Abstract
For years, it has been known that the “onset” of the antipsychotic response is “delayed,” and this notion is expressed in many major textbooks, informs clinical decisions and has even led to the search for biological markers responsible for this delayed onset. But is the onset of antipsychotic action really delayed? In this review, we bring together data from several recent studies of antipsychotic drugs that show that the onset of the antipsychotic effect is within the first day; the effect is distinguishable from behavioural sedation; is specific to antipsychotic drugs; is seen with oral and parenteral preparations; and is seen with typical and atypical antipsychotics. More anti- “psychotic” improvement is seen within the first 2 weeks than in any other 2-week period thereafter, and more improvement is seen in the first month than in the rest of the year of follow-up. This body of data convincingly refutes the notion of “delay” in the onset of antipsychotic action and suggests an “early” onset instead. The implications of this finding for clinical decision-making, mechanisms of antipsychotic action and drug discovery are discussed.
Medical subject headings: antipsychotic agents, dopamine antagonists, drug therapy, schizophrenia
Abstract
On sait depuis des années que « l'apparition » de la réponse aux antipsychotiques est « tardive » et ce concept exprimé dans nombre de manuels réputés éclaire des décisions cliniques et est même à l'origine de la recherche de marqueurs biologiques responsables de cette apparition tardive. L'apparition de l'effet des antipsychotiques est-elle toutefois véritablement tardive? Dans cette critique, nous réunissons des données provenant de plusieurs études récentes sur des antipsychotiques qui montrent que leur effet se fait sentir au cours de la première journée, qu'il est possible de distinguer l'effet de la sédation comportementale, que l'effet est spécifique aux antipsychotiques, qu'on le constate avec des préparations orales et parentérales et qu'il se fait sentir avec des antipsychotiques typiques et atypiques. On constate davantage d'améliorations anti « psychotiques » au cours des deux premières semaines qu'au cours de toute autre période de deux semaines par la suite et une amélioration plus marquée au cours du premier mois que pendant le reste de l'année de suivi. Cette masse de données réfute de façon convaincante le concept d'apparition « tardive » de l'effet antipsychotique et indique plutôt une apparition « précoce ». Les répercussions de cette constatation sur la prise de décisions cliniques, les mécanismes de l'effet antipsychotique et la découverte des médicaments font l'objet de discussions.
Introduction
Psychopharmacological treatment of schizophrenia that targets the elimination of psychotic symptoms started only in the second half of the 20th century.1 Until that time, pharmacotherapeutic interventions in schizophrenia focused on alterations of psychophysiological functioning: fever, sleep, coma and convulsions.2 Chlorpromazine, the first antipsychotic medication, was given to a psychiatric patient on Jan. 19, 1952, marking the beginning of the modern era of psychiatric pharmacotherapy.3 Because chlorpromazine acted on a large variety of molecular targets, it was difficult to decode which was relevant to its therapeutic effect. Stabilization of the cell membrane, inhibition of the N-methyl-transferase enzyme system and a decrease in adenosine triphosphate utilization were some of the suggested mechanisms of action for this antipsychotic medication.4
The search for an antipsychotic target was narrowed down by Carlsson and Lindqvist5 who reported that chlorpromazine and haloperidol increased the production of normetanephrine and methoxytyramine, metabolites of epinephrine and dopamine, respectively. To explain the increased production of these metabolites, these authors suggested that “the most likely [mechanism] appears to be that chlorpromazine and haloperidol block monoaminergic receptors in [the] brain; as is well known, they block the effects of accumulated 5-hydroxytryptamine.” The link to dopamine and dopamine receptors was unambiguously outlined by Van Rossum6 (see also Baumeister and Francis7), who stated that:
The hypothesis that neuroleptic drugs may act by blocking dopamine receptors in the brain has been substantiated by preliminary experiments with a few selective and potent neuroleptic drugs. There is an urgent need for a simple isolated tissue that selectively responds to dopamine so that less specific neuroleptic drugs can also be studied and the hypothesis further tested … When the hypothesis of dopamine blockade by neuroleptic agents can be further substantiated it may have forgoing consequences for the pathophysiology of schizophrenia. Overstimulation of dopamine receptors could then be part of the etiology.
Thus, while these developments hinted at what the target might be, it was Seeman et al8,9 who used in-vitro radioreceptor assays to detect the dopamine receptor directly and to demonstrate antipsychotic selectivity for the dopamine receptor.
Although on the one hand, the search for an antipsychotic mechanism was focusing on molecular targets, at a clinical level the effort was focused on examining the rate and determinants of behavioural response. The earliest reports of chlorpromazine treatment in the 1950s3,10–12 described responses within days. Some of these 1950s studies report an early anti-“psychotic” response that is over and above changes in sedation or level of agitation. These reports described changes in the thought content of the psychotic patient within days after administration of the medications: “Robert S., 21 years old: … treatment with 4560 RP permitted in the first days (“premiers jours”) a return of calm, interrupted by a few logorrheic episodes.”3 In fact, the 1952 report by Delay et al3 showed that within 3 days chlorpromazine alleviated hallucinations and stopped internal “voices” in 8 patients, a dramatic finding. Over subsequent years, several other authors have also raised the issue of an early onset of antipsychotic response in different settings (e.g., Stern et al,13,14 McDermott et al,15 Garver et al16,17 and Keck et al18).
