Abstract
Wnt signaling is essential for T cell development in the thymus, but the stages in which it occurs and the molecular mechanisms underlying Wnt responsiveness have remained elusive. Here we examined Wnt signaling activity in both human and murine thymocyte populations by determining β-catenin levels, Tcf-reporter activation and expression of Wnt-target genes. We demonstrate that Wnt signaling occurs in all thymocyte subsets, including the more mature populations, but most prominently in the double negative (DN) subsets. This differential sensitivity to Wnt signaling was not caused by differences in the presence of Wnts or Wnt receptors, as these appeared to be expressed at comparable levels in all thymocyte subsets. Rather, it can be explained by high expression of activating signaling molecules in DN cells, e.g., β-catenin, plakoglobin, and long forms of Tcf-1, and by low levels of inhibitory molecules. By blocking Wnt signaling from the earliest stage onwards using overexpression of Dickkopf, we show that inhibition of the canonical Wnt pathway blocks development at the most immature DN1 stage. Thus, responsiveness to developmental signals can be regulated by differential expression of intracellular mediators rather than by abundance of receptors or ligands.
Keywords: double negative cells, T cell development, β-catenin, Dickkopf
Thymocyte differentiation in the thymus involves a series of discrete phenotypic stages that correspond to developmental checkpoints (1). In both human and mouse, thymocytes are subsequently CD4−CD8− (double negative, DN), CD4+CD3− (human immature single positive, ISP) or CD8+CD3− (mouse ISP), CD4+CD8+ (double positive, DP), and finally CD4+CD3+ or CD8+CD3+ (single positive, SP). In the mouse, the DN subset can be further subdivided into four stages: CD44+CD25− (DN1), CD44+CD25+ (DN2), CD44−CD25+ (DN3), and CD44−CD25− (DN4). In the human thymus, CD34+CD1a− cells correspond to murine DN1 and DN2 thymocytes and CD34+CD1a+ cells are homologous to DN3 (2). The proliferation, differentiation, and T cell receptor rearrangement steps that occur in the thymus are tightly regulated by a number of molecular pathways. One such pathway that is increasingly recognized to be important for T cell development is the Wnt signaling cascade (3).
Wnt proteins are secreted molecules that regulate cell-to-cell interactions in many different cell types and various species. In the thymus, Wnts are produced mainly by the thymic epithelium (4). Wnts bind to a receptor complex consisting of a member of the Frizzled (Fz) family of seven transmembrane proteins and the low-density lipoprotein receptor-related proteins LRP-5 or LRP-6. Because the high redundancy of the different Wnts and Fz in the thymus (3) obscures their importance when studied in straight knockouts, overexpression of negative regulators of the Wnt pathway may be more informative. For instance, soluble Fz receptors have been shown to block early thymocyte development in fetal thymic organ culture (FTOC) at the DN-to-DP transition (5). These studies have indicated an important role for the membrane proximal components of the Wnt pathway, but the exact contribution of the different Wnts, Fz, and LRPs that are expressed by thymic stroma and thymocyte subsets in human and mouse are unknown.
After binding of a Wnt protein to a Fz receptor, a complex cascade of events is initiated, finally leading to the inhibition of the negative regulatory kinase GSK-3β (6) which, in a complex together with Axin and adenomatous polyposis coli (APC), phosphorylates β-catenin. Inhibition of β-catenin phosphorylation prevents its ubiquitinylation and proteasomal breakdown. Dephosphorylated β-catenin is targeted to the nucleus by Pygopus and Legless/BCL9 (7), where it binds to Tcf/Lef transcription factors (8), in thymocytes predominantly Tcf-1. Young Tcf-1 deficient mice have an incomplete block at the DN1, DN2, and ISP stages, whereas older mice display a complete block at the DN1 stage of thymocyte development (9, 10).
In the absence of β-catenin, Tcf-1 functions as a repressor of transcription (11). Binding of β-catenin to Tcf/Lef recruits chromatin-modifying and -remodeling complexes to transcribe Wnt target genes (12), which in the most immature thymocytes regulate proliferation and cell adhesion (13).
