Abstract
Psychostimulant-induced alteration of dendritic spines on dopaminoceptive neurons in nucleus accumbens (NAcc) has been hypothesized as an adaptive neuronal response that is linked to long-lasting addictive behaviors. NAcc is largely composed of two distinct subpopulations of medium-sized spiny neurons expressing high levels of either dopamine D1 or D2 receptors. In the present study, we analyzed dendritic spine density after chronic cocaine treatment in distinct D1 or D2 receptor-containing medium-sized spiny neurons in NAcc. These studies made use of transgenic mice that expressed EGFP under the control of either the D1 or D2 receptor promoter (Drd1-EGFP or Drd2-EGFP). After 28 days of cocaine treatment and 2 days of withdrawal, spine density increased in both Drd1-EGFP- and Drd2-EGFP-positive neurons. However, the increase in spine density was maintained only in Drd1-EGFP-positive neurons 30 days after drug withdrawal. Notably, increased ΔFosB expression also was observed in Drd1-EGFP- and Drd2-EGFP-positive neurons after 2 days of drug withdrawal but only in Drd1-EGFP-positive neurons after 30 days of drug withdrawal. These results suggest that the increased spine density observed after chronic cocaine treatment is stable only in D1-receptor-containing neurons and that ΔFosB expression is associated with the formation and/or the maintenance of dendritic spines in D1 as well as D2 receptor-containing neurons in NAcc.
The mesolimbic dopaminergic pathway is composed of neurons in the ventral tegmental area that innervate the nucleus accumbens (NAcc), olfactory tubercle, prefrontal cortex, and amygdala (1), whereas nigrostriatal dopaminergic neurons in the substantia nigra (pars compacta) provide an ascending projection to dorsal striatum (2). Psychostimulants elevate synaptic concentrations of dopamine in NAcc: cocaine, by blocking dopamine uptake from the synaptic cleft, and amphetamine, by promoting dopamine release from nerve terminals (3–5). Repeated, intermittent administration of psychostimulants results in augmented behavioral responses (sensitization) to the acute stimulatory effects of these drugs (6–8). Most lines of evidence suggest that adaptive changes in the ventral tegmental area–NAcc dopaminergic system are central to alterations in the experience-dependent plasticity that underlies drug-induced behavior.
In addition to dopamine, glutamate is required for the development of behavioral sensitization in response to psychostimulants (9, 10). Medium-sized spiny neurons (MSNs) in ventral striatum receive excitatory glutamatergic projections from prefrontal cortex that synapse onto the heads of dendritic spines. MSNs also are the major target for dopaminergic axons that synapse onto spine necks (1, 11, 12). Therefore, dendritic spines in MSNs represent the cellular compartment where dopaminergic and glutamatergic transmission are initially integrated.
Dopamine acts on two major receptor subfamilies, the D1 subfamily (D1 and D5 subtypes) and the D2 subfamily (D2, D3, and D4 subtypes) (13). In dorsal striatum, anatomical studies have shown that striatonigral MSNs contain high levels of D1 receptors (together with substance P and dynorphin), whereas striatopallidal MSNs predominantly express D2 receptors (together with enkephalin) (14–17). The projections from NAcc are more complex than in dorsal striatum, with the shell and core parts of the NAcc projecting to distinct subregions of the ventral pallidum and to the ventral tegmental area and substantia nigra (18). Whereas D2 receptors and enkephalin are highly expressed in projections to the ventral pallidum, D1 receptors and substance P are found equally distributed in projections to ventral pallidum and ventral tegmental area (19). Studies of agonists and antagonists selective for D1 or D2 receptors showed that both D1 and D2 receptors are required for psychostimulant-dependent behavioral changes (20–25). However, the roles of these receptors appear to be different. For example, stimulation of D1 receptors attenuates cocaine seeking induced by cocaine priming injections and cocaine-related environmental cues, whereas stimulation of D2 receptors facilitates cocaine-induced reinstatement (26–28).
The behavioral abnormalities associated with psychostimulant addiction are extremely long-lived. Therefore, there has been considerable interest in identifying long-lasting drug-induced changes at the molecular and structural level in neuronal circuits regulated by dopamine and glutamate (29–32). Notably, long-term exposure to cocaine or amphetamine has been found to increase the number of dendritic branch points and spines of MSNs in NAcc (33–35). These structural changes have been shown to persist for up to ≈1–3.5 months after the last drug exposure (30, 35) and have been suggested to underlie long-lasting alterations in synaptic plasticity associated with psychostimulant exposure.
The goal of the present study was to examine cocaine-induced structural alterations of dendritic spines in subpopulations of accumbal MSNs that express either D1 or D2 receptors. In these studies, we have used bacterial artificial chromosome (BAC) transgenic mice that express EGFP under the control of either the D1 (Drd1-EGFP) or D2 (Drd2-EGFP) dopamine receptor promoter (36). The results indicate that, although increased spine density initially occurs in D1 receptor-containing MSNs and D2 receptor-containing MSNs, the altered spine density is stable only in D1 receptor-containing neurons. Moreover, we find similar changes in the expression of the transcription factor ΔFosB, suggesting that ΔFosB may be involved in the formation and/or maintenance of dendritic spines in D1 as well as D2 receptor-containing neurons in NAcc.
Results
Analysis of MSNs in Drd1-EGFP and Drd2-EGFP BAC Transgenic Mice.