Thus, while one can find a sprinkling of findings supporting an early onset of action all through the literature, during the 1970s the notion started arising that the onset of antipsychotic action is delayed and that it takes 2–3 weeks before the onset of therapeutic benefits is produced.19,20 It is hard to pinpoint where this notion actually arose, but it clearly gained credence within the field because leading basic researchers started looking for an explanation for the delayed antipsychotic response.21,22 The suggested explanation for this delayed onset of action was the “depolarization block” theory. This hypothesis, which was based on preclinical studies that involved recordings of dopamine neuron firing in paralyzed anesthetized rats, suggested that the effect of repeated antidopaminergic (i.e., antipsychotic) administration on dopaminergic neurons in the brain is inactivation of firing and that this inactivation takes place only after 3 weeks (21 d) of continuous treatment. This delay in the onset of the biological marker for 3 weeks was thought to coincide with, and explain, the delay in onset of both the therapeutic effect and the neurological side effects of these drugs on patients with schizophrenia.23–25
This idea of a clinical “delayed onset” of antipsychotic action and the depolarization block that explains the delay is now firmly embedded in standard psychiatric textbooks. Over the last 3 decades, more than 105026 articles have cited the main research papers that describe the depolarization block hypothesis.19–22,25,27–35 But is the onset really delayed?
An important distinction must be made between a delayed “onset” and a delayed realization of full improvement. Whereas there can be no debate that the complete therapeutic benefits of antipsychotic medications take several weeks to be realized, this does not imply a delay in the “onset” of action. That treatments take time to reach their full therapeutic benefit is true in almost every area of medicine. For example, a cast put on a broken leg is removed in 6 weeks, a time when the healing has reached a certain level of structural sufficiency. However, no one would claim a delay in the “onset” of fracture healing, which starts at a histopathological level within days of the fracture. The above considerations lead to 2 competing hypotheses: an early onset with progressive accumulation or a true delay in onset (depicted in Fig. 1). Both of these hypotheses can account for the observation that sufficient clinical improvement takes weeks to achieve, but they differ substantially regarding what happens in the first few weeks (Fig. 1).
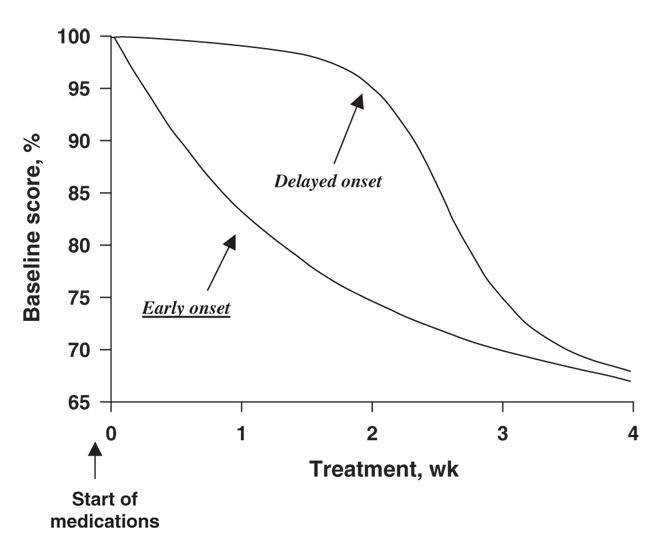
Fig. 1: Delayed-onset hypothesis versus early onset hypothesis.
Questioning the idea of delayed onset
One of the major issues raised by the idea of the delayed onset was the disconnection between the onset of treatment and the onset of therapeutic effect. The question is thrust into an even sharper contrast by the data available from brain imaging studies. Brain imaging has provided a direct window onto the dopamine blockade system in humans. Nordstrom et al36 observed the speed of onset of dopamine blockade in response to receiving haloperidol, and Tauscher et al37 have reported the effects of the atypical antipsychotic medications risperidone and olanzapine. These studies show a robust blockade of the dopamine system within hours after drug administration, and this blockade of the dopamine system is sustained through the next day. Subjects reach significant (~60%) and sustained levels of dopamine-2 (D2) occupancy within the first day or two. Given that the blockade of the dopamine system is essentially immediate, the idea of a delayed onset provides an obstacle for the theory of a direct relation between dopamine, dopamine blockade, psychosis and antipsychotics. A number of recent findings strongly question this delayed-onset notion.
Agid et al38 reviewed the data published in English from controlled, double-blind studies of antipsychotic treatment in patients with schizophrenia spectrum disorders during the first 4 weeks of antipsychotic treatment. Articles for the review were obtained from a search of several databases: MEDLINE, CINAHL, EMBASE, EBMZ (Evidence Based Medicine Reviews), ACP Journal Club, the Cochrane Database of Systematic Reviews and the Database of Abstracts of Reviews of Effectiveness (DARE). Studies were included in the meta-analysis if they included data regarding the efficacy of medication during the first 4 weeks of treatment and described the assessment of efficacy of the drug treatment using the Brief Psychiatric Rating Scale39 (BPRS) or the Positive and Negative Syndrome Scale40 (PANSS) rating scales. Forty-two double-blind controlled studies including 7450 patients were identified. Meta-analysis of the data shows that overall clinical improvement within the first week of antipsychotic treatment was significantly greater than that which was observed in later weeks. Most notably, the decrease in scores over the first week was almost 3 times as great as the observed effect in weeks 3 and 4.
In order to investigate whether the onset of action of the antipsychotics was early versus delayed, tests of overall clinical improvement were undertaken to determine whether greater improvement is seen in the first 2 weeks (as per the early onset hypothesis) or the next 2 weeks (as per the delayed-onset hypothesis). These tests found a significant difference (p < 0.0001), indicating that the decline in scores within the first 2 weeks of treatment (21.9%) was significantly greater than the decline observed in the third and fourth weeks (9.8%) (Fig. 2A).
