Abstract
The placenta is essential for the success of therian mammalian reproduction. Intense selective pressure has shaped changes in placental anatomy and function during mammalian cladogenesis. Here we challenge the view that the hemochorial placenta is a derived feature in haplorhine primates. Using phylogenetic and statistical analyses of molecular and morphological data, we demonstrate that the ancestral eutherian mammalian placenta had the distinctive features of (i) hemochorial placental interface, (ii) a discoid shape, and (iii) a labyrinthine maternofetal interdigitation. These results reveal that the first eutherians had a deeply invasive placenta and imply that the major role of the placenta in sustaining pregnancy and promoting gestational development existed throughout the eutherian lineage that descended to humans from the last common ancestor of placental mammals. The ancestral state reconstructions demonstrate both clade-specific patterns of placentation and specific cases of convergent evolution within individual eutherian clades. Determining the mammalian pattern of change in placental morphology is important for understanding the evolutionary pressures faced by these lineages. The effects of selection pressures on the efficiency of placentation may stem from changes in nutritional demand, gestational length, number of embryos per pregnancy, uterine shape, and maternal body constitution. The influence of these factors on placental development needs further investigation.
Keywords: discoid shape, Eutheria, hemochorial, maternofetal interdigitation, villous type
Recent advances in the understanding of mammalian phylogeny combined with studies of comparative placentation in eutherian mammals reveal the pattern of evolution for the structural characteristics of eutherian placentation. The mammalian chorioallantoic placenta is essential for the growth and development of the embryo and fetus and distinguishes eutherian mammals from other organisms (1).
Placental morphology is characterized by five major features (2). Three have been extensively studied (descriptions of their morphology have been presented in Supporting Text, which is published as supporting information on the PNAS web site): (i) the definitive type of placental interface (called placental barrier by others, e.g., epitheliochorial, endotheliochorial, and hemochorial); (ii) fetomaternal interdigitation (e.g., folded, lamellar, villous, trabecular, and labyrinthine); and (iii) placental shape (e.g., diffuse, cotylendonary, zonary, and discoidal). The other features have been studied to a much lesser degree: (iv) fetomaternal blood flow interrelations (e.g., concurrent, countercurrent, crosscurrent, and multivillous) and (v) neonatal/placental weight ratio. This basic scheme of placental morphology has been in use for nearly a century, and, although much more sophisticated tissue analysis methods are available (e.g., electron microscopy), the basic terminology remains useful for understanding placental anatomy (2–4).
Analysis of placental functional morphology and physiology has focused mainly on two parameters: (i) the extent of the fetomaternal contact (according to the shape and interdigitation of the placenta) (5) and (ii) the amount of maternal–fetal exchange (e.g., nutrient and gas exchange, hormonal actions, etc.) according to the type of placental interface and fetomaternal blood flow interrelation (4–8).
Molecular phylogenetic studies reconstruct four major placental mammalian groups (i.e., clades): Afrotheria (elephants, sirenians, hyraxes, aardvarks, elephant shrews, tenrecs, and golden moles); Xenartha (armadillos, sloths, and anteaters); Laurasiatheria (carnivores, pangolins, bats, soricid shrews, moles, hedgehogs, cetartiodactyls, and perrisodactyls), and Euarchontaglires (primates, rodents, rabbits, treeshrews, and flying lemurs) (9). Clades Laurasiatheria and Euarchontaglires group together to form Boreoutheria. Although most published molecular studies support a sister-group relationship between Laurasiatheria and Euarchontaglires, disputes exist as to the relationships among Boreoeutheria, Afrotheria, and Xenartha (10–13). In reconstructing character state evolution, we employ each of the three sister groupings for these major eutherian clades. Our aim is to use the molecular evidence on eutherian phylogeny to determine when the discoid hemochorial placenta first evolved and subsequently shaped the development of primate embryos and fetuses.
Results
Multiple topologies inferred (Fig. 1) from the molecular data set were evaluated because of the inability of these data to resolve the order of initial branching events within Eutheria (10–13). Table 1 shows that the molecular data set has statistically indistinguishable (when ambiguous characters are excluded) parsimony and likelihood scores when Xenartha is the sister clade to all other placental groups (i.e., Epitheria) or when Afrotheria is the sister clade to other placental mammals (i.e., Notolegia). The Afrotheria + Notolegia tree has the optimal maximum likelihood score. The parsimony score for the tree in which Boreoeutheria is sister to a clade made up of Afrotheria + Xenartha is significantly longer and was not considered in subsequent analyses.