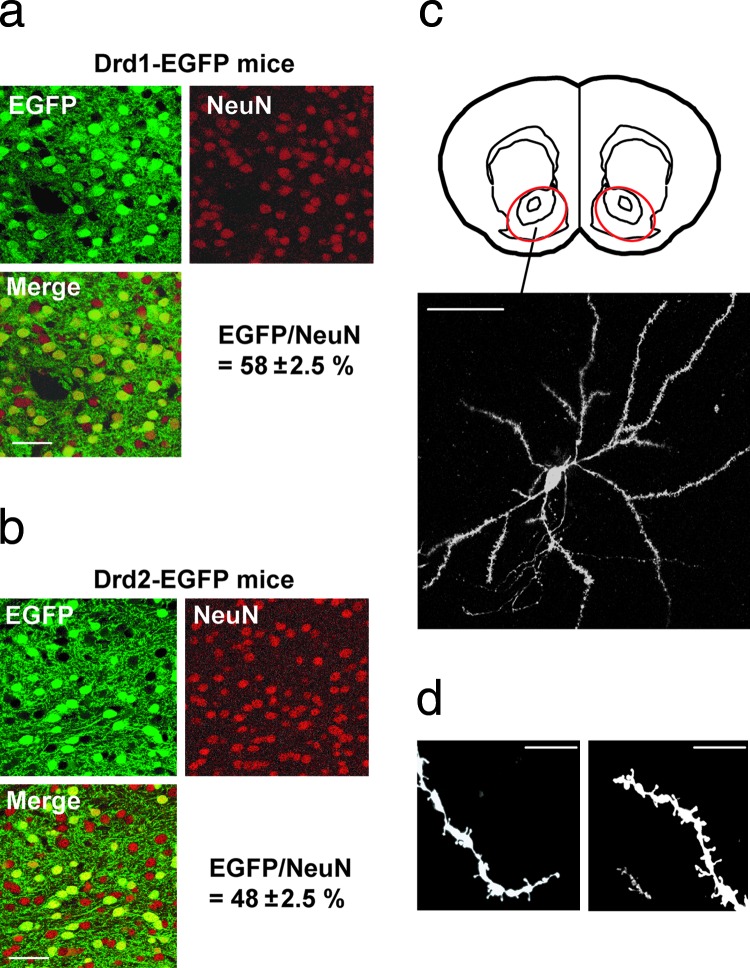
The projection pattern of MSNs from dorsal and ventral striatum in Drd1-EGFP or Drd2-EGFP BAC transgenic mice has been characterized through analysis of GFP expression (36). The differential expression of GFP in MSNs of dorsal striatum corresponds generally to that of endogenous D1 or D2 receptors, respectively (36). We further analyzed differential expression of GFP in NAcc in Drd1-EGFP or Drd2-EGFP mice (Fig. 1a and b). Although ≈58% of neurons in NAcc expressed GFP in Drd1-EGFP mice (Fig. 1a), ≈48% of neurons in NAcc expressed GFP in Drd-2-EGFP mice (Fig. 1b). MSNs represent 90–95% of all neurons in NAcc (12, 37). D1 receptors are expressed only in MSNs, and D2 receptors are expressed in MSNs and in cholinergic interneurons, which represent 1–3% of striatal neurons (37). Taking these factors into account, the results suggest that, minimally, ≈10–15% of MSNs in NAcc are likely to express both D1 and D2 receptors.
Fig. 1.
Analysis of MSNs in Drd1-EGFP and Drd2-EGFP mice. (a and b) Fixed brain slices from NAcc of Drd1-EGFP (a) or Drd2-EGFP (b) BAC transgenic mice were immunostained for GFP and NeuN (as a general neuronal marker). The merged images show, in yellow, colocalization of GFP and NeuN. Of neurons in NAcc of Drd1-EGFP mice, 58% expressed GFP, whereas 48% of neurons in NAcc of Drd2-EGFP mice expressed GFP. The numbers of GFP-positive and NeuN-positive neurons were counted independently; all GFP-positive neurons were also NeuN-positive. (c) Visualization of DiI in a single MSN in NAcc. The region of NAcc analyzed in Lower is indicated in the schematic in Upper (red circle) (Bregma, 1.34 mm). Twenty pictures were taken at different z levels (0.5- to 1-μm intervals); the images were then stacked and flattened. (d) Examples of distal dendrites visualized with DiI and used for analysis of spines. (Scale bars: a–c, 50 μm; d, 10 μm.)
Analysis of Dendritic Spines in Drd1-EGFP and Drd2-EGFP Mice.
GFP expression in the Drd1-EGFP and Drd2-EGFP mice was useful to stain neuronal cell bodies. However, the GFP signal in dendrites and dendritic spines was too weak to allow their analysis after immunostaining with anti-GFP antibodies. Particle-mediated ballistic delivery of fluorescent dyes has recently been used to label neuronal populations in a rapid and efficient manner (38). Entire neurons can be labeled using this technique, and the method appears to be comparable to Golgi–Cox staining. To analyze the dendritic morphology of neurons in NAcc, fixed accumbal slices were labeled with the lipophilic fluorescence dye 1,1′-diotadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) by using a gene gun. An example of a DiI-stained MSN is shown in Fig. 1c. Under the conditions used, we generally observed labeled neurons without any overlapping dendrites from other labeled neurons. At higher magnification, detailed dendritic morphology, including dendritic spines, could be observed (Fig. 1d).
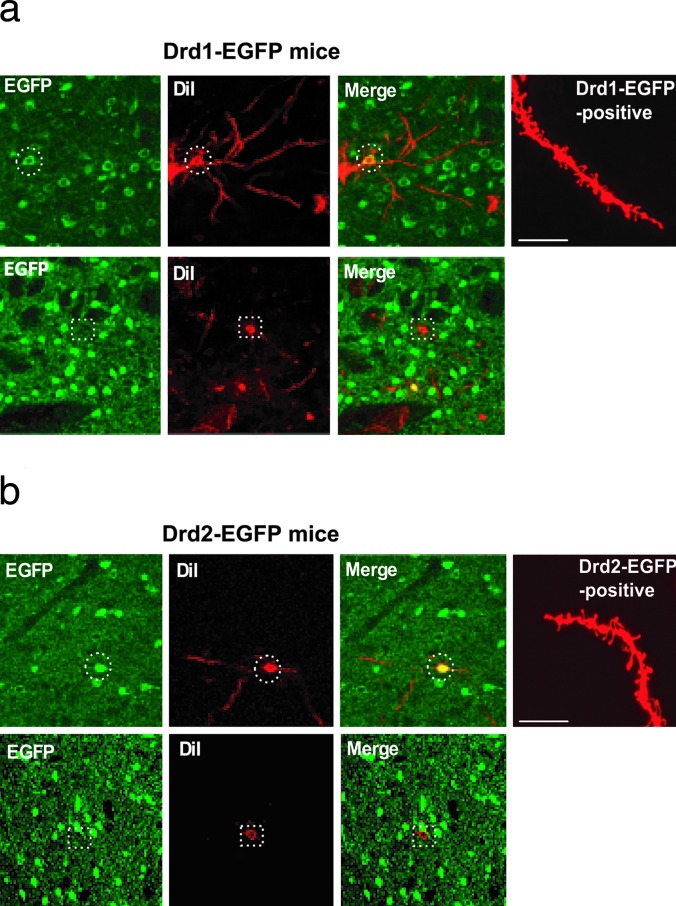
We then used a combination of DiI labeling and immunohistochemistry for GFP in either Drd1-EGFP or Drd2-EGFP transgenic mice, which was made possible by using a low concentration of detergent for tissue permeabilization (see Methods). Through careful comparison of the DiI stain and GFP expression in the cell bodies of MSNs, we could identify DiI- and GFP-positive or DiI-positive and GFP-negative neurons in Drd1-EGFP (Fig. 2a) or Drd2-EGFP (Fig. 2b) mice. For the following studies, we analyzed dendritic morphology in only DiI- and GFP-positive neurons from Drd1-EGFP or Drd2-EGFP mice.