Although the phenotype of the Tcf-1-deficient mouse suggests a role for Wnt signaling in the early stages of T cell development, definite proof for a role of Wnt signaling is not provided by such mice, as the block might be a result of abolishment of the repressive functions or other Wnt independent functions of Tcf-1 (reviewed in ref. 14). Additional roles for Wnt signaling have been reported in more advanced stages of thymocyte development, including differentiation into DP cells (15), survival of DP cells (16) and generation of mature CD8+ thymocytes (17). It has remained unclear during which stages of T cell development Wnt signaling occurs and how it is regulated.
Results
Wnt Signaling Occurs Mainly in the Early DN Stages.
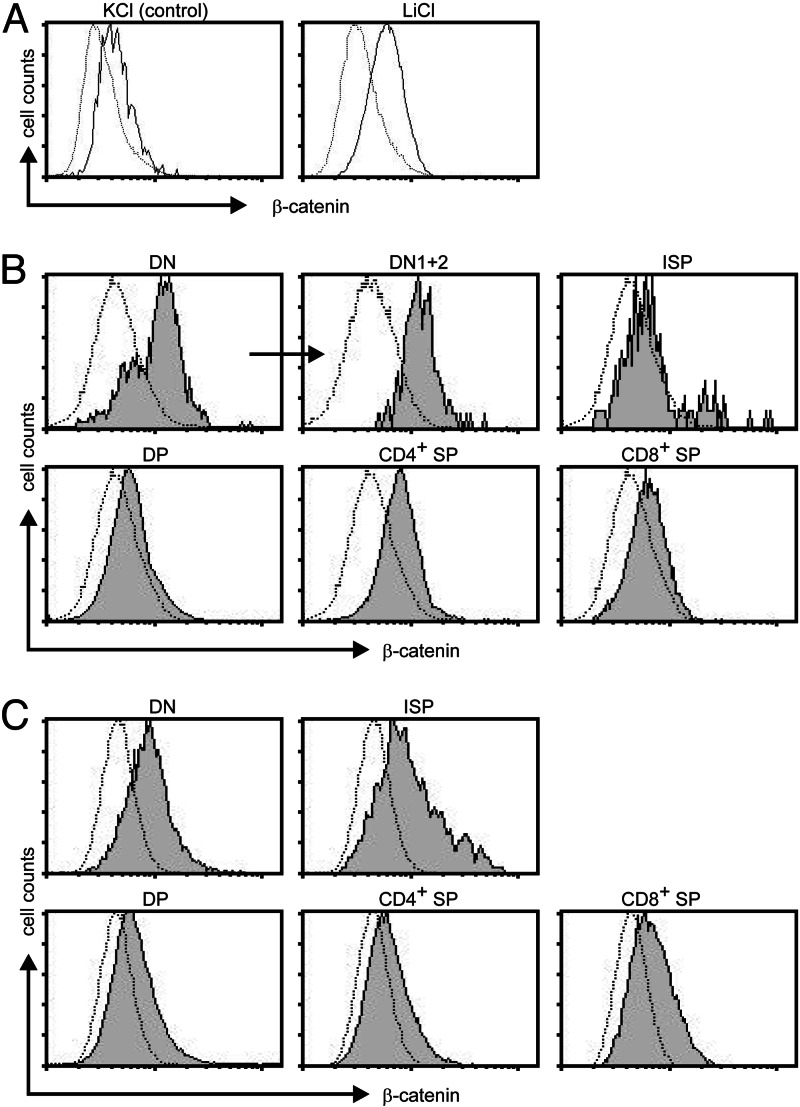
To study where Wnt signaling occurs during T cell development, we have used three readouts for active Wnt signaling, namely levels of β-catenin, the activation of a Tcf reporter, and the transcription of known thymic Wnt target genes. Because dephosphorylated β-catenin is highly stable and not degraded in the proteosome, elevated levels of total β-catenin are an indication that Wnt signaling occurs in a cell. We have developed a flow-cytometric assay to determine the presence of β-catenin in thymocyte subsets. This assay was validated by treating Jurkat cells with lithium chloride, which inhibits GSK-3β and leads to a profound increase in β-catenin levels (Fig. 1A). In the human thymus, the highest levels of β-catenin were observed in the DN cells and especially in the DN CD34+ fraction, which corresponds to murine DN1–3 cells (2) (Fig. 1B). This finding suggested that Wnt signaling is active preferentially in these cells. Similarly, in the adult murine thymus, β-catenin was present predominantly at the DN and ISP stages (Fig. 1C). In fetal thymic lobes of embryonic day 14, 15, and 16, high levels of β-catenin were present. Levels were higher at the DN1 and DN2 stages than at the DN3 and DN4 stages and clearly decreased in the DP cells that arose on embryonic day 16 (Fig. 6, which is published as supporting information on the PNAS web site).
