Abstract
It has been hypothesized that a decrease in the synthesis of new neurons in the adult hippocampus might be linked to major depressive disorder (MDD). This hypothesis arose after it was discovered that antidepressant medications increased the synthesis of new neurons in the brain, and it was noted that the therapeutic effects of antidepressants occurred over a time span that approximates the time taken for the new neurons to become functional. Like antidepressants, exercise also increases the synthesis of new neurons in the adult brain: a 2–3-fold increase in hippocampal neurogenesis has been observed in rats with regular access to a running wheel when they are compared with control animals. We hypothesized, based on the adult-neurogenesis hypothesis of MDD, that exercise should alleviate the symptoms of MDD and that potential mechanisms should exist to explain this therapeutic effect. Accordingly, we evaluated studies that suggest that exercise is an effective treatment for MDD, and we explored potential mechanisms that could link adult neurogenesis, exercise and MDD. We conclude that there is evidence to support the hypothesis that exercise alleviates MDD and that several mechanisms exist that could mediate this effect through adult neurogenesis.
Medical subject headings: beta-endorphin, brain-derived neurotrophic factor, depression, exercise, neurogenesis, serotonin, vascular endothelial growth factor
Abstract
On a émis l'hypothèse qu'une réduction de la synthèse de nouveaux neurones dans l'hippocampe d'un adulte pourrait être liée au trouble dépressif majeur (TDM). On est arrivé à cette hypothèse après avoir découvert que les antidépresseurs augmentaient la synthèse de nouveaux neurones dans le cerveau, et on a remarqué que les antidépresseurs produisaient leurs effets thérapeutiques à peu près dans le même temps que prenaient les nouveaux neurones pour devenir fonctionnels. Tout comme les antidépresseurs, l'exercice augmente la synthèse de nouveaux neurones dans un cerveau adulte : on a observé une augmentation de deux à trois fois supérieure de la neurogénèse hippocampique chez des rats ayant accès à une roue par rapport aux animaux témoins. On a supposé, à partir de l'hypothèse de la neurogénèse adulte liée au TDM, que l'exercice devrait atténuer les symptômes du TDM et que des mécanismes potentiels sont susceptibles d'expliquer cet effet thérapeutique. Nous avons donc examiné des études laissant entendre que l'exercice est un traitement efficace du TDM, et nous avons étudié des mécanismes potentiels susceptibles d'établir un lien entre la neurogénèse adulte, l'exercice et le TDM. Selon nous, des preuves appuient l'hypothèse voulant que l'exercice atténue le TDM et que plusieurs mécanismes peuvent déclencher cet effet durant la neurogénèse adulte.
Introduction
Adult neurogenesis refers to the growth of new neurons in the adult brain. Initially, reports of adult neurogenesis were viewed with a great deal of skepticism, because neurogenic activity had been assumed to be limited to the developmental period;1 however, the legitimacy of adult neurogenesis in some brain areas has since gained wide acceptance. It is now clear that some regions of the adult mammalian brain contain populations of active progenitor cells that can give rise to new neurons and glia.2–8 Indeed, adult neurogenesis seems to be a characteristic of all mammalian brains, including human brains.9 Still, fundamental questions about adult neurogenesis have yet to be answered: for example, why does adult neurogenesis occur in only some areas of the mammalian brain, and what purposes do these new neurons serve? Studies of neurogenic activity in the hippocampi of laboratory animals aim to answer some of these questions.
Recently, it has been proposed that a deficit in adult neurogenesis may result, or be somehow involved, in major depressive disorder (MDD) and that the mechanism of action for antidepressant medications may involve promoting neurogenesis.10–14 The discovery that exercise increases the rate of adult neurogenesis in the hippocampus of mice and rats also suggests a mechanism for the claimed therapeutic effect of exercise on MDD.15,16 In this article, we summarize the process of adult neurogenesis as it is currently understood and briefly describe the evidence that implicates neurogenesis in MDD. We then review studies of the antidepressant effects of exercise on MDD and consider molecular factors that could link adult neurogenesis, exercise and MDD.
Where in the brain does adult neurogenesis occur?
New neurons are not created equally throughout the adult mammalian brain; only certain regions possess populations of active progenitor cells.17–20 Substantial adult neurogenesis occurs in only the dentate gyrus of the hippocampus and the subventricular zone adjacent to the lateral ventricles.3,4,8 It has been estimated that about 9000 new neurons are created in the mature rat dentate gyrus every day.21 The number of new neurons created in the subventricular zones has not yet been estimated, but it is likely to exceed the number created in the dentate gyrus. Although most work regarding adult neurogenesis has been done in rats and mice, current information seems to support both the dentate gyrus and subventricular zone of the lateral ventricles as sites of neurogenesis in both nonhuman primates8,22 and humans.9
The hippocampus is a bilateral limbic structure that plays a role in certain learning and memory processes.23–26 It is composed of 2 substructures that are physiologically distinct (Fig. 1A). One is the cornu Ammonis, whose subfields (CA1, CA2, CA3) contain pyramidal cells that exhibit regional differences in their size, afferent input and efferent projections. The CA1 subfields interlock with the second substructure of the hippocampus, the dentate gyrus. The dentate gyrus is a C-shaped structure that is composed of small, round granule cells. New neurons and glia seem to be created from progenitor cells just below this granule cell layer in what is termed the “subgranular zone” (Fig. 1B).
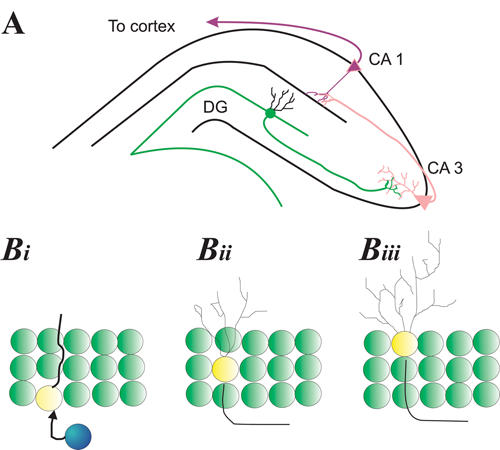
Fig. 1: Hippocampal neurogenesis. (A) Schematic of the hippocampus illustrating the relation between granule cells in the dentate gyrus (DG) and pyramidal cells in the cornu Ammonis (CA1, CA3). DG granule cells extend axons from the inner granule zone that project onto dendrites of pyramidal cells in the CA3 layer of the cornu Ammonis (pink). CA3 pyramidal neurons extend axons to CA1 dendrites (black); CA1 cells then extend axons to multiple neural areas, in particular to the cortex. (Bi) Progenitor cells (blue) in the subgranular zone of the DG give way to immature neurons (yellow with extension) that initially extend a proboscis (primary dendrite) through the granular cell layer as well as an axon toward the CA3 region. (Bii) Dendritic complexity increases as neurons mature and migrate into the granule cell layer. (Biii) Some new neurons migrate to the outer granular zone and have dendrites that no longer possess a primary dendrite but, rather, have a more bush-like appearance.
