isolated as of this writing, studies with neutralizing antibodies to murine IL-32 in mouse models of arthritis could not be included in the present study.
The importance of IL-32 for the inflammatory reactions in RA gains further support from two other studies. In one study, IL-32 was the gene most strongly correlated with the inflammation of RA (33). In that study, the expression of a large number of proinflammatory genes was analyzed by microarray comparing genes expressed synoviocytes from eight patients with RA to those in nine patients with osteoarthritis. IL-32 was the most prominently differentially expressed gene in RA but not in osteoarthritis (33). A second study demonstrated that overexpression of human IL-32 promotes the development of murine type II collagen-induced arthritis (34). Consistent with present study, it was shown that IL-32 promotes proliferation and expression of proinflammatory cytokines in murine macrophages.
In conclusion, the clinical data presented in this study and those of other investigators (33) as well as the ability of IL-32 to exert arthritogenic properties in mice (34) implicate IL-32 as a proinflammatory cytokine participating in the synovitis of RA and possibly of the destruction component on cartilage. Because IL-32 expression correlates with clinical and histological markers of disease severity as well as the presence of cytokines known to be important in the pathogenesis of RA, reducing IL-32 activity may provide benefit to patients with RA.
Materials and Methods
Patients.
Twenty-nine consecutive patients with RA were enrolled in the study. All patients met the American College of Rheumatology criteria for RA (35). The disease activity was calculated by using the disease activity score, and, for the current study only, patients with a disease activity score >3.2 were included (15, 16). The therapeutic regimens of all patients were recorded before blood sampling. Patients receiving prednisone within 6 weeks or biologic therapies, including anti-TNFα or IL-1 receptor antagonist, before the study were not included. Percutaneous biopsies of the knee joint were performed with a Parker–Pearson needle after local administration of anesthesia. An average of 30 samples was obtained at each procedure. Before the biopsy, the ESR was measured, and knee joints were scored by an experienced rheumatologist (P.B.) for the absence (score of 0) or presence of local pain, swelling, or effusion (score of 1, respectively, for each of these). The three scores were added, and patients were classified as having no (score 0), moderate (score 1–2), or severe (score 3) knee-joint arthritis. The synovial tissue from healthy individuals and patients with osteoarthritis was isolated during arthroscopic procedures performed by orthopedic surgeons. The Medical Ethics Committee of the Radboud University Nijmegen Medical Centre approved the study protocol.
Antibodies for Detection of IL-32, IL-18, IL-1β, and TNFα.
Monoclonal anti-human IL-32 was generated as described (13). Affinity-purified goat anti-human IL-32 was used to detect IL-32 expression in RA synovial tissue specimens (13). Monoclonal antibodies against human TNFα (IgG1 and 52B83) were obtained from Monosan (Uden, The Netherlands). Monoclonal antibodies against human IL-1β (IgM and AB-1) were obtained from Oncogene Science, and monoclonal antibodies against human IL-18 (IgG and AF 318) were purchased from R & D Systems. Biotinylated swine anti-rabbit Ig affinity-isolated F(ab′)2 was obtained from DAKO. Avidin peroxidase (Elite kit) was obtained from Vector Laboratories.
Immunohistochemical Analysis of RA Synovial Tissues.
Tissue samples were immediately fixed with 4% formaldehyde and embedded in paraffin. After dewaxing and dehydration, sections were blocked with normal swine serum followed by 60 min of incubation with the antibodies against IL-32, IL-18, IL-1β, and TNFα at a concentration of 0.3 μg/ml. The secondary antibody, biotinylated swine anti-rabbit Ig, was added to the incubation for 30 min. Slides were stained with streptavidin peroxidase, developed with diaminobenzidine, and counterstained with hematoxylin for 30 s. Sections were coded and randomly analyzed by two blinded observers (B.O.-W. and L.A.B.J.). Inadequate sections, which lacked synovial lining, were left out of the analysis. Staining for IL-32 was semiquantitatively scored on a five-point scale (range 0–4) at ×200 magnification; a score of 0 represented no or minimal staining, a score of 1 indicated 10–20% positive cells, a score of 2 indicated 30–40% positive cells, a score of 3 indicated 50–60% positive cells, and a score of 4 represented staining of >60% of the cells. Staining for IL-1β, IL-18, and TNFα was performed as described (15, 16).
PGE2 Production by Human PBMC.