Fig. 2.
Analysis of dendritic spines in Drd1-EGFP and Drd2-EGFP mice. Neurons in NAcc of either Drd1-EGFP mice (a) or Drd2-EGFP mice (b) were first labeled with DiI (red) and then subjected to immunohistochemistry using an anti-GFP antibody (EGFP, green). Only MSNs were labeled with DiI. Images of DiI staining and GFP staining were overlaid (Merge). (a Upper) The dashed circles show an example of double labeling of a DiI-positive and Drd1-EGFP-positive cell. (a Lower) The dashed squares show a lack of double labeling of cells in slices from Drd1-EGFP mice. (b Upper) The dashed circles show an example of double labeling of a DiI-positive and Drd2-EGFP-positive cell. (b Lower) The dashed squares show a lack of double labeling of cells in slices from Drd2-EGFP mice. The rightmost images in a Upper and b Upper show examples of DiI-stained distal dendrites in Drd1-EGFP or Drd2-EGFP mice, respectively. (Scale bars: 10 μm.) The examples shown were from saline-treated mice.
Chronic Cocaine Treatment Results in Increased Spine Density in Accumbal MSNs Expressing Either Drd1-EGFP or Drd2-EGFP.
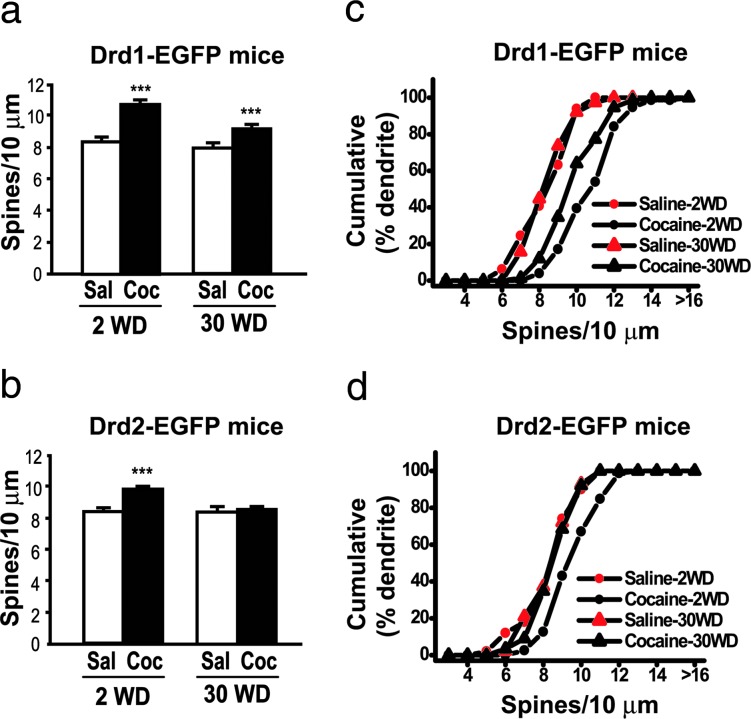
Drd1-EGFP or Drd2-EGFP mice were injected repeatedly with cocaine (30 mg/kg) or saline for four consecutive weeks (see Methods). Two days (2WD) or 30 days (30WD) after the last drug treatment, brains were processed for DiI labeling and immunohistochemistry as described above. A previous study reported that chronic treatment with amphetamine increased spine density on distal but not proximal dendrites of MSNs in NAcc (35). We therefore limited our analysis to distal dendrites (i.e., those with second- or third-order branches), including terminal regions. When analyzed at 2WD, spine density was found to increase in Drd1-EGFP-positive MSNs (128% of saline group) (Fig. 3a and c) and to a lesser extent in Drd2-EGFP-positive neurons (115% of saline group) (Fig. 3 b and d). After 30WD, increased spine density was maintained in Drd1-EGFP-positive neurons (118% of saline control) (Fig. 3 a and c) but not in Drd2-EGFP-positive neurons (Fig. 3 b and d).
Fig. 3.
Chronic cocaine-induced increases in spine density in Drd1-EGFP- or Drd2-EGFP-positive MSNs in NAcc. (a and b) Drd1-EGFP (a) or Drd2-EGFP (b) mice were treated with saline (Sal) or cocaine (Coc, 30 mg/kg) for 4 weeks. Mouse brains 2WD or 30WD were processed for DiI labeling and immunohistochemistry as shown in Fig. 2. All spine-like protrusions on distal dendrites were included in the analysis. Data are expressed as the number of spines per 10 μm of dendritic length (mean ± SEM); ∗∗∗, P < 0.001 versus saline treated group, Kolmogorov–Smirnov test. (c and d) Cumulative frequency plots showing the distribution of spine density from individual neurons that were analyzed in a and b. One to two dendrites from one neuron in 10–15 animals per group were analyzed. The total numbers of dendrites analyzed were as follows. For D1: saline, 49 (2WD) and 38 (30WD); cocaine, 74 (2WD) and 75 (30WD). For D2: saline, 50 (2WD) and 43 (30WD); cocaine, 79 (2WD) and 89 (30WD).
The morphology of dendritic spines is variable in terms of their length and the width of the spine head. We therefore classified dendritic protrusions into four spine classes (stubby, mushroom, thin, and filopodia) at 2WD from cocaine (data not shown). The density of mushroom-type (119.7 ± 4.0%, P < 0.01) and thin spines (120.0 ± 3.4%, P < 0.01) was increased by cocaine treatment in Drd1-EGFP-positive MSNs, whereas the density of stubby (182.4 ± 21.6%, P < 0.05) and mushroom spines (122.5 ± 5.0%, P < 0.01) was increased in Drd2-EGFP-positive MSNs. There was no significant increase in stubby spines in Drd1-EGFP-positive neurons or of thin spines in Drd2-EGFP-positive neurons.