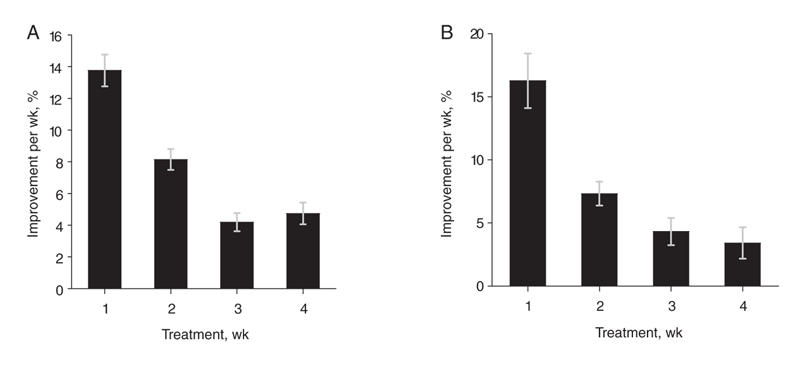
Fig. 2: Response to antipsychotic treatment over time. (A) Mean (and standard error [SE]) overall clinical improvement over time (total score) (p < 0.001). (B) Mean (and SE) change in core psychotic symptoms over time (p < 0.01). The p values are for the main effect of time. The error bars represent standard error. Figure reproduced with permission from the American Medical Association (Arch Gen Psychiatry 2003;60:1228-3538).
Change in core psychotic symptoms over time was measured by change in the BPRS thought subscale and the PANSS positive subscale. The decline in these scores was considerably greater over the first week than in later weeks (p < 0.01). As predicted by the early onset hypothesis, the decline in scores within the first 2 weeks (24.4%) of the initial treatment was almost 3 times as much as the decline observed in the third and fourth weeks (7.7%) (p < 0.01) (Fig. 2B).
To account for the placebo effect, the mean weekly improvement obtained in the placebo-treated group was removed from that observed in the drug-treated group. After subtracting the placebo group response, the improvement in scores on active antipsychotic treatment remained significantly greater in the first week than in the third week (p < 0.001). The improvement in the second week was significantly larger than the effect in the third week (p < 0.001) and the fourth week (p < 0.01). Improvement in the first 2 weeks (17.2% after subtracting the placebo effect) was significantly higher than in the subsequent 2 weeks (6.7%, difference p < 0.001) (Fig. 3A). The rate of decline in the core psychotic symptoms after removal of the placebo effect was also greater within the first 2 weeks of treatment. A contrast of the average effect observed during the first 2 weeks of treatment versus the following 2 weeks confirms that the psychotic items also show a decidedly early onset of improvement (p = 0.019) (Fig. 3B).
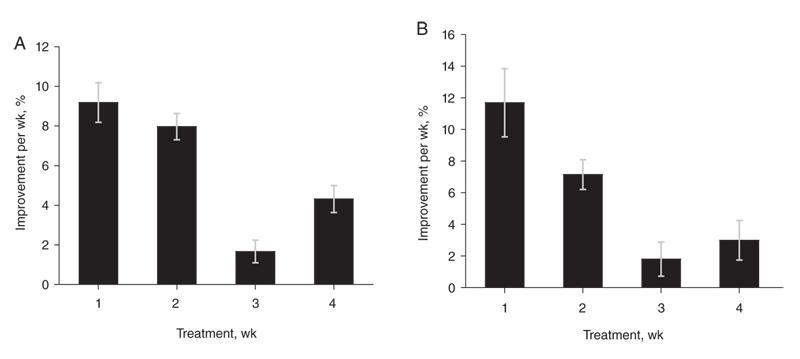
Fig. 3: Response to antipsychotic treatment over time after removal of the placebo effect. (A) Mean (and standard error [SE]) weekly overall clinical improvement (p < 0.001). (B) Mean (and SE) weekly change in core psychotic symptoms (p = 0.08). The p values are for the main effect of time. The error bars represent standard error. Figure reproduced with permission from the American Medical Association (Arch Gen Psychiatry 2003;60:1228-3538).
The delayed-onset hypothesis — tested and rejected
Leucht et al41 replicated the study by Agid et al38 using a different approach. Original patient data from 7 randomized, double-blind studies of the efficacy of amisulpride in acutely ill patients with schizophrenia spectrum disorders were pooled for a post hoc analysis. Data for 1708 patients with psychotic symptoms were examined for the incremental reduction in percentage of the BPRS scores over time.
Whereas the study by Agid et al38 used meta-analysis techniques, Leucht et al41 overcame some of the methodological limitations of the meta-analytic approach by analyzing a large homogeneous database of individual patient data using only 1 rating scale. This individual patients' database enabled the researchers to analyze data in a more homogeneous way, eliminating the need to rely on mean values and often-incomplete presentation of the data in published reports and also addressed the calculations concerning those patients who dropped out of the study more efficiently.41
An additional difference was that Leucht and colleagues chose to investigate amisulpride, an antipsychotic medication with a unique receptor-binding profile of pure D2 blockade with negligible action on other receptors.42 Amisulpride is clearly associated with lower use of antiparkinsonian medication and fewer dropouts due to adverse events than conventional antipsychotic drugs.43,44 Because it is often suggested that improvement in patients with psychosis in the early weeks of treatment results from nonspecific treatment effects (e.g., sedation), the low side-effect profile of amisulpride facilitates investigation of the early effects of the medication on the psychotic symptoms. A final advantage of the study by Leucht et al included the extension of the analysis to 1 year of treatment.