Fig. 1.
Phylogenetic relationships among major placental mammalian groups. Four major superordinal placental (i.e., eutherian) mammalian clades are supported by molecular data (9, 13). These clades are the Afrotheria, Xenartha, Euarchontaglires, and Laurasiatheria. All studies also support the grouping of Euarchontaglires and Laurasiatheria as sister taxa in a larger clade (Boreoeutheria). The phylogenetic branching order at the root of the tree is controversial (9–13). (A) Depiction of the Afrotheria as sister to the remaining three clades (i.e., Notolegia). (B) Depiction of the Xenartha as sister to the remaining three clades. A third choice in which Boreoeutheria is sister to a clade that consists of Xenartha and Afrotheria is not supported by parsimony analysis (Table 1).
Table 1.
Morphological hypotheses tested with DNA sequence data
| Hypothesis | Parsimony tests |
Likelihood tests |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Templeton |
Winning sites |
−ln L* | Diff −ln L¶ | KH test P | SH test P | ||||||
| Length, £ | Rank sums† | n | z | P‡ | Counts | P‡ | |||||
| Murphy et al. (8) tree (Fig. 1A) | 41,976 | 7,656 | 173 | −0.2281 | 0.8196 | 88 | 0.8791 | 211,110.54 | (best) | — | — |
| −7,395 | −85 | ||||||||||
| Xenartha + Epitheria (Fig. 1B) | 41,973 | (best) | 211,119.66 | 9.1 | 0.13 | 0.66 | |||||
| Boreoeutheria (Xenaertha + Afrotheria) | 42,014 | 5,984 | 135 | −3.5287 | 0.0004§ | 88 | 0.0006§ | 211,115.93 | 5.4 | 0.43 | 0.73 |
| −3,196 | −47 | ||||||||||
| Epitheliochorial monophyly | 42,789 | 598,052.5 | 1,208 | −21.5483 | <0.0001§ | 993 | <0.0001§ | 213,457.27 | 2,346.7 | <0.001§ | <0.001§ |
| −132,183.5 | −215 | ||||||||||
| Endotheliochorial monophyly | 43,092 | 879,382.5 | 1,430 | −26.1785 | <0.0001§ | 1,230 | <0.0001§ | 214,338.33 | 3,227.8 | <0.001§ | <0.001§ |
| −143,782.5 | −200 | ||||||||||
| Hemoochorial monophyly | 43,080 | 960,091 | 1,514 | −25.343 | <0.0001§ | 1,264 | <0.0001§ | 214,276.12 | 3,165.6 | <0.001§ | <0.001§ |
| −186,764 | −250 | ||||||||||
| Epitheliochorial, endotheliochorial, and hemoochorial monophyly | 43,340 | 1,073,312.5 | 1,586 | −26,1054 | <0.0001§ | 1,331 | <0.0001§ | 214,629.71 | 3,519.2 | <0.001§ | <0.001§ |
| −185,178.5 | −255 | ||||||||||
A significant result indicates the rejection of the stated hypothesis. Tests were conducted by using paup*. KH, Kishino-Hasegawa; SH, Shimodaira-Hasegawa. £, number of steps in the phylogenetic tree.
*−Log likelihood.
†The Wilcoxon signed-ranks test statistic is the smaller of the absolute values of the two rank sums.
‡Approximate probability of getting a more extreme test statistic under the null hypothesis of no difference between the two trees (two-tailed test).
§P < 0.05.
¶The difference between the maximum likelihood tree −In L and the alternative hypothesis −In L.
Evolution of the Mammalian Placental Interface.
The hypotheses that taxa with hemochorial, endotheliochorial, and epitheliochorial placentas comprise monophyletic groups was rejected whether each state was considered individually or in concert with the others (parsimony, P < 0.0001; likelihood, P < 0.01; Table 1).
Parsimony and likelihood ancestral states were reconstructed for the two statistically equivalent tree topologies (i.e., Afrotheria or Xenartha as sister to other eutherians). The parsimony reconstructions according to both tree topologies unambiguously infer the hemochorial placenta as the ancestral state for eutherian mammals. The Markov model reconstructions define the hemochorial placental interface as the most likely ancestral eutherian placental type both when Afrotheria is the sister to other taxa [proportional likelihood (P.L.) = 0.75; Table 2] and when Xenartha is the sister to other taxa (P.L. = 0.87; Table 2); however, only the Xenartha model reaches statistical significance.
Table 2.
