Abstract
Introduction
To review systematically the effect of interventions aimed at hemodynamic optimization and to relate this to the quality of individual published trials.
Methods
A systematic, computerized bibliographic search of published studies and citation reviews of relevant studies was performed. All randomized clinical trials in which adult patients were included in a trial deliberately aiming at an optimized or maximized hemodynamic condition of the patients (with oxygen delivery, cardiac index, oxygen consumption, mixed venous oxygen saturation and/or stroke volume as end-points) were selected. A total of 30 studies were selected for independent review. Two reviewers extracted data on population, intervention, outcome and methodological quality. Agreement between reviewers was high: differences were eventually resolved by third-party decision. The methodological quality of the studies was moderate (mean 9.0, SD 1.7), and the outcomes of the randomized clinical trials were not related to their quality.
Results
Efforts to achieve an optimized hemodynamic condition resulted in a decreased mortality rate (relative risk ratio (RR) 0.75 (95% confidence interval (CI) 0.62 to 0.90) in all studies combined. This was due to a significantly decreased mortality in peri-operative intervention studies (RR 0.66 (95% CI 0.54 to 0.81). Overall, patients with sepsis and overt organ failure do not benefit from this method (RR 0.92 (95% CI 0.75 to 1.11)).
Conclusion
This systematic review showed that interventions aimed at hemodynamic optimization reduced mortality. In particular, trials including peri-operative interventions aimed at the hemodynamic optimization of high-risk surgical patients reduce mortality. Overall, this effect was not related to the trial quality.
Introduction
It has been shown that, in critically ill patients, impaired cardiovascular function has a role in the development of organ failure. Our understanding of the underlying mechanism responsible for this dysfunction has changed over the past 10 years. Previously, correction of disturbed hemodynamics to normal values in the peri-operative phase was considered standard care in the treatment of surgical patients. However, clinical signs of hypovolemia are non-specific and non-sensitive [1]. Moreover, because the mean values of commonly used parameters, such as central venous pressure and pulmonary artery occlusion pressure, are similar between survivors and non-survivors, the value of correcting these parameters to normal values is questionable [2]. The same is true for critically ill patients treated for sepsis at an intensive care unit [1].
A report by Shoemaker and colleagues [3] changed the prevailing views on the hemodynamic treatment of the critically ill patient. In this report the authors observed that 'normal' values are 'abnormal' in post-operative, trauma and critically ill patients. In comparison with non-surviving patients, surviving trauma patients had above-normal oxygen delivery and oxygen consumption values. These 'supra-normal' values may reflect an ability of these patients to respond adequately to the 'stress' of the trauma.
There have been a considerable number of randomized, controlled, clinical studies investigating the role of improving patients' hemodynamic condition by increasing oxygen delivery to the tissues to supranormal levels or by other goals. Heyland and colleagues published a review in 1996 evaluating studies that included patients for whom supranormal oxygen delivery was the goal of treatment [4]. This review, including a total of 1,291 patients, found no difference in outcome but identified a relation between outcome and trial quality [4]. In two recent meta-analyses, Kern and Shoemaker [5] and Boyd and Hayes [6] found a significant reduction in mortality, but they did not report data on quality analysis.
We therefore decided to perform a systematic review of the effects of interventions aimed at hemodynamic optimization and to examine their relation to the quality of the individual published trials. We hypothesized that a reduced trial quality would be related to a greater reported survival difference.
Materials and methods
Study identification
Three methods were used to retrieve information for this review [7,8]. First, MEDLINE and EMBASE databases for the years 1980 to 2005 were searched, with the following mesh headings: 'oxygen consumption' or 'hemodynamics' or 'dobutamine' or 'fluid therapy', exploding with 'randomized controlled trials' (publication type) and 'intensive care', 'critical care' or 'intensive care unit' or 'surgery' or 'peri-operative care'. The second method used was to search personal files and communications to find additional citations and to search Current Contents for recently published studies. Third, the reference lists of the articles found with the above-mentioned methods were searched for additional articles.
Study selection
The articles found using this search method were classified into original articles, reviews and others (such as letters). Studies were selected if they involved a randomized controlled trial with fluid and/or additional vasoactive therapy to optimize or maximize the hemodynamic condition of the patients (end-points: oxygen delivery, cardiac index, oxygen consumption, mixed venous oxygen saturation and/or stroke volume). Moreover, the studies included had to have been performed either among an adult intensive care unit population or an adult surgical population. Studies with zero mortality in both treatment arms were not excluded from the meta-analysis.
Methodological quality assessment
A methodological scoring system (Table 1) was used to give a relative assessment of the quality of the primarily selected studies [9]. The scoring system was based on the system proposed and validated by Chalmers [9] and previously used by Heyland and colleagues [4]. The scores for the individual studies were compared between two independent observers, and in the event of disagreement a third (non-involved) person decided on the score assigned to the study. Because not all studies aimed at the reduction of mortality as a primary end-point, a scoring distinction was made between studies aiming primarily at reducing mortality (two points) and those having a reduced mortality as a secondary end-point (one point). The presence of crossover is defined as a patient achieving the hemodynamic goals of the opposite group from that to which he or she had been allocated (that is, a patient in the control group achieving the oxygen delivery goal defined for the treatment group, without additional treatment).
Table 1.
