Abstract
Introduction
Monitoring of the cardiac output by continuous arterial pulse contour (COPiCCOpulse) analysis is a clinically validated procedure proved to be an alternative to the pulmonary artery catheter thermodilution cardiac output (COPACtherm) in cardiac surgical patients. There is ongoing debate, however, of whether the COPiCCOpulse is accurate after profound hemodynamic changes. The aim of this study was therefore to compare the COPiCCOpulse after cardiopulmonary bypass (CPB) with a simultaneous measurement of the COPACtherm.
Methods
After ethical approval and written informed consent, data of 45 patients were analyzed during this prospective study. During coronary artery bypass graft surgery, the aortic transpulmonary thermodilution cardiac output (COPiCCOtherm) and the COPACtherm were determined in all patients. Prior to surgery, the COPiCCOpulse was calibrated by triple transpulmonary thermodilution measurement of the COPiCCOtherm. After termination of CPB, the COPiCCOpulse was documented. Both COPACtherm and COPiCCOtherm were also simultaneously determined and documented.
Results
Regression analysis between COPACtherm and COPiCCOtherm prior to CPB showed a correlation coefficient of 0.95 (P < 0.001), and after CPB showed a correlation coefficient of 0.82 (P < 0.001). Bland-Altman analysis showed a mean bias and limits of agreement of 0.0 l/minute and -1.4 to +1.4 l/minute prior to CPB and of 0.3 l/minute and -1.9 to +2.5 l/minute after CPB, respectively. Regression analysis of COPiCCOpulse versus COPiCCOtherm and of COPiCCOpulse versus COPACtherm after CPB showed a correlation coefficient of 0.67 (P < 0.001) and 0.63 (P < 0.001), respectively. Bland-Altman analysis showed a mean bias and limits of agreement of -1.1 l/minute and -1.9 to +4.1 l/minute versus -1.4 l/minute and -4.8 to +2.0 l/minute, respectively.
Conclusion
We observed an excellent correlation of COPiCCOtherm and COPACtherm measurement prior to CPB. Pulse contour analysis did not yield reliable results with acceptable accuracy and limits of agreement under difficult conditions after weaning from CPB in cardiac surgical patients. The pulse contour analysis thus should be re-calibrated as soon as possible, to prevent false therapeutic consequences.
Introduction
Measurement of cardiac output (CO) is widely used in cardiac surgical patients. Over recent decades the main device for determination of CO has been the pulmonary artery catheter (PAC). The use of the PAC has been decreasing over recent years in surgical and cardiac surgical patients, however, as the benefit of guiding therapy with this device is unclear and the use of the PAC might even lead to increased morbidity, as shown in one large trial [1]. Other randomized studies indicate no clear evidence of benefit or harm by managing critically ill patients with a PAC [2,3].
Aortic transpulmonary thermodilution, a less invasive technique for determination of the CO, was therefore developed and has gained increasing acceptance in clinical practice [4-6]. Only an arterial line and a central venous line are needed to determine the CO by this method [7]. Several investigators found a good correlation between these two methods of CO determination [4-6,8]. The device mostly used also offers continuous CO determination by arterial pulse contour analysis. Stroke volume calculation and CO calculation by pulse contour analysis was developed years ago and underwent several methodological improvements of the algorithm [9,10]. Monitoring of the CO by continuous arterial pulse contour analysis (COPiCCOpulse) is a widely used and clinically validated procedure proved to be an alternative to the pulmonary artery catheter thermodilution CO (COPACtherm) in cardiac surgical patients [4,11]. Pulse contour monitoring demonstrated accuracy comparable with that of pulmonary artery thermodilution using a clearly less invasive approach [5,11,12]. There is ongoing debate, however, of whether the COPiCCOpulse is accurate and reliable after profound changes of the hemodynamic situation, such as after cardiopulmonary bypass (CPB) [4,13].
The aim of this study was therefore to compare the bias and the limits of agreement (two standard deviations) of the COPiCCOpulse after CPB, with a simultaneous measurement of the COPACtherm, as the gold standard of CO measurement.
Materials and methods
Patients
Following ethical committee approval and written informed consent, 50 patients were considered eligible for this clinical trial from February to November 2004. The inclusion criteria were age >18 and <75 years, and elective coronary artery bypass graft surgery. The exclusion criteria were withdrawal of consent, valve pathologies, a left ventricular ejection fraction <40% and symptomatic peripheral artery stenosis.
Perioperative management
Oral premedication was 0.1 mg/kg midazolam. In all patients a femoral artery was cannulated with a 4-Fr cannula (Pulsiocath; Pulsion Medical AG, Munich, Germany) prior to induction of anesthesia. A central venous catheter and a pulmonary artery catheter (Thermodilution Catheter; Arrow, Reading, PA, USA) were inserted via the right internal jugular vein.