Briefly, PBMC were isolated from heparinized venous blood by using density-gradient centrifugation over Histopaque (Sigma) The interphase was collected and washed twice with citrated PBS. After washing, PBMC were adjusted to 5 × 105 cells per ml in RPMI medium 1640 supplemented with antibiotics and 10% heat-inactivated FCS (Life Technologies, Breda, The Netherlands). The PBMC were incubated with IL-32γ (10 ng/ml) for 24 h at 37°C. PGE2 concentrations were measured by enhanced electrochemiluminescence with acetylcholinesterase-conjugated tracer used for quantification (36, 37). The sensitivity of the assay was 25 pg/ml.
Animals.
Male C57/Bl6 mice were obtained from Charles River Wiga (Sulzfeld, Germany). C3H/HeJ mice were obtained from The Jackson Laboratory. Toll-like receptor 4 gene-deficient mice (C57/Bl6 background) were a kind gift of S. Akira (Osaka University, Osaka). Breeder pairs of TNFα-deficient mice (129Sv/C57/Bl6 background) were obtained from G. Kollias (Biomedical Sciences Research Center, Athens, Greece). The mice were housed in filter-top cages, and water and food were provided ad libitum. Care was taken to house all of the deficient and control littermate mice under identical conditions. The mice were used at the age of 10–12 weeks.
Cytokine Production by Murine Peritoneal Macrophages.
To investigate the capacity of recombinant IL-32 to stimulate proinflammatory mediators in mice, peritoneal macrophages of C3H/HeJ mice were stimulated with purified recombinant IL-32. The cells were harvested 10 min after the i.p. injection of 4 ml of sterile PBS containing 0.38% sodium citrate. After centrifugation and washing, the cells were resuspended in RPMI medium 1640. Cells were cultured in 96-well microtiter plates at 1 × 105 cells per well in a volume of 100 μl. After stimulation for 24 h with recombinant IL-32 (10 ng/ml and 20 ng/ml), the proinflammatory cytokines TNFα and MIP-2 were measured by electrochemiluminescence as described (13, 14).
i.a. Injection of Human IL-32 in Murine Knee Joints.
Recombinant human IL-32γ was i.a. injected in the right knee joint of C57/Bl6 mice at a concentration of 100 ng per 7 μl. IL-32γ was preincubated with polymyxin B (1 μg/ml) for 30 min at room temperature. As control, 7 μl of polymyxin B (1 μg/ml) was injected in the left knee joint. Murine IL-1β and TNFα were obtained from R & D Systems. Joint swelling and histopathology were examined at days 1, 2, 4, and 7 after injection of human IL-32γ. To exclude a potential effect of LPS contamination of the recombinant human IL-32γ, Toll-like receptor 4 gene-deficient mice were injected i.a. with 100 ng of human IL-32γ. To determine the contribution of endogenous TNFα in IL-32-mediated joint pathology TNFα gene-deficient mice were included in the study.
Measurement of Murine Joint Inflammation.
Joint inflammation was quantified by the 99mTc-uptake method (26, 27). This method measures by external γ counting the accumulation of a small radioisotope at the site of inflammation because of local increased blood flow and tissue swelling. The severity of inflammation is expressed as the ratio of the 99mTc uptake in the right (inflamed) over the left (control) knee joint. All values exceeding 1.10 were assigned as inflammation.
Histology.
Mice were killed by ether anesthesia. Thereafter, whole knee joints were removed and fixed for 4 days in 4% formaldehyde. After decalcification in 5% formic acid the specimens were processed for paraffin embedding. Tissue sections (7 μm) were stained with H&E (cell influx) or Safranin O (cartilage proteoglycan depletion). Histopathological changes were scored by using the following parameters. Infiltration of cells was scored on a scale of 0–3, depending on the amount of inflammatory cells in the synovial cavity and synovial tissues. The loss of proteoglycans was scored on a scale of 0–3, ranging from full stained cartilage to destained cartilage or complete loss of articular cartilage. Histopathological changes in the knee joints were scored in the patella/femur region on five semiserial sections of the joint spaced 70 μm apart. Scoring was performed on decoded slides by two observers, as described (26, 27, 38).
Statistical Analysis.
Analysis was performed by using prism, version 4.0 for Windows (GraphPad, San Diego). Data are expressed as mean or as median as appropriate. Within-groups comparisons were analyzed by paired Student's t test or Mann–Whitney rank-sum test. Correlations are expressed by using the Spearman's rank correlation coefficient. Only P values <0.05 were considered significant.
Acknowledgments
M.G.N. and F.A.J.v.d.L. were supported by a VIDI grant from The Netherlands Organization for Scientific Research. This study was supported by National Institutes of Health Grants AI-15614, HL-68743, and CA-046934 (to C.A.D.).