Fig. 1.
Differential expression of β-catenin in thymic subsets. Intracellular β-catenin levels were determined by flow cytometry in Jurkat cells stimulated for 24 h with either KCl or LiCl (A), freshly isolated human thymocytes (B), freshly isolated adult murine thymocyte (C). All thymus samples were counterstained to distinguish different subsets. Dotted lines represent isotype (human) or unstained (mouse) controls.
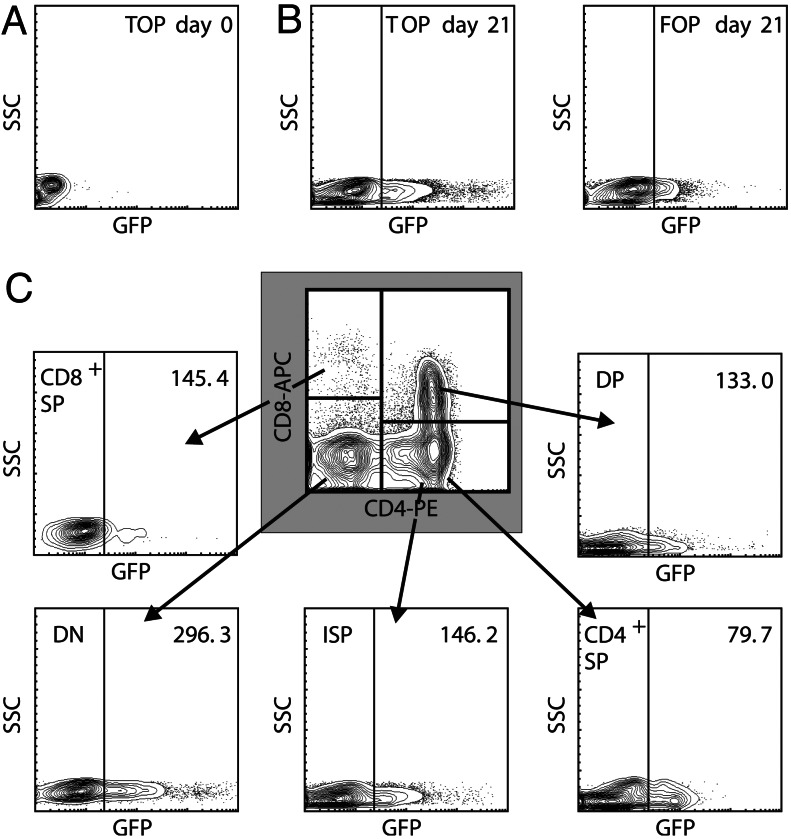
To study the transcriptional activation of the Tcf/β-catenin complex at sequential stages of T cell development, we used a lentiviral Tcf reporter system, in which expression of GFP is driven by a Tcf/Lef-responsive promoter (TOP-GFP) (18). As a negative control, we used a vector containing mutated Tcf binding sites (FOP-GFP). Hematopoietic stem cells from human cord blood were infected with these lentiviruses and induced to develop into T cells in vitro in FTOC. After 2 days of transduction, none of the cells expressed GFP (Fig. 2A), indicating that in vitro cultured HSC do not undergo active Wnt signaling. However, after inducing T cell development in FTOC, a population of GFPhigh cells could be observed that was absent from the FOP-GFP control (Fig. 2B). GFP levels were analyzed in DN, ISP, DP, and SP thymocyte subsets (Fig. 2C). Surprisingly, Tcf-dependent signaling was detected in all thymocyte subsets, but was highest in the DN cells.
Fig. 2.