General anesthesia was induced with etomidate (0.2 mg/kg), 5 μg/kg fentanyl and 0.1 mg/kg pancuronium. Maintenance was with infusion of 5–10 μg/kg per hour fentanyl, boluses of 0.1 mg/kg midazolam, 0.03 mg/kg pancuronium and 0.6–1% end-tidal isofluorane. All patients were ventilated with an oxygen–air mixture (inspiratory oxygen fraction, 0.5) to maintain an end-tidal partial pressure of carbon dioxide of 35–45 mmHg. The CPB technique was normothermic using intermittent antegrade warm blood cardioplegia as described by Calafiore and colleagues [14]. Transfusion management was performed according to our standard operating procedure [15]. The durations of anesthesia, surgery and aortic occlusion and the number of coronary artery bypass grafts were recorded.
Determination of cardiac output
Prior to CPB, the COPiCCOtherm and the COPACtherm were determined immediately after sternotomy under stable hemodynamic conditions.
All volume substitution was stopped during the measurements. The COPACtherm and the COPiCCOtherm were measured by triple injection of 10 ml iced isotone sodium chloride solution into the central venous line of the PAC. The COPACtherm and the COPiCCOtherm were calculated by commercially available monitors (CCO module, Solar 8000; Marquette Hellige, Freiburg, Germany; and PiCCO CCO monitor; Pulsion Medical AG). In case of a deviation >10% of a measurement, five measurements were performed and the highest and lowest were rejected. The COPiCCOpulse measurement was automatically calibrated by the COPiCCOtherm measurement. The COPACtherm and the COPiCCOtherm measurements were carried out simultaneously.
The measurement after CPB was carried out 15 minutes after decanulation of the aorta. The prerequisite for this measurement was an optimized preload and stable hemodynamic condition with no damping of the arterial pressure line, which could be achieved in all patients. At this time the COPiCCOpulse was documented. Simultaneously, the COPiCCOtherm and COPACtherm were determined by thermodilution measurement as already described.
Statistical analysis
All data are expressed as the mean and standard error of the mean. Statistical analysis was performed by linear regression analysis. The bias and limits of agreement (LOA) (two standard deviations) were assessed according to the method described by Bland and Altman [16]. All numerical calculations were carried out with SPSS for WINDOWS (release 11.5.1, ©1989–2002; SPSS Inc, Chicago, IL, USA).
Results
Anesthesia and surgery were uncomplicated in all patients analyzed during this study. Five patients had to be excluded due to their impossibility to achieve a valid COPACtherm or COPiCCOtherm measurement. Therefore, 45 patients remained in the study for analysis. Basic patient characteristics are presented in Table 1. Hemodynamic data are presented in Table 2. The heart rate, COPACtherm and COPiCCOtherm increased significantly compared with the pre-CPB values. The systemic vascular resistance decreased significantly compared with the baseline measurement.
Table 1.
Patient characteristics
| Mean | Standard error of the mean | |
| Age (years) | 62 | 1 |
| Height (m) | 1.77 | 0.01 |
| Body weight (kg) | 91 | 2 |
| Body mass index (kg/m2) | 29.1 | 0.6 |
| Number of grafts (n) | 3 | 0 |
| Duration of anesthesia (minutes) | 314 | 7 |
| Duration of surgery (minutes) | 201 | 6 |
| Temperature prior to cardiopulmonary bypass (°C) | 35.2 | 0.1 |
| Temperature after cardiopulmonary bypass (°C) | 36.1 | 0.1 |
| Cardiopulmonary bypass time (minutes) | 71 | 3 |
| Aortic clamping time (minutes) | 44 | 2 |
Table 2.