Quality control criteria for methodology of the studies
| Score | |||
| Criterion | 0 | 1 | 2 |
| Method | |||
| Randomization | Not randomized | Randomized | |
| Blinding | Not blinded | Double-blind | |
| Analysis | Other | Intention-to-treat | |
| End-point mortality | No mortality as end point | Secondary end-point | Primary end-point |
| Population | |||
| Patient selection | Selected patients or unclear | Consecutive eligible patients | |
| Comparability at baseline | No or unclear | Yes | |
| Extent of follow-up | Incomplete | Complete | |
| Intervention | |||
| Treatment protocol | Unclear | Reproducible | |
| Co-interventions | Not described | Described, but not equal or unclear | Well described and equal |
| Crossover | Not described | >10% | <10% |
Statistical analysis
Data are shown as percentages or absolute numbers ± SD. A statistical meta-analysis was performed with Review Manager 4.2. The primary outcome was the overall mortality rate reported at 28 to 30 days. The relative risk ratios for the individual studies and the overall relative risk ratios with 95% confidence intervals (CIs) were calculated by means of the method developed by Mantel and Haenszel. To assess the heterogeneity between studies, we used the method developed by DerSimonian and Laird [10]. If no significant heterogeneity was found, a fixed-effects model was used to calculate pooled relative risk and 95% CIs.
Several subset analyses were performed. One subset analysis compared the results for 'peri-operative' and 'sepsis' patients included in the various studies. The two patient groups (peri-operative patients and patients with sepsis and organ failure) were separated by using the inclusion criteria from the original studies, based on pathophysiological differences [11]. This subset therefore differentiates between the effects of optimization techniques in peri-operative patients and in patients with organ failure or sepsis and organ failure. The hypothesis tested in this subset analysis was that hemodynamic optimization to values above normal improves the outcome in peri-operative patients (including post-traumatic patients), but has no effect in patients with sepsis and organ failure.
A second subset analysis included the studies using the original 'supranormal' hemodynamic optimization criteria proposed by Shoemaker and colleagues (that is, cardiac index > 4.5 l min-1 m-2, oxygen delivery > 600 ml min-1 m-2 or oxygen consumption (VO2) > 170 ml min-1 m-2) [3,12-28]. The other studies used a variety of therapeutic goals, including mixed venous oxygen saturation (SvO2) [22,29-31], left-ventricle stroke work index [32], stroke volume [33,34], or cardiac index values lower than 4.5 l min-1 m-2 [35-40]. For the purpose of this subset analysis, the study by Gattinoni and colleagues [22] was divided into two datasets. One included the patients for whom cardiac index was the goal of treatment. This dataset was included in the subset of studies using the original criteria proposed by Shoemaker and colleagues [3]. The patients for whom SvO2 was the goal of treatment were included in the other study subset.
In addition, subset analyses were conducted to investigate the effects of the methodological quality criteria. One subset analysis compared studies having a quality score above 10, indicating adequate trial quality, with those having a quality score below 10. This cutoff value for the methodological quality was determined from the peak incidence of quality scores. Finally, the individual quality items of using the presence of mortality as an end-point, blinding and crossover were tested separately in a subset analysis.
Results
Study inclusion and allocation
After initial screening and a subsequent more detailed evaluation of retrieved randomized trial reports, 32 candidate trials were identified. A total of 30 studies were included in the analysis. Two studies were omitted from the analysis after careful review of the methodology: the study by Garrison and colleagues [41] was a case-control study, and the study by Blow and colleagues [42] used no randomization. Of the 30 remaining trials, 21 involved surgery or trauma patients who were hemodynamically optimized peri-operatively, and 9 involved patients with sepsis and/or organ failure.
Study results
The total number of patients included in the studies was 5,733. The median number of patients who were randomized was 75 (range 30 to 1,994; Tables 2 and 3). The mean score on the methodological quality assessment in the included studies was 9.1 (95% CI 7 to 12.7), which is 57% of the maximum score of 16. The duration of follow-up, up to 28 or 30 days, was specified in all trials. Other characteristics of the trials are shown in Tables 2 and 3.
Table 2.
Attributes of included trials with peri-operative patients
| Study | Population | Intervention | Blinding | Allocation concealment | Co-interventions | Crossover | Mortality end-point | Score | Goals of treatment |
| Schultz et al. 1985 [32] | Hip fractured patients | Fluids and inotropes peri-operatively | No | Adequate | Not described | Unclear | Yes | 8 | LVSW/PCWP optimized according to normogram |
| Shoemaker et al. 1988 [3] | High-risk surgical patients | Fluids and inotropes begun pre-operatively | No | Inadequate | Not described | Unclear | Yes | 5 | CI > 4.5, DO2 > 600, VO2 > 170 |
| Berlauk et al. 1991 [35] | Peripheral vascular surgical patients | Fluids, afterload reduction and inotropes | No | Adequate | Not described | Unclear | Yes | 9 | CI > 2.8, 8 < PCWP < 15, SVR 1,100 |
| Fleming et al. 1992 [24] | Trauma patients | Fluids, blood and dobutamine | No | Inadequate | Not described | >10% | Yes | 7 | CI > 4.5, DO2 > 670, VO2 > 166 |