Tcf-reporter expression during human thymocyte development. CD34+ precursor cells from human cord blood were transduced with a lentiviral vector encoding a Tcf reporter (TOP) or a reporter containing mutated Tcf binding sites (FOP) and cultured in FTOC. (A and B) Tcf-dependent GFP expression after 2 days of transduction (A) and after 3 weeks of FTOC (B). (C) Tcf-dependent GFP expression in the different thymocyte subsets after 3 weeks of FTOC. The mean fluorescence of the GFP positive population is indicated in each graph. Mean fluorescence of GFP-positive cells transduced with FOP was similar in all subsets (≈60). Results are representative of two experiments performed with cord blood of different donors.
Cells undergoing Wnt signaling are expected to induce expression of Tcf-dependent target genes. The previously identified Wnt target genes c-fos, CL100 PTPase, and hsp70 (13) were expressed preferentially in the most immature DN populations, whereas fosB and c-jun were higher expressed in the more mature stages (Fig. 7, which is published as supporting information on the PNAS web site).
Microarray studies of the murine thymocyte subsets have been performed by Petrie and coworkers (19). By mining these data for thymic Wnt target genes, we found that p150,95, fos, and jun were expressed at high levels at the DN1 stage and rapidly decreased in the more mature stages (Fig. 7). Taken together, these results point to a high level of Wnt signaling preferentially in the most immature DN, and to a lesser extend ISP thymocyte subsets.
Wnts and Receptors Are Present in All Thymocyte Subsets.
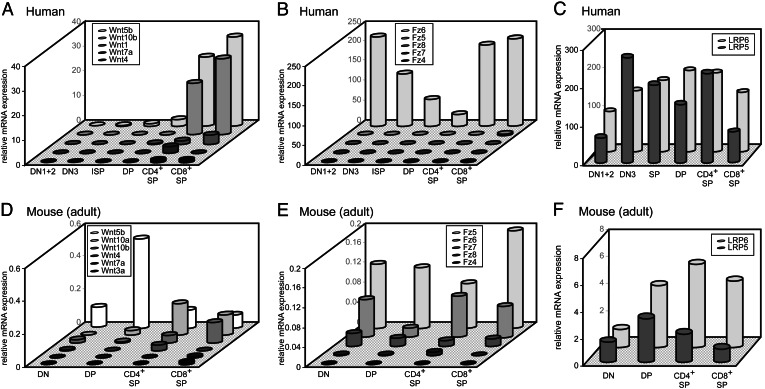
To investigate which factors determine whether cells are receptive to Wnt signaling, we examined the presence of several Wnt signaling components in the different thymocyte subsets using real-time quantitative PCR (RQ-PCR). In man and mouse, most Wnts investigated appeared to be expressed in all thymocyte subsets, but at different levels. In human and murine thymus, Wnt5 and Wnt10 were the most prominent Wnts (Fig. 3). In human and mouse, Wnt expression was most marked in the SP subsets, although in mice, Wnt5b was expressed at high levels in the DP subset as well. In the thymic stroma, high levels of Wnt5a (human) and Wnt4, Wnt5b, and Wnt10 (mouse) were detected, generally at levels significantly higher than in thymocytes (data not shown).
Fig. 3.
Expression of Wnts, Fz and LRPs in thymic subsets. mRNA levels of different Wnts and Fz were determined by RQ-PCR. All graphs show results of a single representative experiment of two to five performed, as replicate experiments always showed the same trend, but absolute expression levels varied. Expression levels are relative to GAPDH expression in the same sample.
Of the Frizzled receptors, Fz6 was by far the most prominent in the human thymus and Fz5 and Fz6 in the murine thymus (Fig. 3). Levels of Fz varied between thymocyte subsets and tended to be lowest in the DP fraction. LRP5 and -6 were expressed at similar levels, both in mouse and in human, and expression was more or less equal between the different thymocyte subsets (Fig. 3). These results largely confirm earlier studies in which Wnt signaling components were investigated by Northern blotting on total thymocytes (5). These studies did not detect Fz6 expression, but confirmed Fz5, Fz7, and F8 expression. Most likely different techniques used (Northern blotting with a Fz consensus probe on total thymocytes vs. RQ-PCR on sorted subpopulations here) explain these differences.
Thus, expression of the Wnts (mainly by the stroma and the more mature SP thymocytes), Fz (variable levels in thymocyte subsets), and LRPs (constitutively expressed), cannot explain that active Wnt signaling occurs predominantly in the DN and ISP subsets.
Expression of Intracellular Signaling Molecules Predisposes DN Thymocytes to Respond Strongly to Wnt Signals.