The subventricular region adjacent to the lateral ventricles is the second major source of new neurons in the adult mammalian brain. In this region, there are numerous proliferative precursor cells that develop into neurons or glia.27 These proliferative cells lie just below the layer of ependymal cells, which line the lateral ventricles.28,29
The subgranular and subventricular precursor cells are close to one another in the mammalian brain; the hippocampus forms the medial wall and floor of the lateral ventricle in each hemisphere. However, these 2 sites of adult neurogenesis are characterized by different patterns of development.30,31 Progenitor cells in the subgranular zone divide, and the resulting daughter cells migrate the short distance (20–30 μm) into the granule cell layer. Once in the granule cell layer, the new cells increase the size and complexity of their dendritic tree and send axons to the CA3 region of the pyramidal layer. This process occurs over a period of about 4 weeks, and it results in the formation of new neurons that exhibit morphological and electrophysiological characteristics like those of mature granule cells.31 In contrast, the daughter cells created in the subependymal zones divide, and the resulting daughter cells migrate about 6 mm to the olfactory bulbs (Fig. 1B).28,32 Once in the olfactory bulbs, these new cells become local interneurons.33
It should be noted that glial cells are also derived from precursor cells in the subependymal and subgranular zones.34,35 Little is known about these new glial cells. It is not known whether they arise from the same precursor cell type as the neurons, or how many new glial cells are born, or what their function may be. Intriguingly, it has also been shown that radial glia themselves may be the progenitor cell in the adult brain.36 Recent demonstrations implicating glial cells in various aspects of neural activity and plasticity suggest that these new glial cells warrant further investigation.37,38
In addition, it has recently been reported that new neurons can, under some conditions, be created in other brain regions, including the neocortex.39–41 However, it remains to be determined whether these become functional neurons.
Environmental influences on adult neurogenesis
There are 3 distinct environmental influences that have been reported to stimulate neurogenesis in adulthood. The first finding was that mice raised in an environment enriched with toys and tunnels had sustained levels of neurogenesis, at least in the dentate gyrus.7 This finding has since been replicated.42,43 The second finding was that learning was capable of stimulating neurogenesis. Rats or mice that were forced to learn a particular task were found to have more new neurons in the dentate gyrus in comparison with rats or mice that did not learn the task.44–46 Interestingly, an investigation into exactly what it was about an enriched environment that increased neurogenesis led to the discovery that voluntary exercise reliably and significantly increases hippocampal neurogenesis.15,16,47,48
Animals that engage in exercise have been reliably shown to undergo a sustained increase in adult neurogenesis in the hippocampus in comparison with control animals that do not engage in exercise15,16,48–50 (Fig. 2). For example, mice given free access to a running wheel for 2–4 months have more than twice the number of new cells in the subgranular zone of the dentate gyrus in comparison with control mice (no access to a running wheel).16 Similar results have also been obtained in rats.47,48 These increases are observed whether animals are housed in small social groups15 or individually.47,48 Increases in adult neurogenesis can be observed as early as 3 days after the start of exercise (unpublished observations) but are more reliably observed after at least a week of exercise.
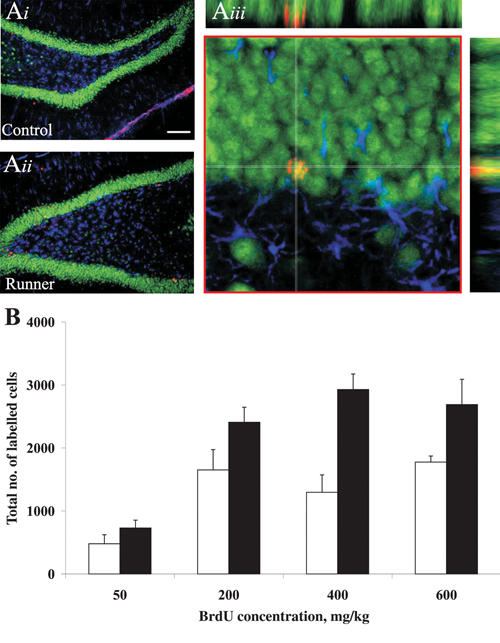
Fig. 2: Exercise increases neurogenesis in adult rats. (Ai–Aiii) Images of 42-day-old cells from a control animal (Ai) and an animal given free access to an exercise wheel (Aii). New neurons are shown in red, whereas mature granule cells are green, and astrocytes are blue. (Aiii) Confocal image of boxed area outlined in red from Ai. For new cells to be considered neurons, they must stain red (bromodeoxyuridine [BrdU], a marker of new cells) and green (Neuronal Nuclei [NeuN], a mature neuronal marker), but not blue (glial fibrillary acidic protein [GFAP], a marker of mature astrocytes). The cell at the centre of the white crosshairs is flipped 90° in both the x and y planes to ensure that the cell is co-labelled for both BrdU and NeuN. (B) Figure shows that exercise (black bars) increases the number of BrdU-positive cells in animals compared with controls (white bars), irrespective of the dose of BrdU administered to the animal (data adapted from Eadie et al, J Comp Neurol 2005;486:39-4747).
MDD and hippocampal pathology
MDD is an affective disorder characterized by depressed mood. This depressed mood can be so severe, enduring and pervasive that it disturbs virtually all daily activities, even such fundamental activities as eating, sleeping and maintaining personal hygiene. About 10% of those diagnosed with MDD commit suicide.51,52 In industrialized Western societies, about 5% of individuals will be diagnosed with MDD at some point in their lives, and the number of people diagnosed with MDD is rising.53,54 Clearly, MDD constitutes a health and social problem of major proportions.55,56
In the last decade, fuelled largely by improvements in brain imaging technology, there has been a concerted effort to identify the neuropathological changes associated with MDD. For example, patients with MDD display a general decrease in cerebral blood flow, as well as reduced glucose metabolism in the amygdala57,58 and the prefrontal cortex.59,60 However, the strongest evidence linking MDD with pathological changes in a specific brain structure involves the hippocampus.61
Several structural magnetic resonance imaging studies have found hippocampal volume is reduced in depressed patients when compared with age-matched and sex-matched controls.62–68 This reduction in hippocampal volume has recently been found in a postmortem study.69 Although reduced neurogenesis may account for some of the loss of hippocampal volume in patients with MDD, there are likely to be other precipitating factors that may contribute to these structural changes. Stress-induced hormones released from the adrenal gland (e.g., the corticosteroids) can affect the hippocampus by decreasing neurogenesis,70–75 and it has been suggested that these hormones play a role in the decreased hippocampal volume associated with MDD.76–79 Indeed, the stress response is tied to the pathophysiology of MDD; too much stress, or allostatic load, can lead to immune dysfunction, depressed mood and changes in brain structure.80 For example, repeated stress is known to cause atrophy of dendrites in the CA3 region.71 The hippocampus has receptors for adrenal steroids, including the mineralocorticoids and glucocorticoids,77,81 and it is known to be involved in the negative feedback loop of the hypothalamic–pituitary–adrenal (HPA) axis. This stress response and the effects on hippocampal neurogenesis may also be differentially regulated in males and females.70 Thus, abnormalities in the regulation of the stress response may be related to decreased hippocampal volume in depressed patients,82 which in turn could further affect the stress response.