Hemodynamic data
| Mean | Standard error of the mean | |
| Heart rate prior to CPB (l/minute) | 69 | 3 |
| Heart rate after CPB (l/minute) | 81* | 2 |
| Mean arterial pressure prior to CPB (mmHg) | 70 | 2 |
| Mean arterial pressure after CPB (mmHg) | 73 | 2 |
| Central venous pressure prior to CPB (mmHg) | 9 | 1 |
| Central venous pressure after CPB (mmHg) | 11 | 1 |
| Mean pulmonary arterial pressure prior to CPB (mmHg) | 21 | 1 |
| Mean pulmonary arterial pressure after CPB (mmHg) | 20 | 1 |
| Pulmonary wedge pressure prior to CPB (mmHg) | 11 | 1 |
| Pulmonary wedge pressure after CPB (mmHg) | 12 | 1 |
| Systemic vascular resistance prior to CPB (dyn/s per cm) | 861 | 53 |
| Systemic vascular resistance after CPB (dyn/s per cm) | 727* | 47 |
| Pulmonary vascular resistance prior to CPB (dyn/s per cm) | 115 | 10 |
| Pulmonary vascular resistance after CPB (dyn/s per cm) | 93 | 8 |
| COPACtherm prior to CPB (l/minute) | 6.2 | 0.4 |
| COPACtherm after CPB (l/minute) | 7.9* | 0.3 |
| COPiCCOtherm prior to CPB (l/minute) | 6.2 | 0.3 |
| COPiCCOtherm after CPB (l/minute) | 7.6* | 0.3 |
| COPiCCOpulse after CPB (l/minute) | 6.5 | 0.3 |
CPB, cardiopulmonary bypass. COPACtherm, pulmonary artery catheter thermodilution cardiac output; COPiCCOtherm, aortic transpulmonary thermodilution cardiac output; COPiCCOpulse, continuous arterial pulse contour analysis cardiac output.
*P < 0.05 compared with baseline.
Prior to CPB, the regression analysis between the COPACtherm and COPiCCOtherm measurements showed an excellent correlation, with a correlation coefficient of 0.95 (P < 0.001). Bland–Altman analysis showed a mean bias and LOA of 0.0 l/minute and -1.4 to +1.4 l/minute. The regression analysis after CPB also showed a good correlation between the COPACtherm and the COPiCCOtherm, with a correlation coefficient of 0.82 (P < 0.001). The Bland–Altman analysis after CPB showed a mean bias and a precision of 0.3 l/minute and -1.9 to +2.5 l/minute.
Comparison of COPiCCOpulse versus COPiCCOtherm and of COPiCCOpulse versus COPACtherm showed only a fair correlation after CPB, with a correlation coefficient of 0.67 (P < 0.001) and 0.63 (P < 0.001), respectively. Bland–Altman analysis showed a mean bias and LOA of -1.1 l/minute and -1.9 to +4.1 l/minute versus -1.4 l/minute and -4.8 to +2.0 l/minute, respectively.
Discussion
The main finding of this study is that the CO measured by pulse contour analysis was considerably different compared with the COPiCCOtherm and the COPACtherm. The COPiCCOtherm and COPACtherm measurements correlated well before and after CPB, indicating that CO measurement by pulse contour analysis needs to be recalibrated after CPB to achieve valid results.
Pulse contour analysis CO has been shown previously to serve as a valid and cost-effective device for CO determination after calibration [17]. In our study we investigated the validity of continuous CO measurement by pulse contour analysis after CPB. The main advantage of COPiCCOpulse measurement after CPB would be the fast determination of CO. As soon as pulsatile flow is restored, the algorithm of the CO monitor automatically starts determination of the CO by continuous pulse contour analysis. Therefore, during a period when the anesthetist's full attention is focused on vasoactive and volume therapy necessary for successful weaning from CPB, a fast and continuous approach such as continuous pulse contour analysis might be much more practical than time-consuming intermittent thermodilution techniques for determination of CO. However, these advantages would only apply if the obtained data are valid.
The initial calibration of the COPiCCOpulse measurement was performed by aortic transpulmonary CO determination prior to CPB. We found an excellent correlation between the COPiCCOtherm and the COPACtherm measurements. This correlation has been described by previous investigators [12]. After CPB the correlation remained good, but Bland-Altman analysis revealed a trend for the COPiCCOtherm to slightly underestimate the CO, with increased LOA compared with the measurements prior to CPB. As we do not know the 'true' CO, it is speculative which CO measurement estimates more precisely the 'true' CO. An explanation for the greater scatter between the two CO measurements after CPB compared with the measurements prior to CPB might be an influx of cold blood. This cold blood might be derived from compartments, which might be hypoperfused during CPB and reperfused in the period after CPB as suggested by previous investigators [4,18]. Even though we performed normothermic CPB management, patients tended to display a slight decrease of their body temperature, worsening the signal-to-noise ratio of the thermal indicator used for determination of the CO by these methods. Better results in this setting might be achieved using an indicator independent from thermal signals. Given the increased LOA of the COPiCCOtherm measurement, therefore, the calibration of the pulse contour analysis with a thermal indicator might be less than ideal in this period and should be repeated early after surgery.
After CPB, the pulse contour CO showed marked differences compared with the COPiCCOtherm and COPACtherm measurements. The COPiCCOpulse measurement systematically underestimated the CO determined by the other two methods. This has been described previously [4]. In our investigation the CO and the heart rate increased significantly after CPB. We also observed a significant decrease in systemic vascular resistance after CPB. Differences between pulse contour CO and thermodilution CO measurements in patients with significant changes of the systemic vascular resistance [13] have already been established in previous investigations. Further studies are therefore needed, addressing also the performance of newly developed pulse contour devices that do not include an independent technique for calibration under difficult clinical settings, such as after CPB.