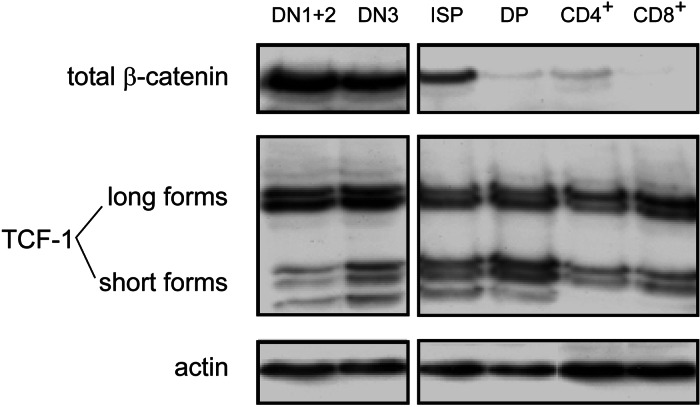
We studied the expression of several intracellular Wnt signaling components using Western blot and microarray analysis. Because there is no antibody against murine Tcf-1 available (20), these experiments were performed by using sorted human thymocyte subsets. Total β-catenin was detected in all thymocyte populations, but mainly in the CD34+ DN1–3 populations and somewhat lower in the ISP subset (Fig. 4A), confirming our results obtained by flow-cytometry (Fig. 1B). The same distribution was detected for active (dephosphorylated) β-catenin, although this was completely absent from the DP stage onwards (data not shown). Staining of human thymus tissue slides with the same antibody against dephosphorylated β-catenin, showed a pattern of nuclear foci in thymocytes (Fig. 8A, which is published as supporting information on the PNAS web site), confirming a function as an active transcription factor in these cells. Expression of total Tcf-1 protein was detected in all populations at similar levels (Fig. 4A). Interestingly, however, the ratio between expression of the long (activating) and short (repressing) splice-isoforms of Tcf-1 differed between the different thymocyte subsets. This ratio was highest in the CD34+CD1a− (DN1 and DN2) cells (Fig. 8B), allowing the final transduction of Wnt signals to occur preferentially in these immature cells.
Fig. 4.
Expression of intracellular Wnt signaling components in the human thymus. DN1/2 thymocytes were defined as CD34+CD1a− and DN3 as CD34+CD1a+. Protein levels in sorted thymocyte subsets determined by Western blot.
Presence of other intracellular Wnt signaling components was extracted from gene expression profiles of human thymocytes subsets (2). Plakoglobin (γ-catenin), another armadillo protein that can activate Tcf/Lef transcription factors (21), showed an expression pattern highly similar to that of β-catenin (Table 1, which is published as supporting information on the PNAS web site), also peaking in the CD34+ (DN1–3) thymocytes. Conversely, expression of Axin, one of the molecules that functions to retain β-catenin in the complex in the cytoplasm, increased gradually during thymocyte maturation. Also HMG2L1, a nuclear negative regulator of Wnt signaling (22), was expressed at lower levels in the most immature (and also the SP) populations (Table 1). Levels of adenomatous polyposis coli, Groucho and transcriptional activators Pygopus and Legless/Bcl9 (7) did not change during thymocyte differentiation (Table 1).
In conclusion, active Wnt signaling in the early thymocyte subsets is determined by elevated levels of activating and lower levels of inhibiting factors in the Wnt signaling cascade (Fig. 8C). Thus, the most immature thymocytes appear to be preprogrammed to respond to Wnt signals.
Wnt Signaling Is Crucial for Early Thymocyte Development.