Evolution of the primate placental morphology from the most recent common eutherian ancestor
| Placental characters |
Interface |
Shape |
Interdigitation |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Crown node | Parsimony | P.L. 1a* | P.L. 1b† | Parsimony | P.L. 1a* | P.L. 1b† | Parsimony | P.L. 1a* | P.L. 1b† |
| Eutheria | Hemochorial | 0.7492 | 0.8651 | Discoid | 0.9740 | 0.9992 | Labyrinthine‡ | 0.9412 | 0.3938 |
| Notolegia | Hemochorial | 0.9267 | — | Discoid | 0.9993 | — | Labyrinthine | 0.9300 | — |
| Epitheria | Hemochorial | — | 0.8673 | Discoid | — | 0.9867 | Labyrinthine | — | 0.9844 |
| Boreoeutheria | Hemochorial | 0.9118 | 0.8684 | Discoid | 0.9999 | 0.9996 | Labyrinthine | 0.9976 | 0.9994 |
| Euarchontaglires | Hemochorial | 0.9679 | 0.9556 | Discoid | 1.0000 | 0.9998 | Labyrinthine | 0.9990 | 0.9992 |
| Euarchonta | Hemochorial | 0.9340 | 0.9257 | Discoid | 0.9965 | 0.9965 | Labyrinthine | 0.9745 | 0.9750 |
| Primates | Hemochorial | 0.8640 | 0.8594 | Discoid | 0.9731 | 0.9731 | Villous | 0.9760 | 0.9763 |
*Afrotheria + Notolegia.
†Xenartha + Epitheria.
‡Equivocal.
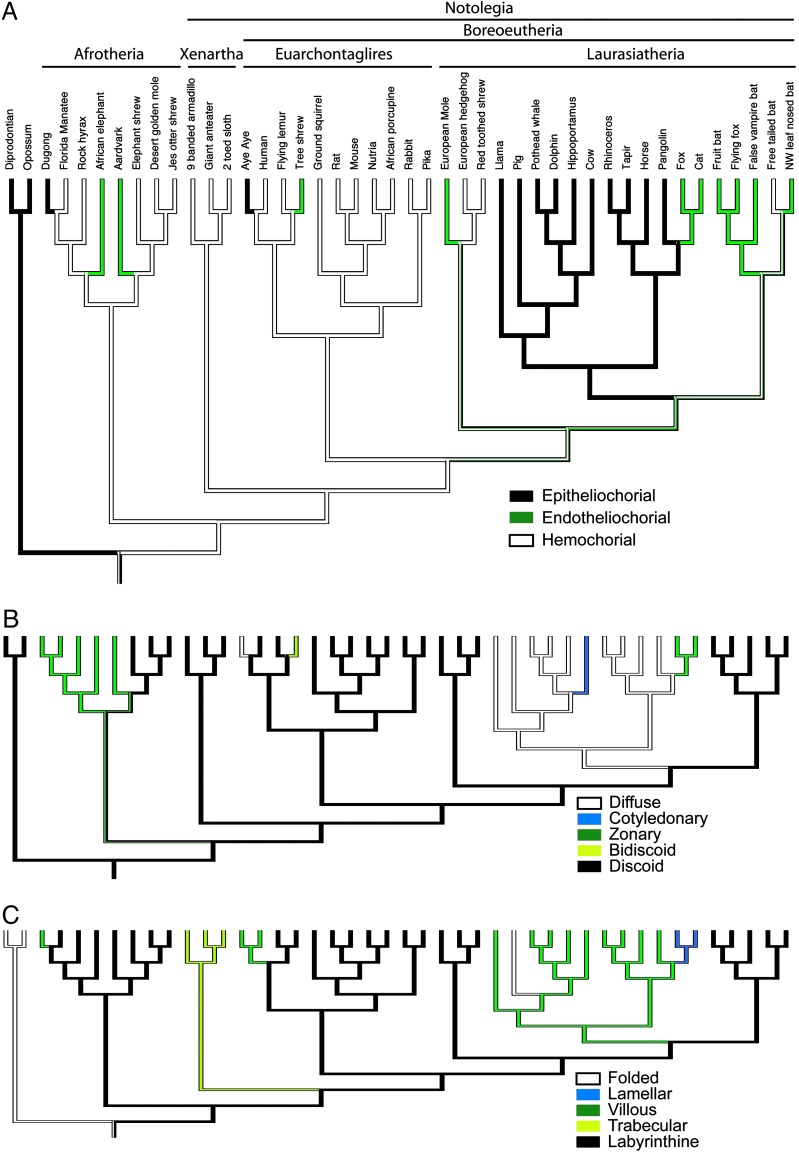
These analyses indicate that at least 11 character state changes (most parsimonious reconstruction) are required to describe the evolution of the placental interface (Fig. 2A). Within the Afrotheria, three evolutionary events are reconstructed: (i) a transition from the hemochorial to endotheliochorial placenta occurred twice [once in the elephants (e.g., Loxodonta africana)] and again in the aardvark, Orycteropus afer]; (ii) a transition from the hemochorial to epitheliochorial state may have occurred in the dugong, Dugong dugon. There is no change within the sampled members of Xenartha in our study (all are hemochorial). Within Euarchontaglires two changes are observed: (i) a transition from hemo- to endotheliochorial placentation in the tree shrew and (ii) a transition from the hemo- to epitheliochorial state in strepsirrhine primates (Fig. 3).