| Boyd et al. 1993 [25] | High-risk surgical patients | Fluids and dopexamine | No | Adequate | Described, but not equal | Unclear | Yes | 10 | DO2 > 600 |
| Bishop et al. 1995 [26] | Cardiac surgical patients | Fluids and dobutamine | No | Adequate | Not described | >10% | Yes | 10 | CI > 4.5, DO2 > 670, VO2 > 166, PCWP 18 |
| Mythen and Webb 1995 [33] | Cardiac surgical patients | Fluids | No | Adequate | Not described | Unclear | Yes | 8 | SV optimized |
| Bender et al. 1997 [36] | Elective vascular surgical patients | Fluids, blood, vasodilators, nitroprusside and dopamine | No | Adequate | Not described | Unclear | Yes | 8 | 8 PCWP 14, CI 2.8, SVR 1,100 |
| Ziegler et al. 1997 [29] | Elective vascular surgical patients | Fluids, blood, inotropes and vasodilators | No | Adequate | Not described | Unclear | Yes | 9 | SvO2 > 65, PCWP > 12, Hb > 10 |
| Sinclair et al. 1997 [34] | Hip fractured patients | Fluids | No | Adequate | Not described | >10% | Yes | 8 | SV optimized to 0.35 < FTc < 0.40 |
| Valentine et al. 1998 [37] | Elective aortic surgical patients | Fluids, nitroprusside, nitroglycerine and dopamine | No | Adequate | Not described | Unclear | Yes | 10 | CI > 2.8, 8 PCWP 15, SVR 1,100 |
| Ueno et al. 1998 [12] | Elective hepatic surgical patients | Fluids and dobutamine | No | Adequate | Not described | Unclear | No | 7 | CI > 4.5, DO2 > 600, VO2 > 170 |
| Boldt et al. 1998 [38] | Pancreatic surgical patients | Dopexamine | Yes | Adequate | Not described | Unclear | No | 8 | MAP 70, CI > 2.5, 12 < PCWP < 14 |
| Wilson et al. 1999 [13] | High-risk surgical patients | Dopexamine or noradrenaline | Yes | Adequate | Described, but not equal | Unclear | Yes | 12 | DO2 > 600 |
| Lobo et al 2000 [23] | High-risk surgical patients | Fluids and dobutamine | No | Adequate | Described, but not equal | >10% | Yes | 11 | DO2 > 600 |
| Velhamos et al. 2000 [14] | Trauma surgical patients | Fluids, blood, inotropes and vasopressors | No | Adequate | Not described | >10% | Yes | 11 | CI > 4.5, DO2 > 600, VO2 > 170, SpO2/FiO2 > 200 |
| Polonen et al. 2000 [31] | Cardiac surgical patients | Fluids, blood and inotropes | No | Adequate | Not described | >10% | Yes, but secondary | 7 | SvO2 > 70, lactate levels < 2.0 |
| Takala et al. 2000 [15] | High-risk surgical patients | Fluids, blood and dopexamine | Yes | Adequate | Not described | >10% | Yes | 13 | DO2 > 600 |
| Bonazzi et al. 2002 [28] | Elective vascular surgical patients | Fluids, inotropes, vasodilators | No | Adequate | Adequate | Unclear | No | 10 | CI > 3.0, 10 < PCWP < 18, SVR < 1,450, DO2 > 600 |
| Conway et al. 2002 [39] | Elective gastro-intestinal surgical patients | Fluids | No | Inadequate | Not described | Unclear | Yes | 8 | CO optimized |
| Sandham et al. 2003 [40] | High-risk surgical patients | Fluids, blood, inotropes, vasodilators, vasopressors | No | Adequate | Not described | <10% | Yes | 11 | 550 < DO2 < 600, 3.5 < CI < 4.5 |
CI, cardiac index (l min-1 m-2); DO2, oxygen delivery (ml min-1 m-2); FTc, corrected flow time; Hb, haemoglobin; LVSW, left ventricular stroke work; MAP, mean arterial pressure (mmHg); PCWP, pulmonary capillary wedge pressure; SpO2/FiO2, ratio of oxygen saturation as measured by pulse-oximetry and inspiration oxygen fraction; SV, stroke volume (ml); SvO2, mixed venous oxygen saturation (%); SVR, systemic vascular resistance (dyn s-1 cm-5); VO2, oxygen consumption (ml min-1 m-2).
Table 3.
Attributes of included trials involving patients with sepsis and organ failure
| Study | Population | Intervention | Blinding | Allocation concealment | Co-interventions | Crossover | Mortality end-point | Score | Goals of treatment |
| Tuchschmidt et al. 1992 [16] | Septic shock patients | Fluids, inotropes | No | Inadequate | Not described | >10% | Yes | 9 | CI > 6, SAP > 90 |
| Yu et al. 1993 [17] | Sepsis, septic shock, ARDS patients | Fluids, blood, inotropes | No | Inadequate | Not described | >10% | Yes | 8 | DO2 > 600 |
| Hayes et al. 1994 [20] | Post-operative patients, sepsis, respiratory failure | Fluids, dobutamine | No | Adequate | Not described | Unclear | Yes | 10 | CI > 4.5, DO2 > 600, VO2 > 170 |
| Gattinoni et al. 1995 [22] | High-risk postoperative patients, sepsis, respiratory failure | Fluids and inotropes | No | Adequate | Described, but not adequate | <10% | Yes | 12 | CI > 4.5 or SvO2 > 70% |
| Yu et al. 1995 [18] | Sepsis, septic shock, ARDS or hypovolemic shock patients | Fluids, inotropes and vasopressors | No | Inadequate | Not described | >10% | Yes | 8 | DO2 > 600 |
| Yu et al. 1998 [19] | SIRS, sepsis, severe sepsis, septic shock, ARDS patients 50–75 years of age | Fluids, afterload reduction, inotropes, amrinone, vasopressors | No | Adequate | Not described | Unclear | Yes | 8 | DO2 > 600 |
| Yu et al. 1998 [19] | SIRS, sepsis, severe sepsis, septic shock, ARDS patients >75 years of age | Fluids, afterload reduction, inotropes, amrinone, vasopressors | No | Adequate | Not described | Unclear | Yes | 8 | DO2 > 600 |
| Durham et al. 1996 [27] | Critically ill patients | Fluids, inotropes and nitroprusside | No | Adequate | Not described | Unclear | Yes | 9 | DO2 > 600, VO2 > 150 |
| Alia et al. 1999 [21] | Septic shock patients or severe sepsis patients | Dobutamine | No | Adequate | Not described | >10% | Yes | 10 | DO2 > 600 |
| Rivers et al. 2001 [30] | Severe sepsis and septic shock | Fluids, blood, inotropes and vasopressors | No | Adequate | Not described | >10% | Yes | 11 | SvO2 > 70% |
ARDS, acute respiratory distress syndrome; CI, cardiac index; DO2, oxygen delivery; SAP, systolic arterial pressure; SIRS, systemic inflammatory response syndrome; SvO2, mixed venous oxygen saturation; VO2, oxygen consumption.