The adult-neurogenesis hypothesis of MDD
According to the hypothesis that implicates adult neurogenesis in MDD, a decreased rate of neurogenesis contributes to the depressed mood observed in patients with MDD.13,83,84 Three lines of evidence support this hypothesis. First, the hippocampus, which is one of only 2 sites of adult neurogenesis in the mammalian brain, has been found to be smaller in some depressed patients.62–68 Because neural degeneration does not appear to be accelerated in depressed patients, at least in the hippocampus,85 the diminished volume of the hippocampus associated with depression could very well be the product of a reduced rate of adult neurogenesis. Second, adult neurogenesis is increased by treatments for MDD. Antidepressants, such as selective serotonin reuptake inhibitors, have been shown to increase the number of new neurons in the adult hippocampus of laboratory animals, as does electroconvulsive shock treatment.10,86–88 This increase is thought to be the result of the downstream effects of increased serotonergic activation, particularly with regard to brain-derived neurotrophic factor.89 Third, new neurons normally take 4 or 5 weeks to become functional,31 which is a latency similar to the onset of therapeutic benefit for most programs of antidepressant medication. This suggests that the relief from depressive symptoms may be dependent on the maturation of new hippocampal neurons and their integration into the existing neural network. Although no direct links between depression and adult neurogenesis have yet been demonstrated,90,91 these 3 lines of indirect evidence warrant further evaluation of the relation between adult neurogenesis and MDD.
Effects of exercise on depression
In the 1980s, reports of the beneficial effects of exercise on MDD began to emerge. Most of these published studies were case studies and epidemiological studies, but by 1999 14 studies that had some degree of experimental control had been published. These 14 studies were reviewed by Lawlor and Hopker,92 who concluded that exercise has antidepressant effects that are of the same magnitude as cognitive therapy. However, they tempered their conclusions by pointing out that the 14 studies had a variety of methodological weaknesses. For example, they tended to lack adequate control conditions and blinded outcome ratings, and in most cases the samples were small.92
Subsequent to the review article by Lawlor and Hopker,92 there has been a marked increase in the study of exercise and depression — perhaps in part stimulated by that article. In general, these recent studies have been of higher quality than earlier ones; that is, they have involved larger samples or better controls, or both. Nevertheless, they have confirmed and extended the results of the earlier studies. For example, the recent epidemiological studies by Kritz-Sliverstein et al,93 Motl et al94 and Strawbridge et al95 have shown that young and elderly individuals who engage in programs of exercise display fewer depressive symptoms and are less likely to subsequently develop MDD. In a second series of studies, Leppamaki et al,96 Penninx et al97 and Dimeo et al98 all found that exercise reduces depressive symptoms in adults who have not been diagnosed with a mental disorder.
Most germane to the present article are the recent studies that have assessed the effects of exercise on patients with MDD. Three articles are of particular interest. First, Dimeo et al99 assessed the efficacy of exercise in the treatment of moderate-to-severe MDD. In this study, exercise significantly alleviated the condition. In the second, Mather et al100 assessed the effects of exercise classes as an adjunct to antidepressant medication. The patients who participated in the exercise classes displayed greater improvement than did the control patients, who attended health education talks. Third, Dunn et al101 assessed the effect of the “dose” of exercise on 80 adults with MDD. There were 3 conditions: moderate aerobic exercise, low-intensity aerobic exercise and flexibility exercise. The moderate aerobic exercise group improved significantly more than the other 2 groups.
The results of 2 recent studies are particularly important, because they indicate that the antidepressant effects of exercise endure after the period of exercise. Remarkably, Singh et al102 and Babyak et al103 found that the antidepressant effects of exercise extend past the treatment period with benefits continuing 21 months102 and 6 months103 after the curtailment of the exercise program, respectively.
Another particularly significant recent study of the therapeutic effects of exercise on MDD is the study by Kubesch et al.104 Most previous studies had focused on various measures of depression; however, the various deficits in executive function that appear to be secondary effects of the depression are particularly problematic for many patients with MDD. Kubesch et al assessed executive function by measuring the reaction times of depressed patients on the Stroop task, the Go/No-Go task, the Task-Switching Paradigm and the Flanker task: reaction times on these tests are seen as a measure of executive function. They found that a single 30-minute aerobic endurance exercise period could significantly improve reaction times on these tests in depressed patients. It should be noted, however, that the immediacy of this result would indicate that the benefits of exercise involve more than just increasing neurogenesis.
In summary, recent studies have confirmed earlier (pre-2000) reports that exercise has a significant beneficial effect on depressive symptoms. Accordingly, there is now substantial evidence of the antidepressant effects of exercise. Although there is still a paucity of parametric studies of exercise and MDD, a few recent studies suggest that greater aerobic exercise is associated with greater benefits, that the antidepressant effects of exercise can far outlast the period of exercise, and that the therapeutic benefits in cases of MDD are not solely restricted to depressive symptoms.
Potential mechanisms for exercise-induced neurogenesis
There are several candidate molecules that could play a role in exercise-induced increases in neurogenesis in the adult brain. To find candidate molecules, we screened the literature for molecules (a) whose levels were affected by exercise and (b) that had an effect on the growth of new neurons in the adult hippocampus. The following 4 molecules were selected: β-endorphins, vascular endothelial growth factor (VEGF), brain-derived neurotrophic factor (BDNF) and serotonin (5-HT).
β-Endorphins
An increase in β-endorphins after exercise was first reported over 20 years ago, when β-endorphins emerged as a candidate mechanism to explain why people seemingly become addicted to running.105–108 β-Endorphins are cleaved from the preprohormone pro-opiomelanocortin (POMC), which is a protein found in the pituitary gland and the brain.109 POMC undergoes cleavage to give way to a number of hormones, including the melanocortins and the opiate peptides. β- Endorphins, a class of opiate peptide, share a common N-terminal sequence, function as neuromodulators and are cleaved from the C-terminus of the POMC protein. When β-endorphins bind to their receptors in neural membranes, cyclic adenosine monophosphate (cAMP) levels in the neurons are reduced, and the conductance of voltage-gated Ca2+ channels is decreased.109 Although β-endorphin is not known to cross the blood–brain barrier, levels of plasma β-endorphin-like immunoactivity may indirectly reflect central opioid activity.110 Importantly, β-endorphins can influence neurogenesis and other hippocampal functions.111,112
Recent studies have shown that an increase in cell proliferation can be produced by the direct infusion of opiates and that opiate receptor antagonists decrease cell proliferation in the dentate gyrus.111,113 Furthermore, when the transcriptional control of enhanced green fluorescent protein (eGFP) is linked to POMC genomic sequences, eGFP is expressed in only new cells in the dentate gyrus.30 Together these results implicate POMC, and possibly β-endorphins, in the genesis, or survival, of new neurons in the dentate gyrus of adult animals.