Abbreviations
- RA
rheumatoid arthritis
- ESR
erythrocyte sedimentation rate
- PBMC
peripheral blood mononuclear cells
- PGE2
prostaglandin PGE2
- H&E
hematoxylin and eosin
- i.a.
intraarticular
- MIP-2
macrophage inflammatory protein
- NOD
nucleotide oligomerization domain.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Arend W. P., Dayer J.-M. Arthritis Rheum. 1995;38:151–160. doi: 10.1002/art.1780380202. [DOI] [PubMed] [Google Scholar]
- 2.Feldmann M., Brennan F. M., Maini R. N. Annu. Rev. Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 3.Miossec P., van den Berg W. Arthritis Rheum. 1997;40:2105–2115. doi: 10.1002/art.1780401203. [DOI] [PubMed] [Google Scholar]
- 4.Bresnihan B., Tak P. P. Baillieres Best. Pract. Res. Clin. Rheumatol. 1999;13:645–659. doi: 10.1053/berh.1999.0051. [DOI] [PubMed] [Google Scholar]
- 5.Goronzy J. J., Weyand C. M. Immunol. Rev. 2005;204:55–73. doi: 10.1111/j.0105-2896.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- 6.Thomas R., MacDonald K. P., Pettit A. R., Cavanagh L. L., Padmanabha J., Zehntner S. J. Leukocyte Biol. 1999;66:286–292. doi: 10.1002/jlb.66.2.286. [DOI] [PubMed] [Google Scholar]
- 7.Santiago-Schwarz F., Anand P., Liu S., Carsons S. E. J. Immunol. 2001;167:1758–1768. doi: 10.4049/jimmunol.167.3.1758. [DOI] [PubMed] [Google Scholar]
- 8.Vossenaar E. R., van Venrooij W. J. Arthritis Res. Ther. 2004;6:107–111. doi: 10.1186/ar1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutton B., Corper A., Bonagura V., Taussig M. Immunol. Today. 2000;21:177–183. doi: 10.1016/s0167-5699(00)01589-9. [DOI] [PubMed] [Google Scholar]
- 10.Leadbetter E. A., Rifkin I. R., Hohlbaum A. M., Beaudette B. C., Shlomchik M. J., Marshak-Rothstein A. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 11.Radstake T. R., Roelofs M. F., Jenniskens Y. M., Oppers-Walgreen B., van Riel P. L., Barrera P., Joosten L. A. B., van den Berg W. B. Arthritis Rheum. 2004;50:3856–3865. doi: 10.1002/art.20678. [DOI] [PubMed] [Google Scholar]
- 12.Roelofs M. F., Joosten L. A. B., Abdollahi-Roodsaz S., van Lieshout A. W., Sprong T., van den Hoogen F. H., van den Berg W. B., Radstake T. R. Arthritis Rheum. 2005;52:2313–2322. doi: 10.1002/art.21278. [DOI] [PubMed] [Google Scholar]
- 13.Kim S. H., Han S. Y., Azam T., Yoon D.-Y., Dinarello C. A. Immunity. 2005;22:131–142. doi: 10.1016/j.immuni.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Netea M. G., Azam T., Ferwerda G., Girardin S. E., Walsh M., Park J. S., Abraham E., Yoon D.-Y., Dinarello C. A., Kim S.-H. Proc. Natl. Acad. Sci. USA. 2005;102:16309–16314. doi: 10.1073/pnas.0508237102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joosten L. A. B., Radstake T. R., Lubberts E., van den Bersselaar L. A., van Riel P. L., van Lent P. L., Barrera P., van den Berg W. B. Arthritis Rheum. 2003;48:339–347. doi: 10.1002/art.10814. [DOI] [PubMed] [Google Scholar]
- 16.Barrera P., Joosten L. A. B., den Broeder A. A., van de Putte L. B., van Riel P. L., van den Berg W. B. Ann. Rheum. Dis. 2001;60:660–669. doi: 10.1136/ard.60.7.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van de Loo F. A., Joosten L. A. B., van Lent P. L., Arntz O. J., van den Berg W. B. Arthritis Rheum. 1995;38:164–172. doi: 10.1002/art.1780380204. [DOI] [PubMed] [Google Scholar]
- 18.Vilcek J., Feldmann M. Trends Pharmacol. Sci. 2004;25:201–209. doi: 10.1016/j.tips.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 19.Dinarello C. A. J. Exp. Med. 2005;201:1355–1359. doi: 10.1084/jem.20050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kontoyiannis D., Pasparakis M., Pizarro T. T., Cominelli F., Kollias G. Immunity. 1999;10:387–398. doi: 10.1016/s1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- 21.Zwerina J., Hayer S., Tohidast-Akrad M., Bergmeister H., Redlich K., Feige U., Dunstan C., Kollias G., Steiner G., Smolen J., Schett G. Arthritis Rheum. 2004;50:277–290. doi: 10.1002/art.11487. [DOI] [PubMed] [Google Scholar]