The odds ratio for all studies combined was 0.61 (95% CI 0.46 to 0.81) with a relative risk of 0.75 (95% CI 0.62 to 0.90; Figure 1). However, the absolute risk reduction was only 0.4% (95% CI -1.7 to 2.6%). Moreover, of the 30 studies included, only 8 showed a significantly greater survival in the optimized patients, whereas one study showed a significantly greater mortality in the optimized patient group, and the other studies did not show a significant difference in survival. For quality control, we correlated the score of the quality assessment with the odds ratio for the individual studies. This correlation was not significant (r = 0.33; p = 0.07).
Figure 1.
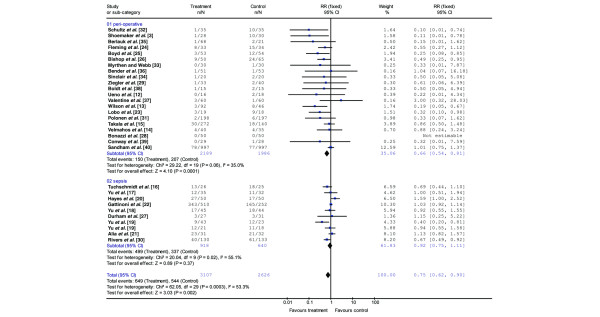
Relative risk determined in individual trials in studies (including subset analysis with patients treated peri-operatively and patients with sepsis and/or organ failure) shown as boxes scaled according to weighting, using the inverse variance method. Error bars indicate 95% confidence intervals (95% CI). A fixed-effects model (peri-operative studies) was used when heterogeneity analysis was not significant, and a random-effects model (sepsis and total included studies) was used when heterogeneity analysis was significant. The pooled relative risk estimates are shown as diamonds that span the 95% CI. n, number of deceased patients in the treatment or control arm; N, total number of patients in treatment or control arm; RR, relative risk ratio.
Subset analysis
Peri-operative and trauma studies versus studies using septic/organ failure patients
There were 4,174 patients enrolled in the studies that used strategies to optimize the hemodynamic condition peri-operatively and during trauma (Table 2). The overall odds ratio for mortality with hemodynamic optimization in this group was 0.43 (95% CI 0.28 to 0.66) with a relative risk ratio of 0.66 (95% CI 0.54 to 0.81; Figure 1). Of the 21 studies, 6 showed a significantly reduced mortality in the treatment group. When using an optimization protocol, 31 patients (95% CI 20 to 63) had to be treated to save one life. The number of patients that must be included in a single study to be able to find this difference is 500, assuming a mortality rate of 15% in the control group.
The overall odds ratio for the 1,558 enrolled patients with septic shock/organ failure was 0.85 (95% CI 0.58 to 1.25) with a relative risk ratio of 0.92 (95% CI 0.75 to 1.11; Figure 1 and Table 3). Of the 10 included studies, 3 found either a tendency towards increased mortality or a significantly increased mortality in the treated patients. Two studies found an improved survival.
The mean quality score for the peri-operative studies did not differ from the mean score for the studies of septic/organ failure patients (9.0 ± 1.9 versus 9.0 ± 1.3; p = 0.9). Neither the peri-operative studies nor the studies including patients with sepsis had a significant correlation between the score and the odds ratio (r = 0.28, p = 0.3, and r = 0.28, p = 0.4, respectively).
Supranormal oxygen delivery as a goal of treatment
Our analysis for all studies combined, but only including those patients optimized by using the criteria proposed by Shoemaker (total number of included patients; n = 2,181), yielded an odds ratio of 0.60 (95% CI 0.42 to 0.88), with a relative risk ratio of 0.75 (95% CI 0.60 to 0.95). This significant effect was not found in the patient group for whom supranormal oxygen delivery was not used as the end-point (relative risk ratio 0.81 (95% CI 0.62 to 1.07); Table 4).
Table 4.
Subset analyses of pooled relative risk of death
| Trial category | Odds ratio (95% CI) | Relative risk (95% CI) | p for heterogeneity |
| Peri-operative trials | |||
| Cardiac index, DO2 or VO2 | 0.41 (0.29–0.59) | 0.49 (0.36–0.65) | 0.1 |
| Other goals | 0.83 (0.62–1.11) | 0.84 (0.64–1.10) | 0.3 |
| Sepsis/organ failure trials | |||
| Cardiac index, DO2 or VO2 | 1.00 (0.77–1.30) | 1.00 (0.90–1.11) | 0.09 |
| Other goals | 0.77 (0.38–1.57) | 0.85 (0.55–1.31) | 0.01 |
| All trials | |||
| Cardiac index, DO2 or VO2 | 0.60 (0.42–0.88) | 0.75 (0.60–0.95) | 0.0003 |
| Other goals | 0.83 (0.68–1.03) | 0.90 (0.80–1.01) | 0.09 |
| Score | |||
| ≥10 | 0.84 (0.66–1.07) | 0.74 (0.51–1.08) | 0.0005 |
| <10 | 0.45 (0.32–0.64) | 0.60 (0.48–0.75) | 0.6 |
| End-point | |||
| Mortality as primary end-point | 0.63 (0.46–0.85) | 0.76 (0.63–0.93) | <0.0001 |
| Secondary or no mortality end-point | 0.34 (0.13–0.93) | 0.36 (0.14–0.94) | 1.0 |
| Blinding | |||
| Yes | 0.61 (0.36–1.04) | 0.64 (0.40–1.03) | 0.1 |
| No | 0.62 (0.46–0.84) | 0.77 (0.63–0.93) | 0.0008 |
| Crossover | |||
| Yes | 0.43 (0.25–0.77) | 0.52 (0.32–0.83) | 0.003 |
| No | 0.85 (0.71–1.01) | 0.86 (0.73–1.02) | 0.05 |
Risk analyses comparing subset including the use of hemodynamic goals with supranormal values (cardiac index, DO2, or VO2) or with other goals both in all trials included, in peri-operative trials, and in studies including patients with sepsis and established organ failure. Risk analysis was also calculated in the subgroup of studies with a quality assessment score of 10 or more, comparing them with the studies with a quality assessment score of less than 10. Individual quality assessment items were also analysed for risk reduction, including the use of mortality as primary end-point in the studies, the use of adequate blinding, and the presence of crossover phenomena. A fixed-effects model for calculating the odds ratio and relative risk ratio was used when heterogeneity analysis (last column) was not significant. 95% CI, 95% confidence interval; DO2, oxygen delivery; VO2, oxygen consumption.