The results of this meta-analysis41 show that the weekly improvement in the total BPRS score and its psychotic subscales in the entire study sample (n = 1708) was significant over time. The mean percentage change in the BPRS total score and the psychotic subscales score up to week 2 of treatment was greater (32.1%) than the additional change up to week 4 of treatment (12.5%). The 1-year subset analysis also revealed that the reduction in the BPRS score acquired during the first 4 weeks of treatment was significantly higher than the additional change in the BPRS during the rest of the year. Accordingly, 68% of the total BPRS effect and 70% of the positive symptoms effect were already achieved after only 4 weeks of treatment (Fig. 4).
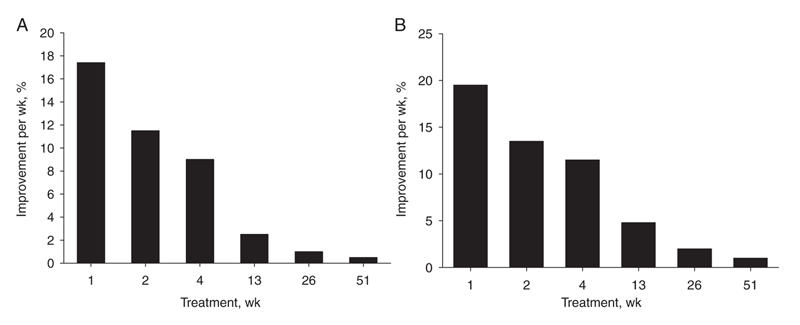
Fig. 4: Response to antipsychotic treatment over time (n = 748). (A) Mean overall clinical improvement over time (total Brief Psychiatric Rating Scale score). (B) Mean change in core psychotic symptoms over time. Figure reproduced with permission from the Society of Biological Psychiatry (Biol Psychiatry 2005;57:1543-941).
Thus, amisulpride, a selective dopamine antagonist medication with negligible action on other receptors, which was not included in the meta-analysis by Agid et al, showed the same early onset of response pattern as the atypical and typical antipsychotic medications that were investigated in that meta-analysis.38
If it's not delayed, how early is it?
The studies described above reviewed research papers that rated the patients every week or every couple of weeks during the first period of treatment. However, in order to find out how quickly antipsychotic medications work, one should investigate what happened to the patients during the first few days and even during the first few hours of treatment. Kapur et al45 investigated the effect of intramuscular antipsychotics on response in an Eli Lilly multicentre study. In this study, patients were rated twice during the first 24 hours after starting treatment. Three-hundred and eleven patients with a diagnosis of schizophrenia spectrum disorder and an acute exacerbation of symptoms were randomly assigned to receive olanzapine, 10 mg intramuscularly (IM) (n = 131), haloperidol, 7.5 mg IM (n = 126), or placebo IM (n = 54). Patients were rated using the PANSS and Clinical Global Impression (CGI) rating scales46 at baseline, 2 hours and 24 hours.
Analysis of the data demonstrates that after 24 hours of treatment, olanzapine and haloperidol show a significant effect on 3 psychotic symptoms (conceptual disorganization, hallucinatory behaviour and unusual thought content) compared with placebo. One might argue that this very early improvement in the BPRS scores is secondary to improvement in the nonspecific behavioural items of the BPRS rating scale. Kapur and colleagues addressed this question by using an analysis of covariance to adjust for the effects of improvement in the nonspecific behavioural factors. There was a statistically significant effect of the antipsychotic medications on the BPRS thought subscale score after correction for the BPRS component dealing with agitation, excitement and hostility.
In addition, the 2-hour change in the BPRS items describing agitation and excitement did not predict the 24-hour change in items describing psychosis (t216 = 1.60, p = 0.11). On the other hand, change in the BPRS psychotic items after 2 hours predicted change in psychosis after 24 hours of treatment, suggesting that the early response in psychosis is distinct from changes in BPRS items describing the agitation and excitement component.
Generalizability to other drugs and settings
Recently, studies exploring second-generation antipsychotic medications indicated an early onset of action for these medications as well.
Quetiapine
The efficacy of the atypical antipsychotic drug quetiapine in treating schizophrenia has been established in 3 double-blind, placebo-controlled randomized trials.47–49 A combined analysis of data from these 3 trials shows that within 1 week after initiating treatment, the overall improvement in symptoms with quetiapine was significantly greater than achieved via placebo, as measured by the total BPRS score.50 The BPRS positive subscale score during the first week of antipsychotic treatment showed a significantly higher proportion of responders (“response” is defined as a 15%–30% reduction in the BPRS positive symptom score) among quetiapine-treated patients versus placebo-treated patients.50
Aripiprazole
In one of the largest 4-week double-blind, placebo-controlled studies testing the efficacy of aripiprazole,51 404 patients were randomly allocated to receive aripiprazole, 20 mg/d (n = 101) or 30 mg/d (n = 101), risperidone, 6 mg/d (n = 99), or placebo (n = 103). Aripiprazole at both doses was significantly better than placebo on PANSS and CGI scores. Separation from placebo occurred already at the first week of treatment according to PANSS total and positive scores. (Mean change in total PANSS score from baseline after 1 week of treatment with aripiprazole, 20 mg/d or 30 mg/d: –9.0 v. –2.0 for placebo, p < 0.001. Mean change in positive PANSS score from baseline after 1 week of treatment with aripiprazole, 20 mg/d or 30 mg/d: –2.5 v. –0.5 for placebo, p < 0.001.)