Vascular endothelial growth factor
VEGF is another molecule that may link exercise and adult neurogenesis.49 VEGF is a protein secreted from blood that acts on endothelial cells to stimulate the formation of blood vessels, and it was first discovered as a vascular permeability factor secreted from cancerous cells.114–116 VEGF family members possess a receptor-binding motif related to that of platelet-derived growth factor.117
VEGF is known to be increased when humans exercise,118 and the infusion of this molecule in the absence of exercise increases neurogenesis in adult rats.119 It is thought that new cells in the dentate gyrus preferentially develop from vascular niches, and it may be the release of VEGF from these blood vessels that leads to an increase in cell numbers in these localized regions.120
Brain-derived neurotrophic factor
BDNF is a member of the neurotrophin family, that is, the same family that includes nerve growth factor and neurotrophins 3 and 4.121 BDNF plays a critical role in the brain throughout development and adulthood by promoting neuronal survival and regeneration.122–125
Exercise leads to increased levels of BDNF.43,126–131 Studies performed in rodents have linked voluntary exercise regimes to increases in BDNF mRNA and BDNF protein. Interestingly, voluntary exercise increases BDNF expression in the central nervous system48,132,133 but not in skeletal muscle,134 indicating that BDNF may play a significant role specifically in the brain. It has recently been found that in “depressed” animals, voluntary exercise can increase both hippocampal cell proliferation and the levels of hippocampal BDNF.135 However, it was concluded that the antidepressant effect of exercise was the result of the enhanced proliferation, because nondepressed animals also exhibited an increase in the levels of BDNF without altering cell proliferation.135 This does not mean, however, that BDNF and exercise do not complement each other in relation to neurogenesis. Indeed, injections of BDNF directly into the hippocampus of rats can enhance proliferation and neurogenesis in the subgranular zone of the dentate gyrus.136 A recent study of mice genetically engineered to express decreased levels of BDNF suggests that BDNF is necessary for the long-term survival of new neurons in the dentate gyrus, because these mice had fewer bromodeoxyuridine (BrdU)-labelled cells 3 weeks after the initial BrdU injection.137 In addition, application of BDNF to cultured dentate gyrus granule cells increases cell survival and differentiation.138 Taken together, these data suggest that exercise might be an effective method for increasing BDNF; BDNF, in turn, can then enhance the survival of newborn cells in the hippocampus.
Serotonin
The link between 5-HT and adult neurogenesis has received a great deal of attention over the past 5 years. The first line of evidence implicating 5-HT in adult neurogenesis was the discovery in rodents that decreases or increases in the brain levels of 5-HT led to decreases and increases in neurogenesis, respectively.86 Currently, specific 5-HT receptor types responsible for regulating both neurogenesis and cell proliferation in the adult brain are beginning to be identified.139 Intriguingly, exercise seems to elevate the levels of tryptophan hydroxylase (the enzyme involved in the rate-limiting step in the synthesis of 5-HT) in the raphe nucleus,140–142 which is an area in the brain stem that is densely populated with serotonergic cells143,144 and sends projections to the hippocampus145,146 that can influence hippocampal activity.147,148 It has also been found that running can increase the levels of tryptophan (which is necessary for the synthesis of 5-HT) in the hippocampus, although this did not increase the levels of 5-HT in the hippocampus.149 Even though there was no specific increase in the levels of 5-HT in the hippocampus, the increased expression of tryptophan might enhance 5-HT production and augment neurogenesis.
Summary
There are several candidate molecules that could play an important role in mediating the effects of exercise on adult neurogenesis. Exercise can increase the levels of β-endorphins, VEGF, BDNF and 5-HT. β-Endorphins might enhance the birth of new neurons in the dentate gyrus, whereas VEGF and BDNF enhance their survival. Exercise also increases the levels of 5-HT in the brain,150–152 which contributes to neurogenesis because 5-HT can increase adult cellular proliferation and neurogenesis in the dentate gyrus.153 Therefore, exercise might have therapeutic effects on MDD, because exercise could increase neurogenesis through the aforementioned putative mechanisms.
Conclusions, limitations and future directions
There is a growing body of evidence that implicates reductions in adult neurogenesis as being associated with MDD. The purpose of this paper was to assess 2 implications of this adult-neurogenesis hypothesis for MDD. First, because exercise has been shown to increase adult neurogenesis in the dentate gyrus, we assessed the evidence for exercise having antidepressant effects. We found substantial evidence that exercise can be an effective treatment for MDD. Second, we reviewed recent studies of adult neurogenesis in laboratory animals to identify molecular mechanisms that might mediate the facilitatory effects of exercise on adult neurogenesis. We identified and discussed 4 molecules that are increased during exercise and have a stimulatory effect on adult neurogenesis: β-endorphins, VEGF, BDNF and 5-HT. These 2 independent lines of evidence together suggest that adult neurogenesis might alleviate the depressive symptoms of MDD.
The major weakness in the empirical support for the adult-neurogenesis hypothesis of MDD is that MDD is a human condition, whereas most of the research on adult neurogenesis has been conducted in nonhuman species. Evidence that exercise increases adult neurogenesis in rodents and decreases depressive symptoms in humans is suggestive but hardly incontrovertible evidence for the hypothesis. In particular, work will need to be done to determine whether the production of new neurons by exercise can play a therapeutic role in alleviating MDD, or whether this phenomenon is merely an unrelated event that is associated with some other factor, such as BDNF, that plays multiple roles in the brain.
Given the inherent difficulties of measuring neurogenesis in human patients, the identification of molecular correlates of adult neurogenesis may prove to be the most beneficial route for future research. However, the evidence thus far provides a hopeful picture for those who have MDD, and further investigations into the role that exercise can play in both structural and functional changes in the brain may help to unveil the nature of MDD in humans and may provide insight into other therapeutic venues for this disorder.
Acknowledgments
This research was supported by Canadian Institutes of Health Research (CIHR), Natural Sciences and Engineering Research Council of Canada (NSERC), Scottish Rite Charitable Foundation of Canada and BC Ministry of Children and Family Development (HELP) awards to Dr. Christie. Mr. Ernst is supported by an NSERC award. Dr. Christie is the BMO Young Investigator at the University of British Columbia Hospital. Dr. Lam has received funding from the CIHR and the Vancouver Hospital Foundation.