The subgroup analysis of the peri-operative studies that included individual studies using the original criteria proposed by Shoemaker (with 1,142 patients) found a relative risk ratio of 0.41 (0.29 to 0.59; Table 4). In these studies, 10 patients (95% CI 7 to 16) needed to be treated to save one life. The quality control score of this subgroup was 9.1 (SD 2.5). Studies using treatment goals other than supranormal oxygen delivery in peri-operative patients found no effect on mortality; the relative risk ratio was 0.84 (0.64 to 1.10).
In the studies including patients with sepsis and organ failure, neither the use of supranormal oxygen delivery nor other specified treatment goals yielded a reduction in mortality; relative risk ratios were 1.00 (95% CI 0.90 to 1.11) and 0.93 (95% CI 0.83 to 1.05), respectively (Table 4).
Quality assessment score
Studies with a high quality assessment (a score of 10 or more) tended to report a higher relative risk ratio, although the difference was not significant (mean 0.84; 95% CI 0.66 to 1.07) than studies with a lower quality assessment score (mean 0.60; 95% CI 0.48 to 0.75; Table 4). In the subset of studies including peri-operative and trauma patients, the overall outcome was not related to the trial quality. The studies with a quality score of 10 or more found a relative risk ratio of 0.60 (95% CI 0.38 to 0.95), compared with a relative risk ratio of 0.27 (95% CI 0.13 to 0.55) in the studies with a quality score of less than 10. Other cutoff points were also tested but produced similar results (data not shown).
Mortality end-point
Relative risk ratios were calculated for 29 of the 30 included studies. In the combined studies that had mortality as the primary end-point, the effect on mortality tended to be lower than that in the remaining studies, although the difference was not significant (Table 4).
Blinding
Only three studies (10%) randomized patients with adequate blinding. The effect on mortality was not significantly different in studies with inadequate blinding from that found in the studies without blinding (Table 4).
Crossover
In the studies in which crossover between the treatment arms was adequately controlled for, no significant effect on mortality was found in comparison with the studies with significant crossover (Table 4).
Discussion
This meta-analysis, for which we conducted a systematic search, selection and quality assessment of the literature, suggests that optimization techniques can improve survival when used in peri-operative and trauma patients without sepsis or multiple organ failure. Overall, patients with sepsis and overt organ failure do not benefit from this method.
The use of hemodynamic optimization as a therapy to improve outcome is controversial. The regimen was originally designed to optimize the hemodynamic status in high-risk surgical patients. The initial studies found an improved outcome, although doubt remained about the methodological quality of these studies. A large number of studies, using different patient populations and optimization techniques, were subsequently conducted. A considerable number of these studies found no improved outcome [14,16,22]. Moreover, one study found an increased mortality rate in the optimized patient group [20]. The meta-analysis by Heyland and colleagues [4], reporting the first seven studies published at that time, found no overall benefit from maximizing oxygen delivery with the aim of improving outcome. This meta-analysis also criticized the quality of the individual studies. A subsequent meta-analysis by Kern and Shoemaker found a significantly lower mortality in patient groups optimized at an early stage (namely surgical patients optimized peri-operatively), but no formal quality analysis was presented [5]. Our meta-analysis represents the most up-to-date evaluation of the issue of hemodynamic optimization in which a quality assessment was performed and related to the outcome of the studies. It suggests that hemodynamic optimization strategies are beneficial in all patient subgroups but that the overall effect is explained by the significant improvement in mortality in those studies including peri-operative and trauma patients.
There are several critical issues to be addressed before valid conclusions can be drawn from the present meta-analysis. The overall trial quality has been called into question previously [4] and we found in our meta-analysis that studies with a high trial quality score (using the cutoff point of 10 out of 16) did not report an improved mortality rate. Fortunately, the trial quality seemed to influence the outcome in the studies including peri-operative patients less than the outcome in the subset of patients with established sepsis and multiple organ failure. In addition, the largest effect on mortality was found in the studies including peri-operative patients.
Another critical point may be the cutoff point that we chose to divide the individual studies between those with a high quality score and those with a low score. However, other cutoff points that we tested produced similar data (data not shown). Moreover, we also tested the effect of individual trial quality features on the outcome of the studies included. Thus, although the overall trial quality is moderate it may be concluded that the impact of this on the outcome of the meta-analysis is not significant.