Ziprasidone
In a 6-week double-blind, placebo-controlled study,52 302 patients with an acute exacerbation of schizophrenia or schizoaffective disorder were randomly allocated to receive ziprasidone, 80 mg/d (n = 106) or 160 mg/d (n = 104), or placebo (n = 92). Both doses of ziprasidone were shown to be significantly more effective than placebo in treating psychosis after 1 week of treatment. Reduction was measured in all assessments of global (BPRS total score) and positive (BPRS core psychotic items) scores. (Mean change from baseline in BPRS total score: –4.0 for ziprasidone, 80 mg/d, –6.0 for ziprasidone, 160 mg/d, v. placebo –0.5; p < 0.05 and p < 0.001, respectively. Mean change from baseline in BPRS core psychotic score: –1.8 for ziprasidone, 80 mg/d, –2.5 for ziprasidone, 160 mg/d, v. placebo –0.8, p < 0.05 and p < 0.001, respectively.)
Dopamine depletion
Although all the efficacy studies described above provide evidence for early change in psychotic symptomatology after administration of an antipsychotic that blocks the D2 receptors, a study using a dopamine depletion agent also demonstrates an early onset of change in psychotic symptoms. Abi-Dargham et al53 examined the effects of AMPT (α-methyl-para-tyrosine), a tyrosine hydroxylase inhibitor known to cause depletion of dopamine levels.53 The subjects were administered AMPT for 2 days, and its effect on dopamine depletion was confirmed with the use of 121I-IBZM single-photon-emission computed tomography imaging. This confirmed that depletion by AMPT induced a significant reduction in the severity of positive psychotic symptoms as early as 72 hours after dopamine depletion. The core psychotic symptoms score as measured by the PANSS positive subscale at baseline was 18.6 (standard deviation [SD] 5.8), and after AMPT 15.0 (SD 6.4) (p = 0.001). Therefore, not only do dopamine blocker agents show an early onset of response, but dopamine depletors do as well.
Relevance and implications
The “delayed onset” idea has greatly influenced psychopharmacological and clinical research. More than 1000 articles, from the 1970s till today, have quoted this hypothesis.54,55 The new data and analyses lead one to reject the delayed-onset hypothesis and provide a revised impetus for basic and clinical research.
Given this evidence for improvement in psychotic symptoms in the first week or two of treatment, why has the concept of delayed onset been so widely accepted? The answer may depend on issues surrounding the power of clinical trials and the issue of clinical relevance of percent changes in PANSS or BPRS scores. From a clinical trial perspective, the erroneous perception of delay may have resulted from confusion between the concept of “onset” of action versus the time required to achieve a given level of improvement or statistical significance. In almost all of the studies that were included in the meta-analysis by Agid et al,38 the antipsychotic group numerically separates from the placebo group in the very first measure (usually in the first week of treatment). The degree of improvement in the first week (13.8% on average) is smaller than the size of total cumulative improvement at the end of the third or fourth week (26.1% and 30.8%, respectively). Because most studies are powered to detect an effect in the range of 25%–30%, they may have inadequate power to declare the early change as significant — even though this was evident in the data from almost each of the trials.
From a clinical perspective, clinicians may not “see” the early response to treatment, because it has not as yet crossed their threshold of clinical noticeable improvement, even though the very same clinicians are rating that improvement on a scale. According to some recent studies,56–58 clinicians declare having observed a “minimal improvement” (using the CGI rating scale) corresponding to a percentage PANSS and BPRS reduction of 19% and 23%, respectively. On the CGI scale, “minimal improvement” is the smallest clinically observable change from baseline. To reach criteria of “much” or “very much” improved, patients have to show 45% or 70% improvement.57,58 Considering the recent data regarding the degree of improvement during the first week of treatment (13.8% on average on a combined BPRS and PANSS scale or 17.7% reduction on the BPRS scale38,41) and second week of treatment (21.8% on average on a combined BPRS and PANSS scale or 30.7% reduction on the BPRS scale38,41), it is possible that the changes during this first week, although highly statistically significant and documented using objective scales, fall below the threshold that clinicians find clinically notable.
At a basic level, apart from the fact that all antipsychotic drugs block the D2 receptor,59 there is little agreement about the molecular and systemic mechanisms mediating the antipsychotic effect. To identify these mechanisms, different authors have sought drug-induced gene-induction, electrophysiological and synaptic alterations. Given that antipsychotic drugs induce hundreds of such changes, the delayed-onset theory has been one way to guide this search. Thus, attention has often focused on biological markers that were absent immediately after the first few doses of a drug, but emerged only after 2–3 weeks of treatment.22,60,61 The current findings call for revision of this strategy. They suggest that if there are simple molecular markers that track antipsychotic response, their course is likely to be as depicted in Figure 1, with features of an early onset, progressive accumulation over repeated dosing and final plateau of an effect.