Footnotes
Contributors: All the authors contributed to the conception and design of the study. Mr. Ernst and Dr. Christie contributed to the acquisition of the data. All the authors contributed to the analysis of the data and to the writing and critical review of the article. All the authors gave final approval for publication.
Competing interests: None declared for Mr. Ernst, Ms. Olson, Dr. Pinel and Dr. Christie. Dr. Lam is on the speaker/advisory boards for AstraZeneca, Biovail, Eli Lilly, GlaxoSmithKline, Janssen, Litebook Company, Inc., Lundbeck, Servier, Shire and Wyeth. He has received research funds from AstraZeneca, Eli Lilly, GlaxoSmithKline, Janssen, Lundbeck, Merck, Roche, Servier and Wyeth.
Correspondence to: Dr. Brian R. Christie, Department of Psychology, University of British Columbia, 2136 West Mall, Vancouver BC V6T 1Z4; fax 604 822-6923; bchristie@psych.ubc.ca
References
- 1.Rakic P. Limits of neurogenesis in primates. Science 1985;227:1054-6. [DOI] [PubMed]
- 2.Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci 1999;2:894-7. [DOI] [PubMed]
- 3.Altman J. Are new neurons formed in the brains of adult mammals? Science 1962;135:1127-8. [DOI] [PubMed]
- 4.Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science 1977;197:1092-4. [DOI] [PubMed]
- 5.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci 1996;16:2027-33. [DOI] [PMC free article] [PubMed]
- 6.Seki T, Arai Y. Age-related production of new granule cells in the adult dentate gyrus. Neuroreport 1995;6:2479-82. [DOI] [PubMed]
- 7.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature 1997; 386:493-5. [DOI] [PubMed]
- 8.Gould E, Reeves AJ, Fallah M, et al. Hippocampal neurogenesis in adult Old World primates. Proc Natl Acad Sci U S A 1999;96:5263-7. [DOI] [PMC free article] [PubMed]
- 9.Eriksson PS, Perfilieva E, Bjork-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med 1998;4:1313-7. [DOI] [PubMed]
- 10.Brezun JM, Daszuta A. Depletion in serotonin decreases neurogenesis in the dentate gyrus and the subventricular zone of adult rats. Neuroscience 1999;89:999-1002. [DOI] [PubMed]
- 11.Kodama M, Fujioka T, Duman RS. Chronic olanzapine or fluoxetine administration increases cell proliferation in hippocampus and prefrontal cortex of adult rat. Biol Psychiatry 2004;56:570-80. [DOI] [PubMed]
- 12.Duman RS, Nakagawa S, Malberg J. Regulation of adult neurogenesis by antidepressant treatment. Neuropsychopharmacology 2001; 25:836-44. [DOI] [PubMed]
- 13.Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry 1997;54:597-606. [DOI] [PubMed]
- 14.Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology 2003;28:1562-71. [DOI] [PubMed]
- 15.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 1999;2:266-70. [DOI] [PubMed]
- 16.van Praag H, Christie BR, Sejnowski TJ, et al. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A 1999;96:13427-31. [DOI] [PMC free article] [PubMed]
- 17.Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron 2004;41:683-6. [DOI] [PubMed]
- 18.Taupin P, Gage FH. Adult neurogenesis and neural stem cells of the central nervous system in mammals. J Neurosci Res 2002;69:745-9. [DOI] [PubMed]
- 19.Steindler DA, Pincus DW. Stem cells and neuropoiesis in the adult human brain. Lancet 2002;359:1047-54. [DOI] [PubMed]
- 20.Gould E, Gross CG. Neurogenesis in adult mammals: some progress and problems. J Neurosci 2002;22:619-23. [DOI] [PMC free article] [PubMed]
- 21.Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol 2001;435:406-17. [DOI] [PubMed]
- 22.Gould E, Vail N, Wagers M, et al. Adult-generated hippocampal and neocortical neurons in macaques have a transient existence. Proc Natl Acad Sci U S A 2001;98:10910-7. [DOI] [PMC free article] [PubMed]
- 23.Milner B, Penfield W. The effect of hippocampal lesions on recent memory. Trans Am Neurol Assoc 1955-6;80th meeting:42-8. [PubMed]
- 24.Penfield W, Milner B. Memory deficit produced by bilateral lesions in the hippocampal zone. AMA Arch Neurol Psychiatry 1958;79:475-97. [DOI] [PubMed]
- 25.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 1957;20:11-21. [DOI] [PMC free article] [PubMed]
- 26.Lisman JE. Relating hippocampal circuitry to function: recall of memory sequences by reciprocal dentate-CA3 interactions. Neuron 1999;22:233-42. [DOI] [PubMed]
- 27.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci 1997;17: 5046-61. [DOI] [PMC free article] [PubMed]
- 28.Garcia-Verdugo JM, Doetsch F, Wichterle H, et al. Architecture and cell types of the adult subventricular zone: In search of the stem cells. J Neurobiol 1998;36:234-48. [DOI] [PubMed]
- 29.Privat A, Leblond CP. The subependymal layer and neighboring region in the brain of the young rat. J Comp Neurol 1972;146:277-302. [DOI] [PubMed]
- 30.Overstreet LS, Hentges ST, Bumaschny VF, et al. A transgenic marker for newly born granule cells in dentate gyrus. J Neurosci 2004;24:3251-9. [DOI] [PMC free article] [PubMed]
- 31.van Praag H, Schinder AF, Christie BR, et al. Functional neurogenesis in the adult hippocampus. Nature 2002;415:1030-4. [DOI] [PMC free article] [PubMed]
- 32.Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science 1996;271:978-81. [DOI] [PubMed]
- 33.Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science 1994;264:1145-8. [DOI] [PubMed]
- 34.Huttmann K, Sadgrove M, Wallraff A, et al. Seizures preferentially stimulate proliferation of radial glia-like astrocytes in the adult dentate gyrus: functional and immunocytochemical analysis. Eur J Neurosci 2003;18:2769-78. [DOI] [PubMed]
- 35.Emsley JG, Hagg T. Endogenous and exogenous ciliary neurotrophic factor enhances forebrain neurogenesis in adult mice. Exp Neurol 2003;183:298-310. [DOI] [PubMed]
- 36.Doetsch F, Caille I, Lim DA, et al. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 1999; 97:703-16. [DOI] [PubMed]
- 37.Aubert A, Costalat R. Interaction between astrocytes and neurons studied using a mathematical model of compartmentalized energy metabolism. J Cereb Blood Flow Metab 2005;25(11):1476-90. [DOI] [PubMed]
- 38.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci 2004;5:347-60. [DOI] [PubMed]