Chronic Cocaine Induces ΔFosB Expression in Drd1-EGFP- or Drd2-EGFP-Positive MSNs in NAcc.
ΔFosB is a member of the Fos family of transcription factors. Whereas acute administration of cocaine induces a rapid and transient induction of several Fos isoforms in NAcc, repeated exposure to cocaine increases the level of ΔFosB. Moreover, the increase in ΔFosB expression persists in NAcc for weeks to months after discontinuation of drug exposure and has been suggested to be involved in long-lasting regulation of gene expression, even after drug taking ceases (29, 39, 40).
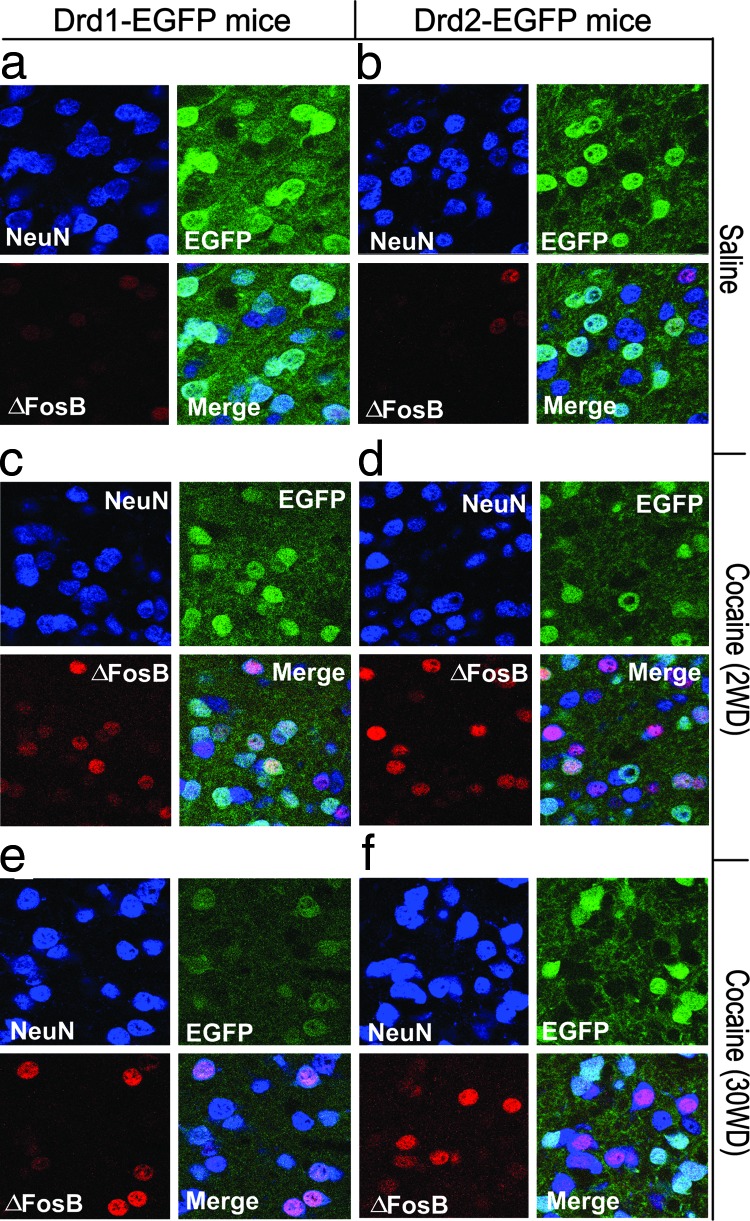
To examine the induction of ΔFosB in NAcc from Drd1-EGFP or Drd2-EGFP mice following cocaine treatment, we analyzed FosB and GFP expression by double labeling (Fig. 4 and Table 1) The anti-FosB antibody recognizes all forms of FosB, but we assume that the increased immunostain represents ΔFosB (see Methods for further discussion). In saline-treated mice, 16% of Drd1-EGFP-positive neurons and 15% of Drd2-EGFP-positive neurons expressed FosB immunoreactivity with relatively weak intensity (Fig. 4 a and b and Table 1). Repeated cocaine treatment followed by 2WD resulted in a significant increase in the number of Drd1-EGFP-positive neurons that coexpressed ΔFosB (55% of GFP-positive neurons) (Fig. 4c and Table 1). A smaller, but still significant increase in ΔFosB expression was found in Drd2-EGFP-positive neurons (25% of GFP-positive neurons) (Fig. 4d and Table 1). As with the changes in spine density, the increased expression of ΔFosB was maintained in Drd1-EGFP-positive neurons (46% of GFP-positive neurons) but not in Drd2-EGFP-positive neurons (15% of GFP-positive neurons) after 30WD (Fig. 4 e and f and Table 1). Note that the increased ΔFosB expression observed in Fig. 4f is present in Drd2-EGFP-negative neurons.
Fig. 4.
Chronic cocaine induces ΔFosB expression in Drd1-EGFP- or Drd2-EGFP-positive MSNs in NAcc. Drd1-EGFP (a, c, and e) or Drd2-EGFP (b, d, and f) mice were treated with saline or chronic cocaine as described in Fig. 3. 2WD (c and d) or 30WD (e and f), the expression of GFP (green) for the identification of Drd1-EGFP- or Drd2-EGFP-positive MSNs and ΔFosB (red) was analyzed by immunohistochemistry. The localization of NeuN (purple) was also analyzed to show the position of neuronal nuclei. NeuN, GFP, and ΔFosB images were merged to examine their coexpression (Merge). Photomicrographs are representative of results from multiple brain sections obtained from three to four animals in each treatment group. Quantitative analysis is shown in Table 1.
Table 1.
Quantification of EGFP-positive neurons expressing ΔFosB
| Mice | ΔFosB/EGFP(+) cells, % |
|---|---|
| Drd1-EGFP | |
| Saline | 16 ± 1.5 |
| Cocaine (2WD) | 55 ± 2.1∗ |
| Cocaine (30WD) | 46 ± 1.4∗ |
| Drd2-EGFP | |
| Saline | 15 ± 1.6 |
| Cocaine (2WD) | 25 ± 2.4∗ |
| Cocaine (30WD) | 15 ± 2.2 |
Animals in the saline groups for the 2WD and 30WD conditions were pooled. Data are expressed as mean ± SEM.