At a clinical level, focusing on a drug's activity in the first few weeks after administration has the potential to provide information regarding predictors of response to antipsychotic treatment. It is a common clinical practice to treat patients for 4–6 weeks with one medication before deciding whether the patient is responding to that drug.62 Although it may well take 4–6 weeks to get a certain degree of response, it is an open question whether one has to wait that long to predict whether a patient will respond to a drug. If the greatest rate of improvement is in the first week or two of treatment, it raises the possibility that early response to treatment may predict the effectiveness of a drug for a given individual. A recent study by Correll et al63 shows this to be the case: 131 acutely ill patients with schizophrenia received 4 weeks of fluphenazine treatment. BPRS scores were obtained at baseline and on a weekly basis thereafter. All the patients who showed 20% (or less) improvement after 1 week of treatment were classified as nonresponders after 4 weeks of treatment. Thus, patients with minimal improvement in the BPRS total score or the BPRS positive symptoms after 1 week of treatment are unlikely to respond to a 4-week treatment trial.63
In summary, several converging lines of evidence have led us to seriously question the conventionally held notion about the delayed onset of antipsychotic action. It is time to give up on that idea. Our work, and that of others, as summarized in this article, proposes an early onset and progressive accumulation hypothesis instead. It should be pointed out that while the new findings may change our understanding about how fast drugs act, this does not by itself change how fast drugs act. The drugs did not act any more slowly when the field believed in the delayed-onset hypothesis, nor will the drugs act any faster just because we have a different way of understanding them. However, the hypothesis generated here opens the door for new basic science findings and new clinical approaches. We hope that some of them will come to fruition.
Acknowledgments
This article is a development of ideas that were presented in a talk given by Drs. Kapur and Seeman at the receipt of the Innovations in Neuropsychopharmacology Award of the Canadian College of Neuropsychopharmacology (CCNP), 2004. Drs. Seeman and Kapur would like to thank the CCNP and their peers who considered their work deserving of the Innovations Award, which in turn made this article possible.
Footnotes
2004 Innovations in Neuropsychopharmacology Award Paper
Contributors: Dr. Kapur conceived of the review. All the authors contributed to the acquisition and interpretation of the data and the writing of the article. All authors gave final approval for the article to be published.
Dr. Kapur's research in this area is supported by a Canada Research Chair and grants from the Canadian Institutes of Health Research (CIHR), a Special Initiative Grant from the Ontario Mental Health Foundation (OMHF), as well as grants from the pharmaceutical industry. Dr. Seeman's work is supported by the OMHF (Regular grant and Special Initiatives grant), the CIHR, the Stanley Medical Research Institute, the National Institute on Drug Abuse, the Medland and O'Rorke families, and the estate of Dr. Karolina Jus.
Competing interests: None declared for Dr. Agid. Dr. Seeman has received speaker fees from AstraZeneca. Dr. Kapur has received grant support from AstraZeneca, Eli Lilly, Janssen, Neuromolecular Inc., Pfizer and Wyeth–Ayerst; speaker fees from AstraZeneca, Bristol–Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen, Pfizer and Wyeth–Ayerst; and has served as scientific advisor to AstraZeneca, Bristol–Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen, Pfizer, Solvay Kingswood and Wyeth–Ayerst.
Correspondence to: Dr. Shitij Kapur, Centre for Addiction and Mental Health, 33 Russell St., Toronto ON M5S 2S1; fax 416 260-4206; shitij_kapur@camh.net
References
- 1.Lehmann HE, Ban TA. The history of the psychopharmacology of schizophrenia. Can J Psychiatry 1997;42:152-62. [DOI] [PubMed]
- 2.Stip E. Happy birthday neuroleptics! 50 years later: la folie du doute. Eur Psychiatry 2002;17:115-9. [DOI] [PubMed]
- 3.Delay J, Deniker P, Harl JM. Traitement des états d'excitation et d'agitation par une méthode médicamenteuse dérivée de l'hibernithérapie. Ann Med Psychol (Paris) 1952;110(2:2):267-73. [PubMed]
- 4.Shen WW. A history of antipsychotic drug development. Compr Psychiatry 1999;40:407-14. [DOI] [PubMed]
- 5.Carlsson A, Lindqvist M. Effect of chlorpromazine or haloperidol on formation of 3-methoxytyramine and normetanephrine in mouse brain. Acta Pharmacol Toxicol (Copenh) 1963;20:140-4. [DOI] [PubMed]
- 6.Van Rossum JM. The significance of dopamine-receptor blockade for the action of neuroleptic drugs. In: Brill H, Cole JO, Deniker P, Hippius H, Bradley PB, editors. Proceedings of the Fifth International Congress of the Collegium Internationale Neuro-Psychopharmacologicum; 1966 Mar 28-31; Washington. Amsterdam: Excerpta Medica Foundation; 1967. p. 321-9.