Fig. 2.
The evolution of morphological placental features in mammals. Parsimony reconstructions of internal nodes are shown for three morphological features (see Materials and Methods for details of reconstruction methodology). Taxon names and branching order are identical in all panels of the figure. Only the Afrotheria + Notolegia tree (i.e., 9) is shown. The data for all tree topologies examined is available as supporting information. (A) The placental interface describes the degree of invasiveness of fetal (i.e., placental) tissue into maternal tissue, with epitheliochorial being least invasive and hemochorial being most invasive. (B) The shape of the contact zone between fetal and uterine tissues. (C) The form of interdigitation between fetal and maternal tissues. Parsimony and Markov model likelihood reconstructions were constructed by using the data file available as supporting information.
Fig. 3.
The evolution of the placental interface in primates. Parsimony reconstructions of internal nodes are shown for the placental interface character (see Materials and Methods for details of reconstruction methodology). Parsimony and Markov model likelihood reconstructions were constructed by using the data file available as supporting information. Strep., Strepsirrhine; NWM, New World monkeys; OWM, Old World monkeys.
The placental interface state is equivocal on the stem laurasiatherian and stem scrotiferan (Laurasatheria less eulipotyphlans, i.e., moles, soricid shrews, and hedgehogs) lineages. An epitheliochorial placental interface is inferred as present on the stem Ferungulata (Scrotifera less bats) lineage. Within Ferungulata the carnivore stem lineage evolved an endotheliochorial interface. The evolution of placental interface types within Yangochirpotera (i.e., most microbats) remains unclear.
Evolution of Placental Shape.
Phylogenetic estimations of the ancestral placental shape suggest this character had a minimum of seven state changes during eutherian evolution (Fig. 2B). A discoid placenta is clearly ancestral for Eutheria (and probably for Metatheria + Eutheria) and is still maintained in most clades. This result is highly supported regardless of tree topology. Transitions of this character state have occurred in afrotherians (change to zonary), in ferungulates (most change to diffuse), and in euarchontans. An expanded primate data set (see Data Sets 1 and 2, which are published as supporting information on the PNAS web site) shows that this feature has changed additionally within the order (e.g., bidiscoid placentas are found among New World platyrrhine monkeys).
Evolution of Maternal–Fetal Interdigitation.
The topology that depicts a branching between Afrotheria and other placentals reconstructs a labyrinthine maternofetal interdigitation (P.L. = 0.99). However, when Xenartha is the sister to other eutherians the ancestral character state for Eutheria cannot be differentiated (the P.L. values are distributed as follows: labyrinthine ancestor, 0.39; trabecular ancestor, 0.39; folded ancestor, 0.19). The possibility that the ancestral eutherian state is either villous or lamellar is rejected. From these two models we can learn that the villous and lamellar forms of interdigitation emerged later during eutherian evolution. The villous type of blood flow exchange from mother to fetus has evolved at least three times independently during the descent of placental mammals from a eutherian most recent common ancestor (Fig. 2C). Changes occurred in the dugong (labyrinthine to villous) and in the stem primates (labyrinthine to villous). As in the previous characters, most change occurred in the laurasiatherians with transitions first from labyrinthine to villous followed by changes from villous to folded (pig) and lamellar (carnivores).
Primate Placental Evolution.