∗, P < 0.01 for cocaine versus saline, Student’s t test. Three to four animals per group and three to four slices from one brain were analyzed. The total numbers of images (110 × 110 μm) analyzed were as follows. D1: saline, 31; cocaine, 29 (2WD) and 25 (30WD); D2: saline, 42; cocaine, 36 (2WD) and 31 (30WD).
Discussion
Long-lasting adaptations in dopaminergic neurotransmission are believed to underlie addictive behaviors associated with psychostimulant drugs. In particular, psychostimulant-induced increases in dendritic spine density of MSNs in NAcc have been hypothesized to be linked to reorganization of synaptic connectivity (30). The NAcc is largely composed of two distinct subpopulations of MSNs expressing high levels of either D1 or D2 dopamine receptors. In the present study, we have analyzed spine density in distinct D1 or D2 receptor-containing MSNs in NAcc after chronic cocaine treatment. The results obtained show that, although increased spine density initially occurs in D1 receptor-containing MSNs and D2 receptor-containing MSNs, altered spine density is stable only in D1-receptor-containing neurons. Moreover, we find a similar pattern of changes in the expression of the transcription factor ΔFosB in D1 and D2 receptor-containing MSNs.
These studies made use of BAC transgenic mice that express GFP in specific subpopulations of MSNs under the control of either the D1 or D2 receptor promoter. Moreover, we developed a double-labeling method that combined immunohistochemistry for GFP with ballistic labeling of neurons using DiI. Previous studies have used the Golgi–Cox method to analyze the effect of psychostimulants on spine density (34), and the DiI method used here gave results that were quantitatively comparable. We developed the double-labeling method because Golgi staining is not compatible with immunohistochemistry. Immunostaining usually requires tissue permeabilization with detergents, a process that typically leads to solubilization of lipophilic dyes from the membrane (38). However, in our current studies, GFP immunostaining did not require a high concentration of detergent for tissue permeabilization and thus could be used in conjunction with lipophilic dye labeling. Our double-labeling method should be generally useful for studies of structural changes in dendritic spines, for example when used for analysis of BAC transgenic mice lines where GFP is expressed in specific populations of neurons in cortex (36).
Although still somewhat controversial, it is believed that D1 and D2 receptors are largely anatomically segregated to the direct (striatonigral) and indirect (striatopallidal) striatal projection neurons, respectively (17, 41). Initial characterization of the localization of GFP in the Drd1-EGFP and Drd2-EGFP mice was consistent with this conclusion (36). Moreover, our analysis of the number of GFP-positive neurons in NAcc from Drd1-EGFP and Drd2-EGFP mice is consistent with the conclusion that ≈50% of MSNs express only D1 receptors, that ≈35–40% express only D2 receptors, and that ≈10–15% coexpress both D1 and D2 receptors. This value of coexpression is similar to that implied by studies of dorsal striatum that combined patch-clamp analysis of single striatal neurons with RT-PCR techniques to isolate and amplify mRNAs (≈17% coexpression of enkephalin and substance P) (42). It should be noted that our current studies do not address the question of expression of D3, D4, and D5 receptors, nor do they address the issue of low levels of expression of D1 receptors in MSNs expressing high levels of D2 receptors or vice versa.
Several previous studies have examined the neuronal localization of psychostimulant-induced Fos expression and the role of D1 and D2 receptors (43–45). Those studies supported the conclusion that Fos and ΔFosB induction is mediated by activation of D1 receptors. However, the cellular localization of Fos expression is influenced by the environmental context in which psychostimulant drugs are administered (46, 47). For example, amphetamine or cocaine given in the home cage induces immediate early genes (including Fos) preferentially in substance P-positive cells that coexpress D1 receptors. In contrast, these drugs can induce Fos expression in both D1 and D2 receptor-containing MSNs when administered in a novel environment. The protocol used in our current studies did not include pairing drug injection with exposure to a novel environment. However, we cannot rule out some sort of context-dependent stress that is responsible for ΔFosB expression in D2 receptor-containing MSNs.
A notable feature of the present results was the parallel pattern of increased spine density and ΔFosB expression. Increased spine density and ΔFosB expression initially occurred in MSNs expressing Drd1-EGFP and Drd2-EGFP. However, these changes were stable only in D1 receptor-containing neurons. One possible explanation for the observation that increased spine density and ΔFosB expression was transiently found in D2 receptor-containing neurons is that this occurred in the small fraction of MSNs that coexpress both D1 and D2 dopamine receptors. Thus, the transient nature of these increases may be associated with antagonistic effects of D2 receptor activation on D1-dependent signaling pathways (48). It is of interest that the changes in spine density and ΔFosB expression were reversible, which may reflect the ability of D2 receptor-dependent signaling pathways to influence the stability of ΔFosB.
The observation that there are parallel changes in the expression of ΔFosB and spine density is consistent with the idea that ΔFosB is involved in the initial formation and the subsequent maintenance of dendritic spines in D1 receptor-containing neurons in NAcc. Expression of ΔFosB is controlled by the D1/DARPP-32/PP1-dependent signaling pathway in MSNs (49). Several studies have shown that ΔFosB plays an important role in the rewarding and locomotor-activating actions of psychostimulants (39), likely by influencing the expression of multiple genes that include neurotransmitter receptors, signaling proteins, and proteins involved in regulation of neuronal morphology (50). However, the specific molecular mechanisms involved in chronic cocaine-induced spine formation are not currently known. Our previous studies have shown that intraaccumbal infusion of the Cdk5 inhibitor roscovitine attenuated cocaine-induced increases in spine density (51). Moreover, Cdk5 is a downstream target gene for ΔFosB and has been implicated in compensatory adaptive changes associated with chronic cocaine treatment (52). Therefore, alteration in Cdk5-dependent phosphorylation is a plausible mechanism underlying cocaine-induced spine formation and/or spine stability. PAK (53), β-catenin (54), PSD-95 (55), and spinophilin (56) are substrates for Cdk5 and are all involved in regulation of spine morphogenesis (57–60). Further characterization of these and other Cdk5 substrates in spines will hopefully shed light on the mechanisms involved in regulation of spine formation by psychostimulants.