- 7.Baumeister AA, Francis JL. Historical development of the dopamine hypothesis of schizophrenia. J Hist Neurosci 2002;11:265-77. [DOI] [PubMed]
- 8.Seeman P, Chau-Wong M, Tedesco J, et al. Brain receptors for antipsychotic drugs and dopamine: direct binding assays. Proc Natl Acad Sci U S A 1975;72:4376-80. [DOI] [PMC free article] [PubMed]
- 9.Seeman P, Lee T, Chau-Wong M, et al. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature 1976;261:717-9. [DOI] [PubMed]
- 10.Delay J, Deniker P, Harl JM. [Therapeutic use in psychiatry of phenothiazine of central elective action (4560 RP)]. Ann Med Psychol (Paris) 1952;110(2:1):112-7. [PubMed]
- 11.Elkes J, Elkes C. Effects of chlorpromazine on the behaviour of chronically overactive psychotic patients. BMJ 1954;4887:560-5. [DOI] [PMC free article] [PubMed]
- 12.Winkelman NW. Chlorpromazine in the treatment of neuropsychiatric disorders. JAMA 1954;155:18-21. [DOI] [PubMed]
- 13.Stern RG, Kahn RS, Davidson M, et al. Early response to clozapine in schizophrenia. Am J Psychiatry 1994;151:1817-8. [DOI] [PubMed]
- 14.Stern RG, Kahn RS, Harvey PD, et al. Early response to haloperidol treatment in chronic schizophrenia. Schizophr Res 1993;10:165-71. [DOI] [PubMed]
- 15.McDermott BE, Sautter FJ, Garver DL. Heterogeneity of schizophrenia: relationship to latency of neuroleptic response. Psychiatry Res 1991;37:97-103. [DOI] [PubMed]
- 16.Garver DL, Kelly K, Fried KA, et al. Drug response patterns as a basis of nosology for the mood-incongruent psychoses (the schizophrenias). Psychol Med 1988;18:873-85. [DOI] [PubMed]
- 17.Garver DL, Steinberg JL, McDermott BE, et al. Etiologic heterogeneity of the psychoses: Is there a dopamine psychosis? Neuropsychopharmacology 1997;16:191-201. [DOI] [PubMed]
- 18.Keck PE Jr, Cohen BM, Baldessarini RJ, et al. Time course of antipsychotic effects of neuroleptic drugs. Am J Psychiatry 1989;146: 1289-92. [DOI] [PubMed]
- 19.Bunney BS. Dopaminergic blocking effects of antipsychotic drugs. J Psychiatr Res 1974;11:72-3. [DOI] [PubMed]
- 20.Bunney BS, Walters JR, Roth RH, et al. Dopaminergic neurons: effect of antipsychotic drugs and amphetamine on single cell activity. J Pharmacol Exp Ther 1973;185:560-71. [PubMed]
- 21.Grace AA. The depolarization block hypothesis of neuroleptic action: implications for the etiology and treatment of schizophrenia. J Neural Transm Suppl 1992;36:91-131. [DOI] [PubMed]
- 22.Grace AA, Bunney BS, Moore H, et al. Dopamine-cell depolarization block as a model for the therapeutic actions of antipsychotic drugs. Trends Neurosci 1997;20:31-7. [DOI] [PubMed]
- 23.Bunney BS. Antipsychotic drug effects on the electrical activity of dopaminergic neurons. Trends Neurosci 1984;7:212-5.
- 24.Bunney BS, Grace AA. Acute and chronic haloperidol treatment: comparison of effects on nigral dopaminergic cell activity. Life Sci 1978;23:1715-27. [DOI] [PubMed]
- 25.Grace AA, Bunney BS. Induction of depolarization block in midbrain dopamine neurons by repeated administration of haloperidol: analysis using in vivo intracellular recording. J Pharmacol Exp Ther 1986;238:1092-100. [PubMed]
- 26. ISI Web of Knowledge. Philadelphia: Thomson Scientific; 2002. Available: www.isiknowledge.com [a subscription is required].
- 27.Brodie MS, Bunney EB. Serotonin potentiates dopamine inhibition of ventral tegmental area neurons in vitro. J Neurophysiol 1996; 76:2077-82. [DOI] [PubMed]
- 28.Grace AA. Cortical regulation of subcortical dopamine systems and its possible relevance to schizophrenia. J Neural Transm Gen Sect 1993;91:111-34. [DOI] [PubMed]
- 29.Harden DG, Grace AA. Activation of dopamine cell firing by repeated L-DOPA administration to dopamine-depleted rats: its potential role in mediating the therapeutic response to L-DOPA treatment. J Neurosci 1995;15:6157-66. [DOI] [PMC free article] [PubMed]
- 30.Hollerman JR, Abercrombie ED, Grace AA. Electrophysiological, biochemical, and behavioral studies of acute haloperidol-induced depolarization block of nigral dopamine neurons. Neuroscience 1992;47:589-601. [DOI] [PubMed]
- 31.Hollerman JR, Grace AA. Acute haloperidol administration induces depolarization block of nigral dopamine neurons in rats after partial dopamine lesions. Neurosci Lett 1989;96:82-8. [DOI] [PubMed]
- 32.Hollerman JR, Grace AA. Subthalamic nucleus cell firing in the 6-OHDA-treated rat: basal activity and response to haloperidol. Brain Res 1992;590:291-9. [DOI] [PubMed]
- 33.Moore H, Todd CL, Grace AA. Striatal extracellular dopamine levels in rats with haloperidol-induced depolarization block of substantia nigra dopamine neurons. J Neurosci 1998;18:5068-77. [DOI] [PMC free article] [PubMed]
- 34.Onn SP, Grace AA. Repeated treatment with haloperidol and clozapine exerts differential effects on dye coupling between neurons in subregions of striatum and nucleus accumbens. J Neurosci 1995;15:7024-36. [DOI] [PMC free article] [PubMed]
- 35.Pucak ML, Grace AA. Evidence that systemically administered dopamine antagonists activate dopamine neuron firing primarily by blockade of somatodendritic autoreceptors. J Pharmacol Exp Ther 1994;271:1181-92. [PubMed]
- 36.Nordstrom AL, Farde L, Halldin C. Time course of D2-dopamine receptor occupancy examined by PET after single oral doses of haloperidol. Psychopharmacology (Berl) 1992;106:433-8. [DOI] [PubMed]
- 37.Tauscher J, Jones C, Remington G, et al. Significant dissociation of brain and plasma kinetics with antipsychotics. Mol Psychiatry 2002;7:317-21. [DOI] [PubMed]
- 38.Agid O, Kapur S, Arenovich T, et al. Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch Gen Psychiatry 2003;60:1228-35. [DOI] [PubMed]
- 39.Overall JEG, Gorham DR. The brief psychiatric rating scale. Psychol Rep 1962;10:799-812.