The hemochorial and discoid placentas found in humans represent ancient mammalian character states that emerged well before the origin of primates (Fig. 3). The epitheliochorial and diffuse placenta of strepsirrhine primates are shared derived features that evolved on the strepsirrhine stem lineage. Strepsirrhines and catarrhines (Old World monkeys, apes, and humans) have villous maternofetal interdigitation, but tarsiers and New World monkeys have trabecular interdigitation. Ancestral state reconstructions for this character are equivocal at the crown primate, crown haplorhine, and crown anthropoid nodes (see Data Set 2). The presence of a villous interdigitation on the crown primate node is supported by two lines of evidence. (i) The P.L. for this state is highest (P.L. = 0.48) for villous in comparison to trabecular (P.L. = 0.25). (ii) The strepsirrhine maternal placental interface and shape are synapomorphies for the clade. The fact they share a villous interdigitation with the catarrhines suggests that the villous form is ancestral and that the trabecular form has evolved twice in primate evolution on the tarsier and New World monkey lineages.
Discussion
Phylogenetic reconstructions demonstrate that the placenta of the ancestral eutherian mammal had a hemochorial placental interface with a discoid shape and a labyrinthine interdigitation. The change from labyrinthine to other forms of interdigitation occurred later during evolution in multiple clades (including primates). These findings challenge the traditional Haeckelian view of eutherian placentation in which the hemochorial placenta evolved from a placenta in which the fetal tissue had a more shallow contact with maternal tissue (14).
The influential monograph of J. P. Hill (15) posited the view that human placental morphology is an advanced, derived state. This perspective was accepted by Le Gros Clark (16) and expanded by Luckett (17, 18) and is widely accepted today. However, the idea that the hemochorial placenta is the ancestral primate state is not new; Wislocki (19) and others have held the mostly ignored position that the strepsirrhine placental interface is secondarily derived. Recently it has been suggested that a hemochorial placental interface is a possible ancestral state among eutherians (10, 20); however, the current study is the first, to our knowledge, to test this hypothesis and provide evidence to support it.
The relationship between the types of placental shape and maternal placental interface suggests that selection pressures constrain the evolution of these features such that the presence of one character state is usually accompanied by the presence of the other. Our findings demonstrate that an association between discoid shape and hemochorial interface had begun as early as the time of the last common ancestor of eutherian mammals. Changes in placental shape along the lineages of the phylogenetic tree usually were accompanied by changes in the maternal placental interface.
Villous maternal–fetal interdigitation evolved concomitantly in the primates and in Ferungulata. Within the latter clade, perrisodactyls, cetartiodactyls, and pholidatans (pangolins, i.e., scaly anteaters) have the villous form of interdigitation. The lamellar interdigitation seen in carnivores is derived in that group. The fact that haplorhine primates and ferungulates have different placental shapes and maternal–fetal interfaces, yet have the same fetal maternal interdigitation, raises questions concerning the selective advantage of maintaining this form of interdigitation in these clades.
Reviewing the reproductive features of clades with a villous type of inderdigitation reveals that many of the species have single offspring and relatively long gestation (between 8 and 18 months). Possibly villous interdigitation imposes less metabolic demand on the mother than labyrinthine interdigitation, thereby enabling her to sustain a longer gestation. In humans, exchange between villous trophoblast and maternal blood is not fully functional until the end of the first trimester (21), and the diabetogenic effect of pregnancy (a state of relative insulin resistance relating to increased fetal glucose transport) becomes effective toward the end of second trimester until delivery (22–26). Human placental lactogens are trophoblast-secreted (27) members of the growth hormone family (28) that play a role in inducing the diabetogenetic effect (29–31) of pregnancy (26, 32, 33). Placental lactogens have evolved independently at least three times during mammalian evolution (34, 35). The increased concentration of placental lactogens in the maternal circulation results in the increased fetal utilization of maternal resources, and therefore maternal–fetal conflict can occur (36, 37). In rodents (with a labyrinthine type of fetomaternal interdigitation), placental lactogens are secreted throughout pregnancy (type I during the first half and type II through the second half of their pregnancy) (38, 39). Conversely, in primates and ruminants (with a villous type of fetomaternal interdigitation) placental lactogens are secreted mainly during the second half of pregnancy (27, 40, 41). This observation implies that during their short gestation rodent fetuses use maternal resources to a much greater extent than do the fetuses of primates and ruminants. This finding coincides with our hypothesis that the evolution of the villous type of maternal–fetal interdigitation represents an evolutionary compromise that helps sustain longer pregnancies without depleting maternal resources to the point of starving the mother. This compromise resolves the maternal–fetal conflict. However, pathologic processes that interfere with this adaptation may result in the development of pregnancy complications (e.g., preterm delivery, intrauterine growth restriction, and stillbirth) (42). Therefore, the success of pregnancy involves maintaining the balance between maternal and fetal demands.