Methods
Animals.
Mice carrying an EGFP transgene under the control of either the D1 or D2 dopamine receptors were generated by the Gensat BAC transgenic project (36). The transgenic mice used in this study were 4–5 weeks old and were on a Swiss–Webster background. Mice were maintained in a 12:12-h light/dark cycle and housed in groups of 2–5 with food and water available ad libitum. All animal protocols were in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Rockefeller University Institutional Animal Care and Use Committee.
Drug Treatment.
Chronic cocaine treatment (30 mg/kg, daily) was reported to produce a robust increase in the spine density of MSNs in both the core and shell of NAcc from rat, but a lower dose (15 mg/kg) increased spine density only in the shell (61). We therefore used the higher dose of cocaine to induce structural modification in both parts of NAcc. Mice received one injection (i.p.) of 30 mg/kg cocaine-HCl (or saline) each day for 5 consecutive days, followed by 2 injection-free days, and this procedure was repeated for 4 consecutive weeks. Injections were carried out in the home cage. 2WD or 30WD, mouse brains were processed for DiI labeling and/or immunohistochemistry.
Ballistic Labeling with the Fluorescent Dye DiI.
Mice were anesthetized with 80 mg/kg sodium pentobarbital and perfused transcardially with 5 ml of PBS, followed by rapid perfusion with 40 ml of 4% paraformaldehyde in PBS (20 ml/min). Brains were quickly removed from the skull and postfixed in 4% paraformaldehyde for 10 min. Brain slices (100 μm) were labeled by ballistic delivery of fluorescent dye DiI (Molecular Probes) as described in ref. 38. A combined DiI labeling–immunohistochemistry method was developed with a low concentration of detergent. DiI-labeled sections were permeabilized with 0.01% Triton X-100 in PBS for 15 min and then incubated in 0.01% Triton X-100 and 10% normal goat serum in PBS for 1 h to minimize nonspecific labeling. Tissue sections were then incubated with 1% normal goat serum/0.01% Triton X-100 and anti-GFP antibody (Abcam, Cambridge, MA) for 2 h at room temperature, washed, and incubated in a 1:1,000 dilution of FITC-conjugated secondary antibody (Molecular Probes). Sections were placed on microscope slides and coverslipped with mounting medium. The ballistic labeling method allowed detailed analysis of dendritic spine structure, and the results obtained were qualitatively and quantitatively comparable with previous studies using the Golgi–Cox impregnation method in rat brain slices (34). However, in contrast to previous studies, we rarely observed two-headed spines in DiI-stained neurons. This difference may be caused by the sensitivity of staining methods or variability of mouse (this study) versus rat tissue (34).
Immunohistochemistry.
Animals were anesthetized and perfused as described above. Brains were removed and stored overnight in 4% paraformaldehyde at 4°C. Brains were transferred to 30% sucrose in PBS solution for cryoprotection. Coronal sections (12 μm) were cut on a freezing microtome (Leica) and then processed for immunohistochemistry. Brain sections were then permeabilized in 0.3% Triton X-100 in PBS for 15 min and rinsed twice in PBS. The sections were preincubated in 10% normal goat serum in PBS for 1 h at 37°C, exposed to primary antibodies (diluted in 1% normal goat serum in PBS) overnight at 4°C, and then rinsed in PBS and incubated with secondary antibodies for 1 h at 37°C. The following antibodies were used: rabbit anti-pan-FosB (SC-48, 1:500; Santa Cruz Biotechnology), mouse anti-NeuN (Chemicon), rabbit anti-GFP, FITC-conjugated anti-rabbit IgG, and rhodamine-conjugated anti-mouse IgG (Molecular Probes). For triple labeling (ΔFosB, NeuN, and GFP), brain sections were first immunostained with anti-pan FosB antibody and anti-NeuN antibody and then incubated with secondary antibodies (rhodamine-conjugated anti-rabbit IgG and cyan-conjugated anti-mouse IgG). Double-stained brain sections were further processed for GFP immunostaining using Zenon labeling technology (Zenon Alexa Fluor 488, Molecular Probes). The anti-pan-FosB antibody was raised to the N terminus of FosB and recognizes ΔFosB and full-length FosB (62). Based on previous studies that showed that ΔFosB but not FosB or other Fos-related antigens is stably expressed after chronic cocaine treatment, we assume that the long-lasting increases in immunoreactivity represent stable expression of ΔFosB. However, the identity of the immunoreactive FosB signal observed in saline-treated mice is unknown. Statistical analysis in Table 1 used the Student’s t test.
Dendritic Spine Analysis.
Individual MSNs in the NAcc were chosen for spine analysis based on several criteria. (i) There was minimal or no overlap with other labeled cells to ensure that processes from different cells would not be confused. (ii) At least three primary dendrites needed to be visible for cells to be used for analysis. (iii) Distal dendrites (terminal dendrites or close to the terminal dendrite) were examined. Dendrites from both MSNs in the core and shell of the NAcc were analyzed. Although we observed sparsely spined MSNs (spiny type II), we analyzed only densely spined MSNs (spiny type I). To calculate spine density, a length of dendrite (>20 μm long) was traced by using a confocal microscope (Zeiss LSM 510) with an oil immersion lens (×40). All images of dendrites were taken at different z levels (0.5–1 μm depth intervals) to examine the morphology of dendritic spines. All measurements were made with metamorph image analysis software (Universal Imaging, Downingtown, PA). Statistical analysis used the Kolmogorov–Smirnov test.
Protrusions from dendrites were classified into four types based on their length as described in refs. 63 and 64. Class 1 protrusions, also called stubby protuberances, were <0.5 μm in length, lacked a large spine head, and did not appear to have a neck; class 2, or mushroom-shaped spines, were between 0.5 and 1.25 μm long and were characterized by a short neck and large spine head; class 3, or thin spines, ranged between 1.25 and 3.0 μm and had elongated spine necks with small heads; class 4, or filopodial extensions, were long filamentous protrusions that lacked a discernible spine head.
Acknowledgments
This work was supported by United States Public Health Service Grant DA10044 (to P.G. and A.C.N.) and by The Simons Foundation, the Peter J. Sharp Foundation, the Picower Foundation, and the F. M. Kirby Foundation.