- 22.Nicklin M. J., Hughes D. E., Barton J. L., Ure J. M., Duff G. W. J. Exp. Med. 2000;191:303–312. doi: 10.1084/jem.191.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horai R., Saijo S., Tanioka H., Nakae S., Sudo K., Okahara A., Ikuse T., Asano M., Iwakura Y. J. Exp. Med. 2000;191:313–320. doi: 10.1084/jem.191.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gracie J. A., Forsey R. J., Chan W. L., Gilmour A., Leung B. P., Greer M. R., Kennedy K., Carter R., Wei X. Q., Xu D., et al. J. Clin. Invest. 1999;104:1393–1401. doi: 10.1172/JCI7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plater-Zyberk C., Joosten L. A. B., Helsen M. M., Sattonnet-Roche P., Siegfried C., Alouani S., van de Loo F. A., Graber P., Aloni S., Cirillo R., et al. J. Clin. Invest. 2001;108:1825–1832. doi: 10.1172/JCI12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joosten L. A. B., Smeets R. L., Koenders M. I., van den Bersselaar L. A., Helsen M. M., Oppers-Walgreen B., Lubberts E., Iwakura Y., van de Loo F. A., van den Berg W. B. Am. J. Pathol. 2004;165:959–967. doi: 10.1016/S0002-9440(10)63357-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joosten L. A. B., Koenders M. I., Smeets R. L., Heuvelmans-Jacobs M., Helsen M. M., Takeda K., Akira S., Lubberts E., van de Loo F. A., van den Berg W. B. J. Immunol. 2003;171:6145–6153. doi: 10.4049/jimmunol.171.11.6145. [DOI] [PubMed] [Google Scholar]
- 28.Kuiper S., Joosten L. A. B., Bendele A. M., Edwards C. K., Arntz O. J., Helsen M. M., van de Loo F. A., van den Berg W. B. Cytokine. 1998;10:690–702. doi: 10.1006/cyto.1998.0372. [DOI] [PubMed] [Google Scholar]
- 29.Lomas-Neira J. L., Chung C. S., Wesche D. E., Perl M., Ayala A. J. Leukocyte Biol. 2005;77:846–853. doi: 10.1189/jlb.1004617. [DOI] [PubMed] [Google Scholar]
- 30.Calkins C. M., Heimbach J. K., Bensard D. D., Song Y., Raeburn C. D., Meng X., McIntyre R. C., Jr. J. Surg. Res. 2001;101:232–237. doi: 10.1006/jsre.2001.6274. [DOI] [PubMed] [Google Scholar]
- 31.McCoy J. M., Wicks J. R., Audoly L. P. J. Clin. Invest. 2002;110:651–658. doi: 10.1172/JCI15528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Westman M., Korotkova M., Af Klint E., Stark A., Audoly L. P., Klareskog L., Ulfgren A. K., Jakobsson P. J. Arthritis Rheum. 2004;50:1774–1780. doi: 10.1002/art.20286. [DOI] [PubMed] [Google Scholar]
- 33.Cagnard N., Letourneur F., Essabbani E., Devauchelle V., Mistou S., Rapinat A., Decraene C., Fournier C., Chiocchia G. Eur. Cytokine Network. 2005;16:289–292. [PubMed] [Google Scholar]
- 34.Shoda H., Fujio K., Yamaguchi Y., Okamoto A., Kitamura T., Yamamoto K. Arthritis Rheum. 2005;52:S158. [Google Scholar]
- 35.Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 36.Lee J.-K., Kim S.-H., Lewis E. C., Azam T., Reznikov L. L., Dinarello C. A. Proc. Natl. Acad. Sci. USA. 2004;101:8815–8820. doi: 10.1073/pnas.0402800101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Netea M. G., Azam T., Lewis E. C., Joosten L. A. B., Wang M., Langenberg D., Mend X., Chan E. D., Yoon D.-Y., Ottenhoff T., et al. PLoS Med. 2006 doi: 10.1371/journal.pmed.0030277. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joosten L. A. B., Heuvelmans-Jacobs M., Lubberts E., van de Loo F. A., Bakker A. C., Helsen M. M., Richards C. D., van den Berg W. B. Arthritis Rheum. 2002;46:1379–1389. doi: 10.1002/art.10233. [DOI] [PubMed] [Google Scholar]