That intimate contact between fetal and maternal blood was established in the last common ancestor of the crown group of Eutheria gives credence to the hypothesis that successful pregnancy requires appropriate allorecognition and tolerance at the maternal–fetal interface. The comparison of the immunological diversity of different mammalian clades (with different placental shape, interface, and interdigitation) can provide a framework for understanding whether there is an association between maternofetal immunological recognition during pregnancy and the characteristics of the placenta.
In summary, we present here an evolutionary view of placental morphology that challenges the view that has existed for over a century. Our proposed view, which is based on our current understanding of the phylogenetic relationships among mammalian orders and within primates, suggests that the last common eutherian ancestor possessed a discoid, hemochorial placenta. The villous type of placental interdigitation is derived from the ancestral state.
Materials and Methods
Data Composition.
Mammalian phylogenetic trees were analyzed by using molecular data (9). Character state distributions were obtained from the literature (2, 4). The molecular data set consists of 44 taxa with at least one representative per eutherian order (n > 1 for Carnivora, Cetartiodactyla, Chiroptera, Eulipotyphla, Lagomorpha, Macroscelidea, Perrisodactyla, Pilosa, Primates, and Rodentia). A marsupial diprodontian and an opossum served as representative members of the metatherian outgroup. Three morphological characters and their states (in parentheses) were examined for the data set: (i) placental interface (epitheliochorial, endotheliochorial, and hemochorial). The hemochorial character state is subdivided into three character states (hemomonochorial, hemodichorial, and hemotrichorial). We performed a subanalysis given these three states. No difference was found whether the state was subdivided or not; therefore, we conducted our analysis with solely a hemochorial placental interface. (ii) Placental shape (diffuse cotylydon, zonary, bidiscoid, and discoid). (iii) Type of maternal–fetal interdigitation (folded, lamellar, villous, trabecular, and labyrinthine). Detailed descriptions of these character states are available (2); data currently available on the description of fetomaternal blood flow interrelation and neonatal/placental weight ratio are insufficient for statistical and phylogenetic analyses. The morphological data set is available as a taxon by character (n = 44) matrix in Data Set 3, which is published as supporting information on the PNAS web site.
Phylogenetic Analyses.
Extrapolating from methods used by Losos et al. (43), parsimony and likelihood scores for the molecular data were calculated according to a variety of tree topologies, including those previously published (9–12), as well as the most parsimonious topological constraint trees in which placental interface character state status was used as the constraining factor. Four constraint trees were analyzed: (i) a monophyly constraint consisting of all taxa with epitheliochorial placental interface; (ii) a monophyly constraint consisting of all taxa with endotheliochorial placental interface; (iii) a monophyly constraint consisting of all taxa with hemochorial placental interface; (iv) three monophyly constraints consisting of all taxa with epitheliochorial, endotheliochorial, and hemochorial placental interfaces. Statistical analysis was conducted to compare these trees (44–46). Tests using parsimony included the Templeton and the winning-sites tests. The likelihood tests were the Kishino–Hasegawa and the Shimodaira–Hasegawa procedures. Optimal trees, depicting three commonly recognized topologies among superordinal clades (Afrotheria, Laurasiatheria, Xenartha, and Euarchontaglires), were compared with trees with topological constraints based on morphological character states.
Character state evolution was reconstructed by using two methods: (i) maximum-likelihood-based discrete Markov k-state 1 and 2 parameter models (45, 47) and (ii) a maximum parsimony approach (44). Parsimony analyses considered character state transformations unordered. The likelihood-based Markov k-state 1 model does not consider any particular state plesiomorphic at the root of the tree, and a character state can change to any other state on any branch of the tree with equal probability. We report P.L. values of states scaled so that the sum of all states is 1. We used a decision threshold of 2.0 in mesquite (45) for statistical considerations. Efforts were made to use the same taxa as were presented in the molecular phylogenetic tree; however, this was not always possible. Only one macroscelidean was included in the morphological analysis; also, two sirenians were included because they may differ so greatly in placental morphology (4). An expanded primate data set was also analyzed given an estimate of phylogenetic relationships within the order (48). This data set is also included in Data Set 1.
Supplementary Material
Acknowledgments
We thank Drs. Joaquin Santolaya-Forgas, Juan C. Opazo, James Schuttle, and Monica Uddin for insightful comments and discussion. Dr. Mark Springer (University of California, Riverside, CA) graciously provided the alignment files of the molecular data. This research was supported in part by the Intramural Research Program of the National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services.