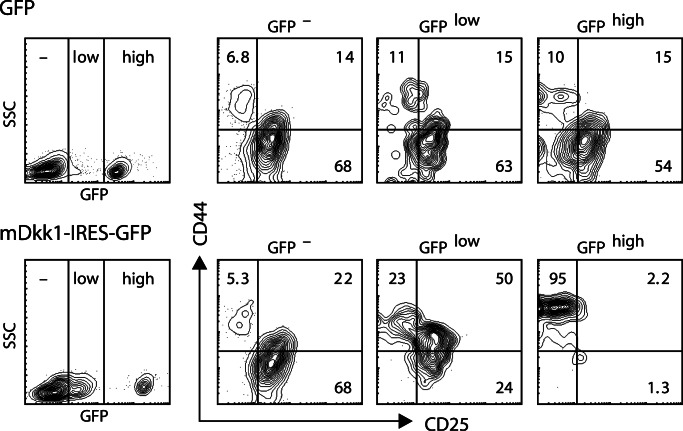
The functional role of Wnt signaling during the DN stages of thymocyte differentiation has not been studied, largely because conditional deletion of Wnt components during thymocyte development relies on Cre-transgenic mice that fail to show significant expression in DN1 and DN2 subsets (e.g., the Lck or CD4 promoter). We used a retroviral vector encoding the gene for mouse Dickkopf-1 (mDkk-1), which binds to the LRP coreceptor for Wnt and thereby potently inhibits Wnt signaling (23). We transduced murine bone marrow and fetal liver cells with the mDkk-1 retrovirus and studied the ability of these cells to develop into T cells in FTOC. In all cultures of mDkk-1-transduced cells, a strong selection against high mDkk-1 expression (i.e., high GFP signals) was detectable (Fig. 9, which is published as supporting information on the PNAS web site). In experiments in which a meaningful number of GFP expressing cells survived, the GFP+ cells exhibited a complete block at the DN1 stage (Fig. 5). This block was less severe for the cells expressing intermediate amounts of GFP. These results show that Wnt signaling is crucial for developing thymocytes, as early as at the DN1 stage.
Fig. 5.
Overexpression of mouse mDkk-1 blocks T cell development at the DN1 stage. Murine fetal liver cells were transduced with a vector containing the mDkk-1 gene in combination with GFP (mDkk-1) or a control vector containing only GFP (GFP). After 9 days of culture in FTOC, thymocyte subset distribution was determined in cells gated for absent, low, and high GFP expression, both for cells transduced with the control vector (Upper) and with mDkk-1 (Lower).
Discussion
We here show that all thymic subpopulations can undergo low levels of Wnt signaling, but the highest levels of active Wnt signaling were detected in the most immature DN cells. This differential responsiveness is not determined by expression of membrane associated factors, but rather by the balance between activating and inhibiting intracellular components of the Wnt pathway. Inhibiting Wnt signaling results in a complete DN1 block. Whether the developmental block caused by overexpression of mDkk-1 is due to inhibited proliferation or differentiation of DN1 thymocytes is difficult to distinguish, because these processes are closely connected at this stage of development. During the DN1 and -2 stages of T cell development, thymocytes undergo massive proliferation to expand the thymocyte pool before the T cell receptor rearrangement and selection processes are initiated (24).
The role of β-catenin during T cell development has been addressed in two different conditional knockout mouse models, which have generated conflicting results. The study by Sen and coworkers (17) indicated that β-catenin-mediated signals are required for normal T cell development, whereas Radtke and colleagues (25) reported that hematopoiesis including T cell development in the thymus proceeded normally in absence of β-catenin. As a possible explanation for their negative results, Radtke and colleagues (25) suggested that expression of plakoglobin may compensate for the absence of its homologue β-catenin. The parallel expression of β-catenin and plakoglobin in our experiments provides evidence that this is a plausible explanation and may account for the relatively mild or absent phenotype of β-catenin deficient mice (25, 26).
The regulation of responsiveness to developmental clues by differential expression of intracellular regulators rather than by abundance of receptors or ligands provides a previously unrecognized mechanism to regulate signal transduction events. To our knowledge, this is the first demonstration that only cells preprogrammed to respond to external stimuli do so during differentiation. It seems likely that such mechanisms operate during other developmental processes in other tissues.
Materials and Methods
Cells and Flow Cytometry.
Human thymus material was obtained from children operated for congenital heart disease, lacking immunohematological problems, according to the informed consent guidelines of Erasmus MC, Rotterdam. All mouse experimentation was done in accordance with legal regulations in The Netherlands. All monoclonal antibodies against human and mouse proteins were obtained from BD PharMingen, except for human CD1a-RD1, which was from Beckman Coulter. For each sorting experiment, frozen thymocytes of five different human donors or fresh thymocytes of four different mice were used. CD34+ human thymocytes were prepurified by AutoMACS (Milteni Biotec) before FACS sorting. Cells were sorted on a FACS Digital Vantage (DiVa) cell sorter (BD Biosciences). Purity of the recovered subpopulations was checked and was always >95%. For intracellular staining of β-catenin, freshly isolated thymocytes were immediately fixed in 2% paraformaldehyde at 37°C and permeabilized in PBS containing 0.5% saponin, 4% FCS, 1 mM glycerolphosphate, and phosphatase inhibitor cocktails I and II (Sigma). Cells were stained for 1 h with an antibody against active β-catenin (clone 8E4, Upstate Biotechnology), washed, and stained with cell surface markers.