One may question our subset analysis, which divided the studies into a subset with studies involving peri-operative and trauma patients and one with studies using septic/organ failure patients. It has been suggested, both in reviews [43,44] and in a previous meta-analysis [6], that the outcome of studies with late interventions should be separated from those of studies with early interventions. A similar distinction was made in the meta-analysis by Heyland and colleagues [4]. In addition, in the meta-analysis by Kern and Shoemaker [5] risk differences (-0.23 ± 0.07) in the subgroup using goals to supranormal values in patients before organ failure were found comparable to the data reported in our study (risk difference -0.12; 95% CI -0.20 to -0.03). Recent publications have indeed reported a pathophysiological basis for this distinction. In an early stage of the disease process of the systemic inflammatory response syndrome, it is possible to prevent or overcome peripheral defects in oxygen delivery, on the basis of decreased flow, hypoxia or hypovolemia. In contrast, persistent defects in oxygen delivery to the tissues during decreased flow or hypovolemia may alter vascular and cellular metabolism. These defects in cellular oxygenation become irreversible as a result of mitochondrial damage, and when they occur in the endothelium they lead to vascular hyporeactivity or 'vasoplegia', resulting in impaired perfusion and organ failure. Moreover, organ function is less likely to recover at this stage because of the relative insensitivity of patients with multiple organ failure to the optimization techniques [11,45,46]. The study by Rivers and colleagues [30], in which early optimization of the hemodynamics led to a reduced mortality even in patients with early septic shock, underlines this point.
The studies in our meta-analysis included different patient groups with varying co-morbidities and expected mortality rates. The differentiation between the patients included in early and late intervention studies partly compensates for this effect. Some studies had a lower statistical power than expected, because of the low mortality rate in control patients. In the study by Takala and colleagues [15], no survival benefit was found in the overall study group, which had low baseline mortality, but a survival benefit was detected in a subgroup with higher baseline mortality (namely emergency surgery).
Conclusion
There is sufficient evidence that aiming for optimized oxygen transport values in patients with high-risk surgery or trauma is beneficial and that the trial quality, although overall only moderate, is not important in these patients. The promising results obtained by Rivers and colleagues [30] and the aggressive early optimization of sepsis deserves further investigation and confirmation. However, patients with established organ failure due to sepsis do not benefit from attempts to optimize oxygen transport values.
Key messages
• Peri-operative interventions aimed at the hemodynamic optimization of high-risk surgical patients reduce mortality.
• The use of hemodynamic optimization as a therapy to improve outcome during sepsis with established organ failure does not reduce mortality.
• Although there is no clear relationship between overall trial quality and outcome of all trials, individual quality assessment items influenced study outcome.
Abbreviations
CI = confidence interval; RR = relative risk ratio; SvO2 = mixed venous oxygen saturation; VO2 = oxygen consumption.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
MP carried out the study gathering, scored the individual trials, participated in its design and coordination and wrote the manuscript. JG participated in its design and coordination and helped to draft the manuscript. GR carried out the study gathering, scored the individual trials, participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Contributor Information
Martijn Poeze, Email: m.poeze@ah.unimaas.nl.
Jan Willem M Greve, Email: j.greve@surgery.azm.nl.
Graham Ramsay, Email: g.ramsay@atriummc.nl.
References
- Wo CC, Shoemaker WC, Appel PL, Bishop MH, Kram HB, Hardin E. Unreliability of blood pressure and heart rate to evaluate cardiac output in emergency resuscitation and critical illness. Crit Care Med. 1993;21:218–223. doi: 10.1097/00003246-199302000-00012. [DOI] [PubMed] [Google Scholar]
- Bishop MH, Shoemaker WC, Appel PL, Wo CJ, Zwick C, Kram HB, Meade P, Kennedy F, Fleming AW. Relationship between supranormal circulatory values, time delays, and outcome in severely traumatized patients. Crit Care Med. 1993;21:56–63. doi: 10.1097/00003246-199301000-00013. [DOI] [PubMed] [Google Scholar]
- Shoemaker WC, Appel PL, Kram HB, Waxman K, Lee TS. Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest. 1988;94:1176–1186. doi: 10.1378/chest.94.6.1176. [DOI] [PubMed] [Google Scholar]
- Heyland DK, Cook DJ, King D, Kernerman P, Brun-Buisson C. Maximizing oxygen delivery in critically ill patients: a methodological appraisal of the evidence. Crit Care Med. 1996;24:517–524. doi: 10.1097/00003246-199603000-00025. [DOI] [PubMed] [Google Scholar]
- Kern JW, Shoemaker WC. Meta-analysis of hemodynamic optimization in high-risk patients. Crit Care Med. 2002;30:1686–1692. doi: 10.1097/00003246-200208000-00002. [DOI] [PubMed] [Google Scholar]