Abbreviation
- P.L.
proportional likelihood.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Archibald J. D., Rose K. D. In: The Rise of Placental Mammals. Rose K. D., Archibald J. D., editors. Baltimore: Johns Hopkins Univ. Press; 2005. pp. 1–8. [Google Scholar]
- 2.Benirschke K., Kaufmann P. Pathology of the Human Placenta. New York: Springer; 2000. [Google Scholar]
- 3.Enders A. C. Am. J. Anat. 1965;116:29–67. doi: 10.1002/aja.1001160103. [DOI] [PubMed] [Google Scholar]
- 4.Mossman H. W. Vertebrate Fetal Membranes: Comparative Ontogeny and Morphology, Evolution, Phylogenetic Significance, Basic Functions, Research Opportunities. New Brunswick, NJ: Rutgers Univ. Press; 1987. [Google Scholar]
- 5.Kaufman P. Placenta Suppl. 1981;1:13–28. [Google Scholar]
- 6.Dantzer V., Leiser R., Kaufmann P., Luckhardt M. Trophoblast Res. 1988;3:235–260. [Google Scholar]
- 7.Grosser O. Eihaute und der Placenta. Vienna: Braumuller; 1909. [Google Scholar]
- 8.Mossman H. W. Contrib. Embryol. 1937;26:133–247. [Google Scholar]
- 9.Murphy W. J., Eizirik E., O’Brien S. J., Madsen O., Scally M., Douady C. J., Teeling E., Ryder O. A., Stanhope M. J., de Jong W. W., Springer M. S. Science. 2001;294:2348–2351. doi: 10.1126/science.1067179. [DOI] [PubMed] [Google Scholar]
- 10.Asher R. J. In: The Rise of Placental Mammals. Rose K. D., Archibald J. D., editors. Baltimore: Johns Hopkins Univ. Press; 2005. pp. 50–70. [Google Scholar]
- 11.Asher R. J., Novacek M. J., Geisler J. H. J. Mamm. Evol. 2003;10:131–194. [Google Scholar]
- 12.Springer M. S., Murphy W. J., Eizirik E., O’Brien S. J. In: The Rise of Placental Mammals. Rose K. D., Archibald J. D., editors. Baltimore: Johns Hopkins Univ. Press; 2005. pp. 37–49. [Google Scholar]
- 13.Waddell P. J., Shelley S. Mol. Phylogenet. Evol. 2003;28:197–224. doi: 10.1016/s1055-7903(03)00115-5. [DOI] [PubMed] [Google Scholar]
- 14.Haeckel E. H. P. A. The Evolution of Man: A Popular Exposition of the Principal Points of Human Ontogeny and Phylogeny. London: Kegan Paul, Trench, and Co.; 1883. [Google Scholar]
- 15.Hill J. P. Philos. Trans. R. Soc. London Ser. B. 1932;221:45–178. [Google Scholar]
- 16.Le Gros Clark W. E. The Antecedents of Man: An Introduction to the Evolution of the Primates. Chicago: Quadrangle; 1960. [Google Scholar]
- 17.Luckett W. P. In: Reproductive Biology of the Primates. Luckett W. P., editor. Vol. 3. Basel: Karger; 1974. pp. 142–234. [Google Scholar]
- 18.Luckett W. P. In: Major Patterns in Vertebrate Evolution. Hecht M. K., Goody P. C., Hecht B. M., editors. New York: Plenum; 1977. pp. 439–516. [Google Scholar]
- 19.Wislocki G. B. Contrib. Embryol. 1929;20:51–80. [Google Scholar]
- 20.Vogel P. Placenta. 2005;26:591–596. doi: 10.1016/j.placenta.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Burton G. J., Jauniaux E. J. Soc. Gynecol. Invest. 2004;11:342–352. doi: 10.1016/j.jsgi.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Knopp R., Montes A., Childs M., Li J. R., Mabuchi H. Clin. Obstet. Gynecol. 1981;24:21–49. doi: 10.1097/00003081-198103000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Baird J. D. J. Endocrinol. 1969;44:139–172. doi: 10.1677/joe.0.0440139. [DOI] [PubMed] [Google Scholar]
- 24.Buchanan T. A., Metzger B. E., Freinkel M., Bergman R. N. Am. J. Obstet. Gynecol. 1990;160:1008–1014. doi: 10.1016/0002-9378(90)91306-w. [DOI] [PubMed] [Google Scholar]