Abbreviations
- NAcc
nucleus accumbens
- MSN
medium-sized spiny neuron
- BAC
bacterial artificial chromosome
- Drd1
dopamine receptor D1 promoter-driven
- Drd2
dopamine receptor D2 promoter-driven
- DiI
1,1′-diotadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate
- 2WD
2 days after last drug treatment
- 30WD
30 days after last drug treatment.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Totterdell S., Smith A. D. J. Chem. Neuroanat. 1989;2:285–298. [PubMed] [Google Scholar]
- 2.Smith Y., Bevan M. D., Shink E., Bolam J. P. Neuroscience. 1998;86:353–387. doi: 10.1016/s0306-4522(98)00004-9. [DOI] [PubMed] [Google Scholar]
- 3.Heikkila R. E., Orlansky H., Cohen G. Biochem. Pharmacol. 1975;24:847–852. doi: 10.1016/0006-2952(75)90152-5. [DOI] [PubMed] [Google Scholar]
- 4.Ritz M. C., Lamb R. J., Goldberg S. R., Kuhar M. J. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- 5.Nestler E. J. Trends Pharmacol. Sci. 2004;25:210–218. doi: 10.1016/j.tips.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Kalivas P. W., Stewart J. Brain Res. Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- 7.Pierce R. C., Kalivas P. W. Brain Res. Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- 8.Robinson T. E., Berridge K. C. Annu. Rev. Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- 9.Wolf M. E., Khansa M. R. Brain Res. 1991;562:164–168. doi: 10.1016/0006-8993(91)91202-c. [DOI] [PubMed] [Google Scholar]
- 10.Vanderschuren L. J., Kalivas P. W. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- 11.Sesack S. R., Pickel V. M. J. Comp. Neurol. 1992;320:145–160. doi: 10.1002/cne.903200202. [DOI] [PubMed] [Google Scholar]
- 12.Smith A. D., Bolam J. P. Trends Neurosci. 1990;13:259–265. doi: 10.1016/0166-2236(90)90106-k. [DOI] [PubMed] [Google Scholar]
- 13.Sibley D. R., Monsma F. J., Jr. Trends Pharmacol. Sci. 1992;13:61–69. doi: 10.1016/0165-6147(92)90025-2. [DOI] [PubMed] [Google Scholar]
- 14.Beckstead R. M., Cruz C. J. Neuroscience. 1986;19:147–158. doi: 10.1016/0306-4522(86)90012-6. [DOI] [PubMed] [Google Scholar]
- 16.Gerfen C. R., Young W. S., III Brain Res. 1988;460:161–167. doi: 10.1016/0006-8993(88)91217-6. [DOI] [PubMed] [Google Scholar]
- 16.Gerfen C. R. Trends Neurosci. 2000;23:S64–S70. doi: 10.1016/s1471-1931(00)00019-7. [DOI] [PubMed] [Google Scholar]
- 17.Gerfen C. R., Engber T. M., Mahan L. C., Susel Z., Chase T. N., Monsma F. J., Jr., Sibley D. R. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- 18.Zahm D. S. Neurosci. Biobehav. Rev. 2000;24:85–105. doi: 10.1016/s0149-7634(99)00065-2. [DOI] [PubMed] [Google Scholar]
- 19.Lu X.-Y., Ghasemzadeh M. B., Kalivas P. W. Neuroscience. 1998;82:767–780. doi: 10.1016/s0306-4522(97)00327-8. [DOI] [PubMed] [Google Scholar]
- 20.Koob G. F., Le H. T., Creese I. Neurosci. Lett. 1987;79:315–320. doi: 10.1016/0304-3940(87)90451-4. [DOI] [PubMed] [Google Scholar]
- 21.Woolverton W. L., Virus R. M. Pharmacol. Biochem. Behav. 1989;32:691–697. doi: 10.1016/0091-3057(89)90019-1. [DOI] [PubMed] [Google Scholar]
- 22.Bergman J., Kamien J. B., Spealman R. D. Behav. Pharmacol. 1990;1:355–363. doi: 10.1097/00008877-199000140-00009. [DOI] [PubMed] [Google Scholar]
- 23.Epping-Jordan M. P., Markou A., Koob G. F. Brain Res. 1998;784:105–115. doi: 10.1016/s0006-8993(97)01190-6. [DOI] [PubMed] [Google Scholar]
- 24.Caine S. B., Negus S. S., Mello N. K., Bergman J. J. Pharmacol. Exp. Ther. 1999;291:353–360. [PubMed] [Google Scholar]
- 25.De Vries T. J., Cools A. R., Shippenberg T. S. NeuroReport. 1998;9:1763–1768. doi: 10.1097/00001756-199806010-00017. [DOI] [PubMed] [Google Scholar]
- 26.Self D. W., Barnhart W. J., Lehman D. A., Nestler E. J. Science. 1996;271:1586–1589. doi: 10.1126/science.271.5255.1586. [DOI] [PubMed] [Google Scholar]
- 27.Khroyan T. V., Barrett-Larimore R. L., Rowlett J. K., Spealman R. D. J. Pharmacol. Exp. Ther. 2000;294:680–687. [PubMed] [Google Scholar]
- 28.Alleweireldt A. T., Weber S. M., Kirschner K. F., Bullock B. L., Neisewander J. L. Psychopharmacology. 2002;159:284–293. doi: 10.1007/s002130100904. [DOI] [PubMed] [Google Scholar]
- 29.Nestler E. J. Nat. Rev. Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- 30.Robinson T. E., Kolb B. Neuropharmacology. 2004;47:33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 31.Kalivas P. W. Curr. Opin. Pharmacol. 2004;4:23–29. doi: 10.1016/j.coph.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Hyman S. E., Malenka R. C. Nat. Rev. Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- 33.Robinson T. E., Kolb B. J. Neurosci. 1997;17:8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson T. E., Kolb B. Eur. J. Neurosci. 1999;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- 35.Li Y., Kolb B., Robinson T. E. Neuropsychopharmacology. 2003;28:1082–1085. doi: 10.1038/sj.npp.1300115. [DOI] [PubMed] [Google Scholar]