- 39.Gould E, Reeves AJ, Graziano MS, et al. Neurogenesis in the neocortex of adult primates. Science 1999;286:548-52. [DOI] [PubMed]
- 40.Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature 2000;405:951-5. [DOI] [PubMed]
- 41.Dayer AG, Cleaver KM, Abouantoun T, et al. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J Cell Biol 2005;168:415-27. [DOI] [PMC free article] [PubMed]
- 42.Brown J, Cooper-Kuhn CM, Kempermann G, et al. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci 2003;17:2042-6. [DOI] [PubMed]
- 43.Nilsson M, Perfilieva E, Johansson U, et al. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J Neurobiol 1999;39:569-78. [DOI] [PubMed]
- 44.Kempermann G, Gage FH. Experience-dependent regulation of adult hippocampal neurogenesis: effects of long-term stimulation and stimulus withdrawal. Hippocampus 1999;9:321-32. [DOI] [PubMed]
- 45.Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci 1998;18:3206-12. [DOI] [PMC free article] [PubMed]
- 46.Gould E, Beylin A, Tanapat P, et al. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci 1999;2:260-5. [DOI] [PubMed]
- 47.Eadie BD, Redila VA, Christie BR. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J Comp Neurol 2005;486:39-47. [DOI] [PubMed]
- 48.Farmer J, Zhao X, van Praag H, et al. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience 2004;124:71-9. [DOI] [PubMed]
- 49.Fabel K, Fabel K, Tam B, et al. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci 2003;18: 2803-12. [DOI] [PubMed]
- 50.Rhodes JS, van Praag H, Jeffrey S, et al. Exercise increases hippocampal neurogenesis to high levels but does not improve spatial learning in mice bred for increased voluntary wheel running. Behav Neurosci 2003;117:1006-16. [DOI] [PubMed]
- 51.Suominen K, Henriksson M, Suokas J, et al. Mental disorders and comorbidity in attempted suicide. Acta Psychiatr Scand 1996;94:234-40. [DOI] [PubMed]
- 52.Beautrais AL, Joyce PR, Mulder RT, et al. Prevalence and comorbidity of mental disorders in persons making serious suicide attempts: a case-control study. Am J Psychiatry 1996;153:1009-14. [DOI] [PubMed]
- 53.Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry 1994;51:8-19. [DOI] [PubMed]
- 54.Klerman GL, Weissman MM. Increasing rates of depression. JAMA 1989;261:2229-35. [PubMed]
- 55.Bland RC, Newman SC, Orn H. Period prevalence of psychiatric disorders in Edmonton. Acta Psychiatr Scand Suppl 1988;338:33-42. [DOI] [PubMed]
- 56.Murphy JM, Laird NM, Monson RR, et al. A 40-year perspective on the prevalence of depression: the Stirling County Study. Arch Gen Psychiatry 2000;57:209-15. [DOI] [PubMed]
- 57.Drevets WC, Videen TO, Price JL, et al. A functional anatomical study of unipolar depression. J Neurosci 1992;12:3628-41. [DOI] [PMC free article] [PubMed]
- 58.Drevets WC, Raichle ME. Neuroanatomical circuits in depression: implications for treatment mechanisms. Psychopharmacol Bull 1992; 28:261-74. [PubMed]
- 59.Baxter LR Jr, Schwartz JM, Phelps ME, et al. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiatry 1989;46:243-50. [DOI] [PubMed]
- 60.Biver F, Goldman S, Delvenne V, et al. Frontal and parietal metabolic disturbances in unipolar depression. Biol Psychiatry 1994;36:381-8. [DOI] [PubMed]
- 61.Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci 2004;29:417-26. [PMC free article] [PubMed]
- 62.Sheline YI, Wang PW, Gado MH, et al. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A 1996;93:3908-13. [DOI] [PMC free article] [PubMed]
- 63.Sheline YI, Sanghavi M, Mintun MA, et al. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci 1999;19:5034-43. [DOI] [PMC free article] [PubMed]
- 64.Steffens DC, Byrum CE, McQuoid DR, et al. Hippocampal volume in geriatric depression. Biol Psychiatry 2000;48:301-9. [DOI] [PubMed]
- 65.Mervaala E, Fohr J, Kononen M, et al. Quantitative MRI of the hippocampus and amygdala in severe depression. Psychol Med 2000; 30:117-25. [DOI] [PubMed]
- 66.Bremner JD, Narayan M, Anderson ER, et al. Hippocampal volume reduction in major depression. Am J Psychiatry 2000;157:115-8. [DOI] [PubMed]
- 67.Campbell S, Marriott M, Nahmias C, et al. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry 2004;161:598-607. [DOI] [PubMed]
- 68.Czeh B, Michaelis T, Watanabe T, et al. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci U S A 2001;98:12796-801. [DOI] [PMC free article] [PubMed]
- 69.Stockmeier CA, Mahajan GJ, Konick LC, et al. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry 2004;56:640-50. [DOI] [PMC free article] [PubMed]
- 70.Gould E, Tanapat P. Stress and hippocampal neurogenesis. Biol Psychiatry 1999;46:1472-9. [DOI] [PubMed]
- 71.Karten YJ, Olariu A, Cameron HA. Stress in early life inhibits neurogenesis in adulthood. Trends Neurosci 2005;28:171-2. [DOI] [PubMed]
- 72.Pham K, Nacher J, Hof PR, et al. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci 2003;17:879-86. [DOI] [PubMed]
- 73.Rosenbrock H, Koros E, Bloching A, et al. Effect of chronic intermittent restraint stress on hippocampal expression of marker proteins for synaptic plasticity and progenitor cell proliferation in rats. Brain Res 2005;1040:55-63. [DOI] [PubMed]
- 74.Tanapat P, Galea LA, Gould E. Stress inhibits the proliferation of granule cell precursors in the developing dentate gyrus. Int J Dev Neurosci 1998;16:235-9. [DOI] [PubMed]
- 75.Westenbroek C, Den Boer JA, Veenhuis M, et al. Chronic stress and social housing differentially affect neurogenesis in male and female rats. Brain Res Bull 2004;64:303-8. [DOI] [PubMed]
- 76.Brown ES, Rush AJ, McEwen BS. Hippocampal remodeling and damage by corticosteroids: implications for mood disorders. Neuropsychopharmacology 1999;21:474-84. [DOI] [PubMed]
- 77.McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci 1999;22:105-22. [DOI] [PubMed]
- 78.Sapolsky RM, Uno H, Rebert CS, et al. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci 1990;10:2897-902. [DOI] [PMC free article] [PubMed]
- 79.Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry 2000;57:925-35. [DOI] [PubMed]
- 80.McEwen BS. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann N Y Acad Sci 2004;1032:1-7. [DOI] [PubMed]