- Boyd O, Hayes M. The oxygen trail: the goal. Br Med Bull. 1999;55:125–139. doi: 10.1258/0007142991902330. [DOI] [PubMed] [Google Scholar]
- de Bie RA. Methodology of systematic reviews: an introduction. Phys Ther Rev. 1996;1:47–51. [Google Scholar]
- Boissel JP. Standards for reporting clinical trials. Clin Trials Metaanal. 1992;28:5–7. [PubMed] [Google Scholar]
- Chalmers TC, Smith H, Jnr, Blackburn B, Silverman B, Schroeder B, Reitman D, Ambroz A. A method for assessing the quality of a randomized control trial. Control Clin Trials. 1981;2:31–49. doi: 10.1016/0197-2456(81)90056-8. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Sibbald WJ, Fox G, Martin C. Abnormalities of vascular reactivity in the sepsis syndrome. Chest. 1991;100:155S–159S. doi: 10.1378/chest.100.3_supplement.155s. [DOI] [PubMed] [Google Scholar]
- Ueno S, Tanabe G, Yamada H, Kusano C, Yoshidome S, Nuruki K, Yamamoto S, Aikou T. Response of patients with cirrhosis who have undergone partial hepatectomy to treatment aimed at achieving supranormal oxygen delivery and consumption. Surgery. 1998;123:278–286. doi: 10.1067/msy.1998.85942. [DOI] [PubMed] [Google Scholar]
- Wilson J, Woods I, Fawcett J, Whall R, Dibb W, Morris C, McManus E. Reducing the risk of major elective surgery: randomised controlled trial of preoperative optimisation of oxygen delivery. BMJ. 1999;318:1099–1103. doi: 10.1136/bmj.318.7191.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velmahos GC, Demetriades D, Shoemaker WC, Chan LS, Tatevossian R, Wo CC, Vassiliu P, Cornwell EE, 3rd, Roth B, Belzberg H, et al. Endpoints of resuscitation of critically injured patients: normal or supranormal? A prospective randomized trial. Ann Surg. 2000;232:409–418. doi: 10.1097/00000658-200009000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takala J, Meier Hellmann A, Eddleston J, Hulstaert P, Sramek V, for the European Multicenter Study Group on Dopexamine in Major Abdominal Surgery Effect of dopexamine on outcome after major abdominal surgery: a prospective, randomized, controlled multicenter study. Crit Care Med. 2000;28:3417–3423. doi: 10.1097/00003246-200010000-00007. [DOI] [PubMed] [Google Scholar]
- Tuchschmidt J, Fried J, Astiz M, Rackow E. Elevation of cardiac output and oxygen delivery improves outcome in septic shock. Chest. 1992;102:216–220. doi: 10.1378/chest.102.1.216. [DOI] [PubMed] [Google Scholar]
- Yu M, Levy MM, Smith P, Takiguchi SA, Miyasaki A, Myers SA. Effect of maximizing oxygen delivery on morbidity and mortality rates in critically ill patients: a prospective, randomized, controlled study. Crit Care Med. 1993;21:830–838. doi: 10.1097/00003246-199306000-00009. [DOI] [PubMed] [Google Scholar]
- Yu M, Takanishi D, Myers SA, Takiguchi SA, Severino R, Hasaniya N, Levy MM, McNamara JJ. Frequency of mortality and myocardial infarction during maximizing oxygen delivery: a prospective, randomized trial. Crit Care Med. 1995;23:1025–1032. doi: 10.1097/00003246-199506000-00006. [DOI] [PubMed] [Google Scholar]
- Yu M, Burchell S, Hasaniya NW, Takanishi DM, Myers SA, Takiguchi SA. Relationship of mortality to increasing oxygen delivery in patients > or = 50 years of age: a prospective, randomized trial. Crit Care Med. 1998;26:1011–1019. doi: 10.1097/00003246-199806000-00018. [DOI] [PubMed] [Google Scholar]
- Hayes MA, Timmins AC, Yau EH, Palazzo M, Hinds CJ, Watson D. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med. 1994;330:1717–1722. doi: 10.1056/NEJM199406163302404. [DOI] [PubMed] [Google Scholar]
- Alia I, Esteban A, Gordo F, Lorente JA, Diaz C, Rodriguez JA, Frutos F. A randomized and controlled trial of the effect of treatment aimed at maximizing oxygen delivery in patients with severe sepsis or septic shock. Chest. 1999;115:453–461. doi: 10.1378/chest.115.2.453. [DOI] [PubMed] [Google Scholar]
- Gattinoni L, Brazzi L, Pelosi P, Latini R, Tognoni G, Pesenti A, Fumagalli R, for the SvO2 Collaborative Group A trial of goal-oriented hemodynamic therapy in critically ill patients. N Engl J Med. 1995;333:1025–1032. doi: 10.1056/NEJM199510193331601. [DOI] [PubMed] [Google Scholar]
- Lobo SM, Salgado PF, Castillo VG, Borim AA, Polachini CA, Palchetti JC, Brienzi SL, de Oliveira GG. Effects of maximizing oxygen delivery on morbidity and mortality in high-risk surgical patients. Crit Care Med. 2000;28:3396–3404. doi: 10.1097/00003246-200010000-00003. [DOI] [PubMed] [Google Scholar]
- Fleming A, Bishop M, Shoemaker W, Appel P, Sufficool W, Kuvhenguwha A, Kennedy F, Wo CJ. Prospective trial of supranormal values as goals of resuscitation in severe trauma. Arch Surg. 1992;127:1175–1181. doi: 10.1001/archsurg.1992.01420100033006. [DOI] [PubMed] [Google Scholar]
- Boyd O, Grounds RM, Bennett ED. A randomized clinical trial of the effect of deliberate perioperative increase of oxygen delivery on mortality in high-risk surgical patients. JAMA. 1993;270:2699–2707. doi: 10.1001/jama.270.22.2699. [DOI] [PubMed] [Google Scholar]
- Bishop MH, Shoemaker WC, Appel PL, Meade P, Ordog GJ, Wasserberger J, Wo CJ, Rimle DA, Kram HB, Umali R, et al. Prospective, randomized trial of survivor values of cardiac index, oxygen delivery, and oxygen consumption as resuscitation endpoints in severe trauma. J Trauma. 1995;38:780–787. doi: 10.1097/00005373-199505000-00018. [DOI] [PubMed] [Google Scholar]