TaqMan-Based RQ-PCR.
One microgram of RNA isolated from sorted subpopulations was treated with 1 unit of DNase I and subsequently transcribed to cDNA with Superscript II Reverse transcriptase (200 units), oligo(dT), and random hexanucleotide primers. A 1/20 cDNA mixture was used for RQ-PCR for each primer/probe set and performed in duplicate. Primer/probe sets are described in Table 2, which is published as supporting information on the PNAS web site.
Retroviral and Lentiviral Transduction and FTOC.
The retroviral mDkk-1 containing plasmid LZRS-mDkk1-IRES-eGFP and control plasmid LZRS-IRES-eGFP were transfected into Phoenix Ecotropic packaging cells. Stable high-titer producer clones were selected with puromycin. Lentiviral Tcf-1 reporter vectors have been described (18). Virus was produced by transfection of 293T cells using third-generation packaging system plasmids (27). Transductions were done on retronectin-coated dishes using 30,000 human CD34+ cells or 50,000 murine FL cells for subsequent FTOC.
Supplementary Material
Acknowledgments
We thank L. Ailles and I. Weissman (Stanford University School of Medicine) for pRLL-TOP and FOP vectors, L. Naldini (Universita Vita Salute, Milan, Italy) for the lentiviral helper plasmids, and C. Niehrs (Deutsches Krebsforschungszentrum, Heidelberg, Germany) for the mDkk1 cDNA. A. Bogers (Erasmus MC) and C. de Groot (Erasmus MC) are acknowledged for providing thymus and cord blood material, respectively. M. Comans-Bitter is thanked for preparing the figures.
Abbreviations
- DN
double negative
- ISP
immature single positive
- DP
double positive
- SP
single positive
- FTOC
fetal thymic organ culture
- RQ-PCR
real-time quantitative PCR.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Staal F. J., Weerkamp F., Langerak A. W., Hendriks R. W., Clevers H. C. Stem Cells. 2001;19:165–179. doi: 10.1634/stemcells.19-3-165. [DOI] [PubMed] [Google Scholar]
- 2.Dik W. A., Pike-Overzet K., Weerkamp F., de Ridder D., de Haas E. F., Baert M. R., van der Spek P., Koster E. E., Reinders M. J., van Dongen J. J., et al. J. Exp. Med. 2005;201:1715–1723. doi: 10.1084/jem.20042524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staal F. J., Clevers H. C. Curr. Opin. Immunol. 2003;15:204–208. doi: 10.1016/s0952-7915(03)00003-7. [DOI] [PubMed] [Google Scholar]
- 4.Pongracz J., Hare K., Harman B., Anderson G., Jenkinson E. J. Eur. J. Immunol. 2003;33:1949–1956. doi: 10.1002/eji.200323564. [DOI] [PubMed] [Google Scholar]
- 5.Staal F. J., Meeldijk J., Moerer P., Jay P., van de Weerdt B. C., Vainio S., Nolan G. P., Clevers H. Eur. J. Immunol. 2001;31:285–293. doi: 10.1002/1521-4141(200101)31:1<285::AID-IMMU285>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 6.Itoh K., Krupnik V. E., Sokol S. Y. Curr. Biol. 1998;8:591–594. doi: 10.1016/s0960-9822(98)70229-5. [DOI] [PubMed] [Google Scholar]
- 7.Townsley F. M., Cliffe A., Bienz M. Nat. Cell Biol. 2004;6:626–633. doi: 10.1038/ncb1141. [DOI] [PubMed] [Google Scholar]