- 81.Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci 2002;3:453-62. [DOI] [PubMed]
- 82.De Kloet ER, Vreugdenhil E, Oitzl MS, et al. Brain corticosteroid receptor balance in health and disease. Endocr Rev 1998;19:269-301. [DOI] [PubMed]
- 83.Coyle JT, Duman RS. Finding the intracellular signaling pathways affected by mood disorder treatments. Neuron 2003;38:157-60. [DOI] [PubMed]
- 84.Santarelli L, Saxe M, Gross C, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003;301:805-9. [DOI] [PubMed]
- 85.Cotter D, Mackay D, Chana G, et al. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex 2002;12:386-94. [DOI] [PubMed]
- 86.Brezun JM, Daszuta A. Serotonin may stimulate granule cell proliferation in the adult hippocampus, as observed in rats grafted with foetal raphe neurons. Eur J Neurosci 2000;12:391-6. [DOI] [PubMed]
- 87.Malberg JE, Eisch AJ, Nestler EJ, et al. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 2000;20:9104-10. [DOI] [PMC free article] [PubMed]
- 88.Duman RS. Depression: A case of neuronal life and death? Biol Psychiatry 2004;56:140-5. [DOI] [PubMed]
- 89.Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci 2004;27:589-94. [DOI] [PubMed]
- 90.Henn FA, Vollmayr B. Neurogenesis and depression: Etiology or epiphenomenon? Biol Psychiatry 2004;56:146-50. [DOI] [PubMed]
- 91.Sapolsky RM. Is impaired neurogenesis relevant to the affective symptoms of depression? Biol Psychiatry 2004;56:137-9. [DOI] [PubMed]
- 92.Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. BMJ 2001;322:763-7. [DOI] [PMC free article] [PubMed]
- 93.Kritz-Silverstein D, Barrett-Connor E, Corbeau C. Cross-sectional and prospective study of exercise and depressed mood in the elderly: the Rancho Bernardo study. Am J Epidemiol 2001;153:596-603. [DOI] [PubMed]
- 94.Motl RW, Birnbaum AS, Kubik MY, et al. Naturally occurring changes in physical activity are inversely related to depressive symptoms during early adolescence. Psychosom Med 2004;66:336-42. [DOI] [PubMed]
- 95.Strawbridge WJ, Deleger S, Roberts RE, et al. Physical activity reduces the risk of subsequent depression for older adults. Am J Epidemiol 2002;156:328-34. [DOI] [PubMed]
- 96.Leppamaki S, Haukka J, Lonnqvist J, et al. Drop-out and mood improvement: a randomised controlled trial with light exposure and physical exercise [ISRCTN36478292]. BMC Psychiatry 2004;4:22. [DOI] [PMC free article] [PubMed]
- 97.Penninx BW, Rejeski WJ, Pandya J, et al. Exercise and depressive symptoms: a comparison of aerobic and resistance exercise effects on emotional and physical function in older persons with high and low depressive symptomatology. J Gerontol B Psychol Sci Soc Sci 2002;57:P124-32. [DOI] [PubMed]
- 98.Dimeo FC, Thomas F, Raabe-Menssen C, et al. Effect of aerobic exercise and relaxation training on fatigue and physical performance of cancer patients after surgery. A randomised controlled trial. Support Care Cancer 2004;12(11):774-9. [DOI] [PubMed]
- 99.Dimeo F, Bauer M, Varahram I, et al. Benefits from aerobic exercise in patients with major depression: a pilot study. Br J Sports Med 2001;35:114-7. [DOI] [PMC free article] [PubMed]
- 100.Mather AS, Rodriguez C, Guthrie MF, et al. Effects of exercise on depressive symptoms in older adults with poorly responsive depressive disorder: randomised controlled trial. Br J Psychiatry 2002;180:411-5. [DOI] [PubMed]
- 101.Dunn AL, Trivedi MH, Kampert JB, et al. Exercise treatment for depression: efficacy and dose response. Am J Prev Med 2005;28:1-8. [DOI] [PubMed]
- 102.Singh NA, Clements KM, Singh MA. The efficacy of exercise as a long-term antidepressant in elderly subjects: a randomized, controlled trial. J Gerontol A Biol Sci Med Sci 2001;56:M497-504. [DOI] [PubMed]
- 103.Babyak M, Blumenthal JA, Herman S, et al. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med 2000;62:633-8. [DOI] [PubMed]
- 104.Kubesch S, Bretschneider V, Freudenmann R, et al. Aerobic endurance exercise improves executive functions in depressed patients. J Clin Psychiatry 2003;64:1005-12. [DOI] [PubMed]
- 105.Colt EW, Wardlaw SL, Frantz AG. The effect of running on plasma beta-endorphin. Life Sci 1981;28:1637-40. [DOI] [PubMed]
- 106.Appenzeller O. What makes us run? N Engl J Med 1981;305:578-80. [DOI] [PubMed]
- 107.Gambert SR, Garthwaite TL, Pontzer CH, et al. Running elevates plasma beta-endorphin immunoreactivity and ACTH in untrained human subjects. Proc Soc Exp Biol Med 1981;168:1-4. [DOI] [PubMed]
- 108.Farrell PA, Gates WK, Maksud MG, et al. Increases in plasma beta-endorphin/beta-lipotropin immunoreactivity after treadmill running in humans. J Appl Physiol 1982;52:1245-9. [DOI] [PubMed]
- 109.Hadley ME, Haskell-Luevano C. The proopiomelanocortin system. Ann N Y Acad Sci 1999;885:1-21. [DOI] [PubMed]
- 110.Inder WJ, Livesey JH, Donald RA. Peripheral plasma levels of beta-endorphin in alcoholics and highly trained athletes and the relationship to a measure of central opioid tone. Horm Metab Res 1998;30:523-5. [DOI] [PubMed]
- 111.Persson AI, Thorlin T, Bull C, et al. Mu-and delta-opioid receptor antagonists decrease proliferation and increase neurogenesis in cultures of rat adult hippocampal progenitors. Eur J Neurosci 2003;17:1159-72. [DOI] [PubMed]
- 112.Gallagher M. Behavioral significance of opioid peptides in relation to hippocampal function. NIDA Res Monogr 1988;82:118-32. [PubMed]
- 113.Persson AI, Thorlin T, Bull C, et al. Opioid-induced proliferation through the MAPK pathway in cultures of adult hippocampal progenitors. Mol Cell Neurosci 2003;23:360-72. [DOI] [PubMed]
- 114.Holmes DI, Zachary I. The vascular endothelial growth factor (VEGF) family: angiogenic factors in health and disease. Genome Biol 2005;6:209. [DOI] [PMC free article] [PubMed]
- 115.Senger DR, Galli SJ, Dvorak AM, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 1983;219:983-5. [DOI] [PubMed]
- 116.Leung DW, Cachianes G, Kuang WJ, et al. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989;246: 1306-9. [DOI] [PubMed]
- 117.Vitt UA, Hsu SY, Hsueh AJ. Evolution and classification of cystine knot-containing hormones and related extracellular signaling molecules. Mol Endocrinol 2001;15:681-94. [DOI] [PubMed]