- 25.Catalano P. M., Tyzbir E. D., Roman N. M., Amino S. B., Sims E. A. H. Am. J. Obstet. Gynecol. 1991;165:1667–1672. doi: 10.1016/0002-9378(91)90012-g. [DOI] [PubMed] [Google Scholar]
- 26.Homko C. J., Sivan E., Reece E. A., Boden G. Sem. Reprod. Endocrinol. 1999;17:119–125. doi: 10.1055/s-2007-1016219. [DOI] [PubMed] [Google Scholar]
- 27.Braunstein G. D., Rasor J. L., Engvall E., Wade M. E. Am. J. Obstet. Gynecol. 1980;138:1205–1213. doi: 10.1016/s0002-9378(16)32793-4. [DOI] [PubMed] [Google Scholar]
- 28.Niall H. D., Hogan M. L., Sauer R., Rosenblum I. Y., Greenwood F. C. Proc. Natl. Acad. Sci. USA. 1971;68:866–870. doi: 10.1073/pnas.68.4.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beck P., Daughaday W. H. J. Clin. Invest. 1967;46:103–110. doi: 10.1172/JCI105503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samaan N., Yen S. C. C., Gonzalez D., Pearson O. H. J. Clin. Endocrinol. Metab. 1968;28:485–491. doi: 10.1210/jcem-28-4-485. [DOI] [PubMed] [Google Scholar]
- 31.Kim Y. J., Felig P. J. Clin. Endocrinol. Metab. 1971;32:864–867. doi: 10.1210/jcem-32-6-864. [DOI] [PubMed] [Google Scholar]
- 32.Kalkhoff R. K., Richardson B. L., Beck P. Diabetes. 1969;18:153–163. doi: 10.2337/diab.18.3.153. [DOI] [PubMed] [Google Scholar]
- 33.Handwerger S., Brar A. Semin. Reprod. Endocrinol. 1992;10:106. [Google Scholar]
- 34.Forsyth I. A., Wallis M. J. Mamm. Gland Biol. Neoplasia. 2002;7:291–312. doi: 10.1023/a:1022804817104. [DOI] [PubMed] [Google Scholar]
- 35.Gootwine E. Anim. Reprod. Sci. 2004;82–83:551–566. doi: 10.1016/j.anireprosci.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Haig D. Quat. Rev. Biol. 1993;68:495–532. doi: 10.1086/418300. [DOI] [PubMed] [Google Scholar]
- 37.Haig D. J. Evol. Biol. 1996;9:357–380. [Google Scholar]
- 38.Robertson M. C., Friesen H. G. Endocrinology. 1981;108:2388–2390. doi: 10.1210/endo-108-6-2388. [DOI] [PubMed] [Google Scholar]
- 39.Robertson M. C., Gellespie B., Friesen H. G. Endocrinology. 1982;111:1862–1866. doi: 10.1210/endo-111-6-1862. [DOI] [PubMed] [Google Scholar]
- 40.Belanger C., Shome B., Friesen H. G., Myers R. E. J. Clin. Invest. 1971;50:2660–2667. doi: 10.1172/JCI106767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelly P. A., Robertson H. A., Friesen H. G. Nature. 1974;248:435–437. doi: 10.1038/248435a0. [DOI] [PubMed] [Google Scholar]
- 42.Romero R. Prenatal Neonatal Med. 1996;1:8–11. [Google Scholar]
- 43.Losos J. B., Jackman T. R., Larson A., Queiroz K., Rodriguez-Schettino L. Science. 1998;279:2115–2118. doi: 10.1126/science.279.5359.2115. [DOI] [PubMed] [Google Scholar]
- 44.Maddison D. R., Maddison W. P. macclade: Analysis of Phylogeny and Character Evolution. Sunderland, MA: Sinauer; 2000. Version 4.08. [DOI] [PubMed] [Google Scholar]
- 45.Maddison W. P., Maddison D. R. mesquite: A Modular System for Evolutionary Analysis. 2005. ( http://mesquiteproject.org/mesquite/mesquite.html), Version 1.06.
- 46.Swofford D. L. paup*: Phylogenetic Analysis Using Parsimony (* and Other Methods) Sunderland, MA: Sinauer; 2002. Version 4.0b10. [Google Scholar]
- 47.Lewis P. O. Syst. Biol. 2001;50:913–925. doi: 10.1080/106351501753462876. [DOI] [PubMed] [Google Scholar]
- 48.Goodman M., Grossman L. I., Wildman D. E. Trends Genet. 2005;21:511–517. doi: 10.1016/j.tig.2005.06.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.