- 8.Behrens J., von Kries J. P., Kuhl M., Bruhn L., Wedlich D., Grosschedl R., Birchmeier W. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 9.Verbeek S., Izon D., Hofhuis F., Robanus-Maandag E., te Riele H., van de Wetering M., Oosterwegel M., Wilson A., MacDonald H. R., Clevers H. Nature. 1995;374:70–74. doi: 10.1038/374070a0. [DOI] [PubMed] [Google Scholar]
- 10.Schilham M. W., Wilson A., Moerer P., Benaissa-Trouw B. J., Cumano A., Clevers H. C. J. Immunol. 1998;161:3984–3991. [PubMed] [Google Scholar]
- 11.Roose J., Molenaar M., Peterson J., Hurenkamp J., Brantjes H., Moerer P., van de Wetering M., Destree O., Clevers H. Nature. 1998;395:608–612. doi: 10.1038/26989. [DOI] [PubMed] [Google Scholar]
- 12.Barker N., Hurlstone A., Musisi H., Miles A., Bienz M., Clevers H. EMBO J. 2001;20:4935–4943. doi: 10.1093/emboj/20.17.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Staal F. J., Weerkamp F., Baert M. R., van den Burg C. M., van Noort M., de Haas E. F., van Dongen J. J. J. Immunol. 2004;172:1099–1108. doi: 10.4049/jimmunol.172.2.1099. [DOI] [PubMed] [Google Scholar]
- 14.Staal F. J., Clevers H. C. Nat. Rev. Immunol. 2005;5:21–30. doi: 10.1038/nri1529. [DOI] [PubMed] [Google Scholar]
- 15.Gounari F., Aifantis I., Khazaie K., Hoeflinger S., Harada N., Taketo M. M., von Boehmer H. Nat. Immunol. 2001;2:863–869. doi: 10.1038/ni0901-863. [DOI] [PubMed] [Google Scholar]
- 16.Ioannidis V., Beermann F., Clevers H., Held W. Nat. Immunol. 2001;2:691–697. doi: 10.1038/90623. [DOI] [PubMed] [Google Scholar]
- 17.Mulroy T., Xu Y., Sen J. M. Int. Immunol. 2003;15:1485–1494. doi: 10.1093/intimm/dxg146. [DOI] [PubMed] [Google Scholar]
- 18.Reya T., Duncan A. W., Ailles L., Domen J., Scherer D. C., Willert K., Hintz L., Nusse R., Weissman I. L. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 19.Tabrizifard S., Olaru A., Plotkin J., Fallahi-Sichani M., Livak F., Petrie H. T. J. Immunol. 2004;173:1094–1102. doi: 10.4049/jimmunol.173.2.1094. [DOI] [PubMed] [Google Scholar]
- 20.Castrop J., van Wichen D., Koomans-Bitter M., van de Wetering M., de Weger R., van Dongen J., Clevers H. Blood. 1995;86:3050–3059. [PubMed] [Google Scholar]
- 21.Maeda O., Usami N., Kondo M., Takahashi M., Goto H., Shimokata K., Kusugami K., Sekido Y. Oncogene. 2004;23:964–972. doi: 10.1038/sj.onc.1207254. [DOI] [PubMed] [Google Scholar]
- 22.Yamada M., Ohkawara B., Ichimura N., Hyodo-Miura J., Urushiyama S., Shirakabe K., Shibuya H. Genes Cells. 2003;8:677–684. doi: 10.1046/j.1365-2443.2003.00666.x. [DOI] [PubMed] [Google Scholar]
- 23.Mao B., Wu W., Li Y., Hoppe D., Stannek P., Glinka A., Niehrs C. Nature. 2001;411:321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- 24.Mori S., Shortman K., Wu L. Blood. 2001;98:696–704. doi: 10.1182/blood.v98.3.696. [DOI] [PubMed] [Google Scholar]
- 25.Cobas M., Wilson A., Ernst B., Mancini S. J., MacDonald H. R., Kemler R., Radtke F. J. Exp. Med. 2004;199:221–229. doi: 10.1084/jem.20031615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu Y., Banerjee D., Huelsken J., Birchmeier W., Sen J. M. Nat. Immunol. 2003;4:1177–1182. doi: 10.1038/ni1008. [DOI] [PubMed] [Google Scholar]
- 27.Dull T., Zufferey R., Kelly M., Mandel R. J., Nguyen M., Trono D., Naldini L. J. Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.