- 118.Schobersberger W, Hobisch-Hagen P, Fries D, et al. Increase in immune activation, vascular endothelial growth factor and erythropoietin after an ultramarathon run at moderate altitude. Immunobiology 2000;201:611-20. [DOI] [PubMed]
- 119.Jin K, Zhu Y, Sun Y, et al. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A 2002;99:11946-50. [DOI] [PMC free article] [PubMed]
- 120.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol 2000;425:479-94. [DOI] [PubMed]
- 121.Barde YA. Trophic factors and neuronal survival. Neuron 1989; 2:1525-34. [DOI] [PubMed]
- 122.Thoenen H. The changing scene of neurotrophic factors. Trends Neurosci 1991;14:165-70. [DOI] [PubMed]
- 123.Horch HW. Local effects of BDNF on dendritic growth. Rev Neurosci 2004;15:117-29. [DOI] [PubMed]
- 124.Vanhoutte P, Bading H. Opposing roles of synaptic and extrasynaptic NMDA receptors in neuronal calcium signalling and BDNF gene regulation. Curr Opin Neurobiol 2003;13:366-71. [DOI] [PubMed]
- 125.Wozniak W. Brain-derived neurotrophic factor (BDNF): role in neuronal development and survival. Folia Morphol (Warsz) 1993;52:173-81. [PubMed]
- 126.Neeper SA, Gomez-Pinilla F, Choi J, et al. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res 1996;726:49-56. [PubMed]
- 127.Widenfalk J, Olson L, Thoren P. Deprived of habitual running, rats downregulate BDNF and TrkB messages in the brain. Neurosci Res 1999;34:125-32. [DOI] [PubMed]
- 128.Russo-Neustadt A, Beard RC, Cotman CW. Exercise, antidepressant medications, and enhanced brain derived neurotrophic factor expression. Neuropsychopharmacology 1999;21:679-82. [DOI] [PubMed]
- 129.Berchtold NC, Kesslak JP, Pike CJ, et al. Estrogen and exercise interact to regulate brain-derived neurotrophic factor mRNA and protein expression in the hippocampus. Eur J Neurosci 2001;14: 1992-2002. [DOI] [PubMed]
- 130.Berchtold NC, Kesslak JP, Cotman CW. Hippocampal brain-derived neurotrophic factor gene regulation by exercise and the medial septum. J Neurosci Res 2002;68:511-21. [DOI] [PubMed]
- 131.Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci 2002;25:295-301. [DOI] [PubMed]
- 132.Gomez-Pinilla F, Ying Z, Roy RR, et al. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol 2002;88:2187-95. [DOI] [PubMed]
- 133.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci 2004;20:2580-90. [DOI] [PubMed]
- 134.Widegren U, Wretman C, Lionikas A, et al. Influence of exercise intensity on ERK/MAP kinase signalling in human skeletal muscle. Pflugers Arch 2000;441:317-22. [DOI] [PubMed]
- 135.Bjornebekk A, Mathe AA, Brene S. The antidepressant effect of running is associated with increased hippocampal cell proliferation. Int J Neuropsychopharmacol 2005;8:357-68. [DOI] [PubMed]
- 136.Scharfman H, Goodman J, Macleod A, et al. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol 2005;192:348-56. [DOI] [PubMed]
- 137.Sairanen M, Lucas G, Ernfors P, et al. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci 2005;25:1089-94. [DOI] [PMC free article] [PubMed]
- 138.Lowenstein DH, Arsenault L. The effects of growth factors on the survival and differentiation of cultured dentate gyrus neurons. J Neurosci 1996;16:1759-69. [DOI] [PMC free article] [PubMed]
- 139.Banasr M, Hery M, Printemps R, et al. Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology 2004;29:450-60. [DOI] [PubMed]
- 140.Lim BV, Jang MH, Shin MC, et al. Caffeine inhibits exercise-induced increase in tryptophan hydroxylase expression in dorsal and median raphe of Sprague-Dawley rats. Neurosci Lett 2001; 308:25-8. [DOI] [PubMed]
- 141.Davis JM, Bailey SP. Possible mechanisms of central nervous system fatigue during exercise. Med Sci Sports Exerc 1997;29:45-57. [DOI] [PubMed]
- 142.Min YK, Chung SH, Lee JS, et al. Red ginseng inhibits exercise-induced increase in 5-hydroxytryptamine synthesis and tryptophan hydroxylase expression in dorsal raphe of rats. J Pharmacol Sci 2003;93:218-21. [DOI] [PubMed]
- 143.Vertes RP, Crane AM. Distribution, quantification, and morphological characteristics of serotonin-immunoreactive cells of the supralemniscal nucleus (B9) and pontomesencephalic reticular formation in the rat. J Comp Neurol 1997;378:411-24. [DOI] [PubMed]
- 144.Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev 1992;72:165-229. [DOI] [PubMed]
- 145.Kohler C, Chan-Palay V, Steinbusch H. The distribution and origin of serotonin-containing fibers in the septal area: a combined immunohistochemical and fluorescent retrograde tracing study in the rat. J Comp Neurol 1982;209:91-111. [DOI] [PubMed]
- 146.Pasquier DA, Reinoso-Suarez F. The topographic organization of hypothalamic and brain stem projections to the hippocampus. Brain Res Bull 1978;3:373-89. [DOI] [PubMed]
- 147.Viana Di Prisco G, Albo Z, Vertes RP, et al. Discharge properties of neurons of the median raphe nucleus during hippocampal theta rhythm in the rat. Exp Brain Res 2002;145:383-94. [DOI] [PubMed]
- 148.Nitz DA McNaughton BL. Hippocampal EEG and unit activity responses to modulation of serotonergic median raphe neurons in the freely behaving rat. Learn Mem 1999;6:153-67. [PMC free article] [PubMed]
- 149.Chaouloff F, Laude D, Elghozi JL. Physical exercise: evidence for differential consequences of tryptophan on 5-HT synthesis and metabolism in central serotonergic cell bodies and terminals. J Neural Transm 1989;78:121-30. [DOI] [PubMed]
- 150.Dey S, Singh RH, Dey PK. Exercise training: significance of regional alterations in serotonin metabolism of rat brain in relation to antidepressant effect of exercise. Physiol Behav 1992;52:1095-9. [DOI] [PubMed]
- 151.Meeusen R, Thorre K, Chaouloff F, et al. Effects of tryptophan and/or acute running on extracellular 5-HT and 5-HIAA levels in the hippocampus of food-deprived rats. Brain Res 1996;740:245-52. [DOI] [PubMed]
- 152.Wilson WM, Marsden CA. In vivo measurement of extracellular serotonin in the ventral hippocampus during treadmill running. Behav Pharmacol 1996;7:101-4. [PubMed]
- 153.Banasr M, Hery M, Printemps R, et al. Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology 2004;29:450-60. [DOI] [PubMed]