- 36.Gong S., Zheng C., Doughty M. L., Losos K., Didkovsky N., Schambra U. B., Nowak N. J., Joyner A., Leblanc G., Hatten M. E., et al. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- 37.Zhou F. M., Wilson C. J., Dani J. A. J. Neurobiol. 2002;53:590–605. doi: 10.1002/neu.10150. [DOI] [PubMed] [Google Scholar]
- 38.Grutzendler J., Tsai J., Gan W. B. Methods. 2003;30:79–85. doi: 10.1016/s1046-2023(03)00009-4. [DOI] [PubMed] [Google Scholar]
- 39.Kelz M. B., Chen J., Carlezon W. A., Jr., Whisler K., Gilden L., Beckmann A. M., Steffen C., Zhang Y. J., Marotti L., Self D. W., et al. Nature. 1999;401:272–276. doi: 10.1038/45790. [DOI] [PubMed] [Google Scholar]
- 40.Nestler E. J. Neuropharmacology. 2004;47:24–32. doi: 10.1016/j.neuropharm.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 41.Le Moine C., Bloch B. J. Comp. Neurol. 1995;355:418–426. doi: 10.1002/cne.903550308. [DOI] [PubMed] [Google Scholar]
- 42.Surmeier D. J., Song W. J., Yan Z. J. Neurosci. 1996;16:6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nye H. E., Hope B. T., Kelz M. B., Iadarola M., Nestler E. J. J. Pharmacol. Exp. Ther. 1995;275:1671–1680. [PubMed] [Google Scholar]
- 44.Gerfen C. R., Keefe K. A., Gauda E. B. J. Neurosci. 1995;15:8167–8176. doi: 10.1523/JNEUROSCI.15-12-08167.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moratalla R., Elibol B., Vallejo M., Graybiel A. M. Neuron. 1996;17:147–156. doi: 10.1016/s0896-6273(00)80288-3. [DOI] [PubMed] [Google Scholar]
- 46.Badiani A., Oates M. M., Day H. E., Watson S. J., Akil H., Robinson T. E. Behav. Brain. Res. 1999;103:203–209. doi: 10.1016/s0166-4328(99)00041-8. [DOI] [PubMed] [Google Scholar]
- 47.Uslaner J., Badiani A., Norton C. S., Day H. E., Watson S. J., Akil H., Robinson T. E. Eur. J. Neurosci. 2001;13:1977–1983. doi: 10.1046/j.0953-816x.2001.01574.x. [DOI] [PubMed] [Google Scholar]
- 48.Huff R. M., Chio C. L., Lajiness M. E., Goodman L. V. Adv. Pharmacol. 1998;42:454–457. doi: 10.1016/s1054-3589(08)60786-3. [DOI] [PubMed] [Google Scholar]
- 49.Zachariou V., Sgambato-Faure V., Sasaki T., Svenningsson P., Berton O., Fienberg A. A., Nairn A. C., Greengard P., Nestler E. J. Neuropsychopharmacology. 2005 Aug 3; doi: 10.1038/sj.npp.1300832. 10.1038/sj.npp.1300832. [DOI] [PubMed] [Google Scholar]
- 50.McClung C. A., Nestler E. J. Nat. Neurosci. 2003;6:1208–1215. doi: 10.1038/nn1143. [DOI] [PubMed] [Google Scholar]
- 51.Norrholm S. D., Bibb J. A., Nestler E. J., Ouimet C. C., Taylor J. R., Greengard P. Neuroscience. 2003;116:19–22. doi: 10.1016/s0306-4522(02)00560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bibb J. A., Chen J., Taylor J. R., Svenningsson P., Nishi A., Snyder G. L., Yan Z., Sagawa Z. K., Ouimet C. C., Nairn A. C., et al. Nature. 2001;410:376–380. doi: 10.1038/35066591. [DOI] [PubMed] [Google Scholar]
- 53.Nikolic M., Chou M. M., Lu W., Mayer B. J., Tsai L. H. Nature. 1998;395:194–198. doi: 10.1038/26034. [DOI] [PubMed] [Google Scholar]
- 54.Kesavapany S., Lau K. F., McLoughlin D. M., Brownlees J., Ackerley S., Leigh P. N., Shaw C. E., Miller C. C. Eur. J. Neurosci. 2001;13:241–247. [PubMed] [Google Scholar]
- 55.Morabito M. A., Sheng M., Tsai L. H. J. Neurosci. 2004;24:865–876. doi: 10.1523/JNEUROSCI.4582-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Futter M., Uematsu K., Bullock S. A., Kim Y., Hemmings H. C., Jr., Nishi A., Greengard P., Nairn A. C. Proc. Natl. Acad. Sci. USA. 2005;102:3489–3494. doi: 10.1073/pnas.0409802102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hayashi M. L., Choi S. Y., Rao B. S., Jung H. Y., Lee H. K., Zhang D., Chattarji S., Kirkwood A., Tonegawa S. Neuron. 2004;42:773–787. doi: 10.1016/j.neuron.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 58.Murase S., Mosser E., Schuman E. M. Neuron. 2002;35:91–105. doi: 10.1016/s0896-6273(02)00764-x. [DOI] [PubMed] [Google Scholar]
- 59.Prange O., Murphy T. H. J. Neurosci. 2001;21:9325–9333. doi: 10.1523/JNEUROSCI.21-23-09325.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feng J., Yan Z., Ferreira A., Tomizawa K., Liauw J. A., Zhuo M., Allen P. B., Ouimet C. C., Greengard P. Proc. Natl. Acad. Sci. USA. 2000;97:9287–9292. doi: 10.1073/pnas.97.16.9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Y., Acerbo M. J., Robinson T. E. Eur. J. Neurosci. 2004;20:1647–1654. doi: 10.1111/j.1460-9568.2004.03612.x. [DOI] [PubMed] [Google Scholar]
- 62.Perrotti L. I., Bolanos C. A., Choi K. H., Russo S. J., Edwards S., Ulery P. G., Wallace D. L., Self D. W., Nestler E. J., Barrot M. Eur. J. Neurosci. 2005;21:2817–2824. doi: 10.1111/j.1460-9568.2005.04110.x. [DOI] [PubMed] [Google Scholar]
- 63.Harris K. M., Jensen F. E., Tsao B. J. Neurosci. 1992;12:2685–2705. doi: 10.1523/JNEUROSCI.12-07-02685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vanderklish P. W., Edelman G. M. Proc. Natl. Acad. Sci. USA. 2002;99:1639–1644. doi: 10.1073/pnas.032681099. [DOI] [PMC free article] [PubMed] [Google Scholar]