- Durham RM, Neunaber K, Mazuski JE, Shapiro MJ, Baue AE. The use of oxygen consumption and delivery as endpoints for resuscitation in critically ill patients. J Trauma. 1996;41:32–39. doi: 10.1097/00005373-199607000-00007. [DOI] [PubMed] [Google Scholar]
- Bonazzi M, Gentile F, Biasi GM, Migliavacca S, Esposti D, Cipolla M, Marsicano M, Prampolini F, Ornaghi M, Sternjakob S, Tshomba Y. Impact of perioperative haemodynamic monitoring on cardiac morbidity after major vascular surgery in low risk patients. A randomised pilot trial. Eur J Vasc Endovasc Surg. 2002;23:445–451. doi: 10.1053/ejvs.2002.1617. [DOI] [PubMed] [Google Scholar]
- Ziegler DW, Wright JG, Choban PS, Flancbaum L. A prospective randomized trial of preoperative 'optimization' of cardiac function in patients undergoing elective peripheral vascular surgery. Surgery. 1997;122:584–592. doi: 10.1016/S0039-6060(97)90132-X. [DOI] [PubMed] [Google Scholar]
- Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M, Early Goal-Directed Therapy Collaborative Group Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- Polonen P, Ruokonen E, Hippelainen M, Poyhonen M, Takala J. A prospective, randomized study of goal-oriented hemodynamic therapy in cardiac surgical patients. Anesth Analg. 2000;90:1052–1059. doi: 10.1097/00000539-200005000-00010. [DOI] [PubMed] [Google Scholar]
- Schultz RJ, Whitfield GF, LaMura JJ, Raciti A, Krishnamurthy S. The role of physiologic monitoring in patients with fractures of the hip. J Trauma. 1985;25:309–316. doi: 10.1097/00005373-198504000-00005. [DOI] [PubMed] [Google Scholar]
- Mythen MG, Webb AR. Perioperative plasma volume expansion reduces the incidence of gut mucosal hypoperfusion during cardiac surgery. Arch Surg. 1995;130:423–429. doi: 10.1001/archsurg.1995.01430040085019. [DOI] [PubMed] [Google Scholar]
- Sinclair S, James S, Singer M. Intraoperative intravascular volume optimisation and length of hospital stay after repair of proximal femoral fracture: randomised controlled trial. BMJ. 1997;315:909–912. doi: 10.1136/bmj.315.7113.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlauk JF, Abrams JH, Gilmour IJ, O'Connor SR, Knighton DR, Cerra FB. Preoperative optimization of cardiovascular hemodynamics improves outcome in peripheral vascular surgery. A prospective, randomized clinical trial. Ann Surg. 1991;214:289–297. doi: 10.1097/00000658-199109000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender JS, Smith-Meek MA, Jones CE. Routine pulmonary artery catheterization does not reduce morbidity and mortality of elective vascular surgery: results of a prospective, randomized trial. Ann Surg. 1997;226:229–236. doi: 10.1097/00000658-199709000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine RJ, Duke ML, Inman MH, Grayburn PA, Hagino RT, Kakish HB, Clagett GP. Effectiveness of pulmonary artery catheters in aortic surgery: a randomized trial. J Vasc Surg. 1998;27:203–211. doi: 10.1016/S0741-5214(98)70351-9. [DOI] [PubMed] [Google Scholar]
- Boldt J, Papsdorf M, Piper S, Padberg W, Hempelmann G. Influence of dopexamine hydrochloride on hemodynamics and regulators of circulation in patients undergoing major abdominal surgery. Acta Anaesthesiol Scand. 1998;42:941–947. doi: 10.1111/j.1399-6576.1998.tb05354.x. [DOI] [PubMed] [Google Scholar]
- Conway DH, Mayall R, Abdul-Latif MS, Gilligan S, Tackaberry C. Randomised controlled trial investigating the influence of intravenous fluid titration using oesophageal Doppler monitoring during bowel surgery. Anaesthesia. 2002;57:845–849. doi: 10.1046/j.1365-2044.2002.02708.x. [DOI] [PubMed] [Google Scholar]
- Sandham JD, Hull RD, Brant RF, Knox L, Pineo GF, Doig CJ, Laporta DP, Viner S, Passerini L, Devitt H, Canadian Critical Care Clinical Trials Group et al. A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med. 2003;348:5–14. doi: 10.1056/NEJMoa021108. [DOI] [PubMed] [Google Scholar]
- Garrison RN, Wilson MA, Matheson PJ, Spain DA. Preoperative saline loading improves outcome after elective, noncardiac surgical procedures. Am Surg. 1996;62:223–231. [PubMed] [Google Scholar]
- Blow O, Magliore L, Claridge JA, Butler K, Young JS. The golden hour and the silver day: detection and correction of occult hypoperfusion within 24 hours improves outcome from major trauma. J Trauma. 1999;47:964–969. doi: 10.1097/00005373-199911000-00028. [DOI] [PubMed] [Google Scholar]
- Poeze M, Greve JW, Ramsay G. Goal-oriented hemodynamic therapy: a plea for a closer look at using peri-operative oxygen transport optimisation. Intensive Care Med. 2000;26:635–637. doi: 10.1007/s001340051216. [DOI] [PubMed] [Google Scholar]
- Poeze M, Greve JW, Ramsay G. Oxygen delivery in septic shock. Chest. 1999;116:1145. doi: 10.1378/chest.116.4.1145. [DOI] [PubMed] [Google Scholar]
- Hollenberg SM, Cunnion RE. Endothelial and vascular smooth muscle function in sepsis. J Crit Care. 1994;9:262–280. doi: 10.1016/0883-9441(94)90006-X. [DOI] [PubMed] [Google Scholar]
- Abid O, Akca S, Haji-Michael P, Vincent JL. Strong vasopressor support may be futile in the intensive care unit patient with multiple organ failure. Crit Care Med. 2000;28:947–949. doi: 10.1097/00003246-200004000-00006. [DOI] [PubMed] [Google Scholar]