- 40.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 1987;13:261-76. [DOI] [PubMed]
- 41.Leucht S, Busch R, Hamann J, et al. Early-onset hypothesis of antipsychotic drug action: a hypothesis tested, confirmed and extended. Biol Psychiatry 2005;57:1543-9. [DOI] [PubMed]
- 42.Perrault G, Depoortere R, Morel E, et al. Psychopharmacological profile of amisulpride: an antipsychotic drug with presynaptic D2/D3 dopamine receptor antagonist activity and limbic selectivity. J Pharmacol Exp Ther 1997;280:73-82. [PubMed]
- 43.Leucht S. Amisulpride a selective dopamine antagonist and atypical antipsychotic: results of a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol 2004;7(Suppl 1):S15-20. [DOI] [PubMed]
- 44.Leucht S, Pitschel-Walz G, Engel RR, et al. Amisulpride, an unusual “atypical” antipsychotic: a meta-analysis of randomized controlled trials. Am J Psychiatry 2002;159:180-90. [DOI] [PubMed]
- 45.Kapur S, Arenovich T, Agid O, et al. Evidence for onset of antipsychotic effects within the first 24 hours of treatment. Am J Psychiatry 2005;162:939-46. [DOI] [PubMed]
- 46.Guy W. Clinical global impression. In: Guy W, editor. ECDEU Assessment Manual for Psychopharmacology. Rockville (MD): US Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, NIMH Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976. p. 218-22.
- 47.Arvanitis LA, Miller BG. Multiple fixed doses of “Seroquel” (quetiapine) in patients with acute exacerbation of schizophrenia: a comparison with haloperidol and placebo. The Seroquel Trial 13 Study Group. Biol Psychiatry 1997;42:233-46. [DOI] [PubMed]
- 48.Borison RL, Arvanitis LA, Miller BG. ICI 204,636, an atypical antipsychotic: efficacy and safety in a multicenter, placebo-controlled trial in patients with schizophrenia. US SEROQUEL Study Group. J Clin Psychopharmacol 1996;16:158-69. [DOI] [PubMed]
- 49.Small JG, Hirsch SR, Arvanitis LA, et al. Quetiapine in patients with schizophrenia. A high-and low-dose double-blind comparison with placebo. Seroquel Study Group. Arch Gen Psychiatry 1997;54:549-57. [DOI] [PubMed]
- 50.Small JG, Kolar MC, Kellams JJ. Quetiapine in schizophrenia: onset of action within the first week of treatment. Curr Med Res Opin 2004;20:1017-23. [DOI] [PubMed]
- 51.Potkin SG, Saha AR, Kujawa MJ, et al. Aripiprazole, an antipsychotic with a novel mechanism of action, and risperidone vs placebo in patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry 2003;60:681-90. [DOI] [PubMed]
- 52.Daniel DG, Zimbroff DL, Potkin SG, et al. Ziprasidone 80 mg/day and 160 mg/day in the acute exacerbation of schizophrenia and schizoaffective disorder: a 6-week placebo-controlled trial. Ziprasidone Study Group. Neuropsychopharmacology 1999;20:491-505. [DOI] [PubMed]
- 53.Abi-Dargham A, Rodenhiser J, Printz D, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci U S A 2000;97:8104-9. [DOI] [PMC free article] [PubMed]
- 54.Grunder G, Siessmeier T, Piel M, et al. Quantification of D2-like dopamine receptors in the human brain with 18F-desmethoxyfallypride. J Nucl Med 2003;44:109-16. [PubMed]
- 55.Kuhar MJ, Joyce AR. Slow onset of CNS drugs: Can changes in protein concentration account for the delay? Trends Pharmacol Sci 2001;22:450-6. [DOI] [PubMed]
- 56.Leucht S, Engel RR. The relative sensitivity of the Clinical Global Impressions Scale and the Brief Psychiatric Rating Scale in antipsychotic drug trials. Neuropsychopharmacology 2006'31(2):406-12. [DOI] [PubMed]
- 57.Leucht S, Kane JM, Kissling W, et al. Clinical implications of Brief Psychiatric Rating Scale scores. Br J Psychiatry 2005;187:366-71. [DOI] [PubMed]
- 58.Leucht S, Kane JM, Kissling W, et al. What does the PANSS mean? Schizophr Res 2005;79:231-8. [DOI] [PubMed]
- 59.Kapur S, Mamo D. Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry 2003;27:1081-90. [DOI] [PubMed]
- 60.Atkins JB, Chlan-Fourney J, Nye HE, et al. Region-specific induction of deltaFosB by repeated administration of typical versus atypical antipsychotic drugs. Synapse 1999;33:118-28. [DOI] [PubMed]
- 61.Lynch MR, Carey RJ. Chronic low-dose haloperidol effects on self-stimulation rate-intensity functions. Psychopharmacology (Berl) 1990;102:122-9. [DOI] [PubMed]
- 62.Kaplan HI, Sadock BJ. Comprehensive textbook of psychiatry. Baltimore (MD): Lippincot Williams & Wilkins; 2000.
- 63.Correll CU, Malhotra AK, Kaushik S, et al. Early prediction of antipsychotic response in schizophrenia. Am J Psychiatry 2003; 160:2063-5. [DOI] [PubMed]