The fact that we failed to determine the CO by a method independent of thermal signals such as echocardiographic or lithium dilution measurement of the CO to validate the thermal dilution measurement [19,20] is a shortcoming of our study. Bearing in mind, however, that we did find an excellent correlation prior to CPB and a good correlation after CPB for the two thermodilution measurements, we believe that the thermodilution methods represent a reliable estimation of the 'true' CO in clinical practice. In case of severe hemodynamic instability after CPB, indicated by the COPiCCOpulse, COPiCCOtherm, COPACtherm or other clinical parameters, echocardiography should be used to guide therapy as suggested previously [21]. It has been established formerly that pulse contour analysis CO is a valid and cost-effective device for CO determination after calibration. Another limitation is that the design of our study does not allow for an ultimate demonstration of a causal relationship between CPB and lack of agreement. However, a number of studies show that pulse contour analysis is valid for at least some hours if there are no severe changes in hemodynamics. The mean time between sternotomy and the start of CPB is about 60 minutes. We therefore think it is reasonable to assume that CPB is mainly responsible for the inaccuracy of the post-CPB pulse contour analysis observed in our study.
Conclusion
In conclusion, we observed an excellent correlation of COPiCCOtherm and COPACtherm measurement prior to CPB. Our study could not prove pulse contour analysis with a modified Wesseling algorithm used in this study to be a method yielding reliable results with excellent accuracy and limits of agreement under difficult conditions after CPB in cardiac surgical patients. Hence, due to the broad distribution and the underestimation of the CO after CPB, the use of the uncalibrated continuous pulse contour cardiac output cannot be recommended after weaning from CPB. A re-calibration in this setting is essential.
Key messages
• We observed an excellent correlation of COPiCCOtherm and COPACtherm measurement prior to CPB.
• Our study could not prove pulse contour analysis with a modified Wesseling algorithm to be a method yielding reliable results under difficult conditions after CPB in cardiac surgical patients.
• Due to the broad distribution and the underestimation of the CO after CPB, the use of the uncalibrated continuous pulse contour cardiac output cannot be recommended after weaning from CPB.
Abbreviations
CO = cardiac output; COPACtherm = pulmonary artery catheter thermodilution cardiac output; COPiCCOpulse = continuous arterial pulse contour analysis cardiac output; COPiCCOtherm = aortic transpulmonary thermodilution cardiac output; CPB = cardiopulmonary bypass; LOA = limits of agreement; PAC = pulmonary artery catheter.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
MS and CvH prepared the manuscript, carried out the cardiac output measurements, conceived of the study and performed the statistical analysis. AF, JG and VvD helped with the recruitment of the patients and the drafting of the manuscript. SD and WFK participated in the study design and helped with the recruitment of patients. CS drafted the manuscript, helped with the study design and coordination. All authors read and approved the final manuscript.
Figure 1.
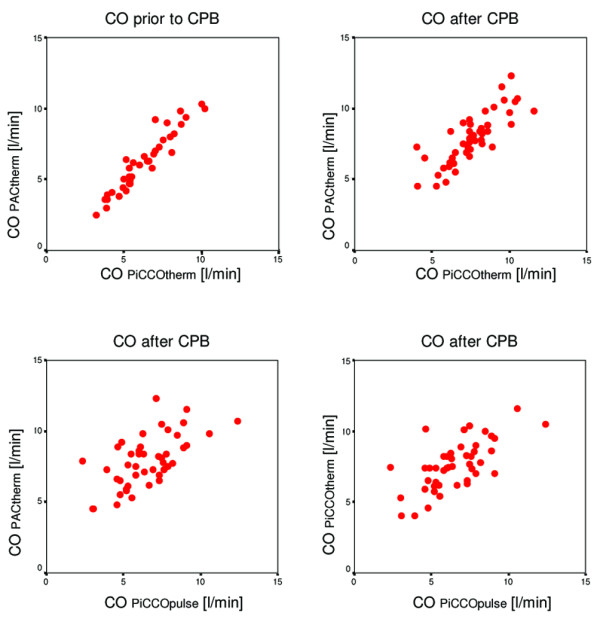
Regression analysis of pulmonary artery catheter thermodilution cardiac output (COPACtherm) versus aortic transpulmonary thermodilution cardiac output (COPiCCOtherm) prior to and after cardiopulmonary bypass (CPB), and regression analysis of continuous arterial pulse contour analysis cardiac output (COPiCCOpulse) versus COPiCCOtherm and versus COPACtherm after CPB.
Figure 2.
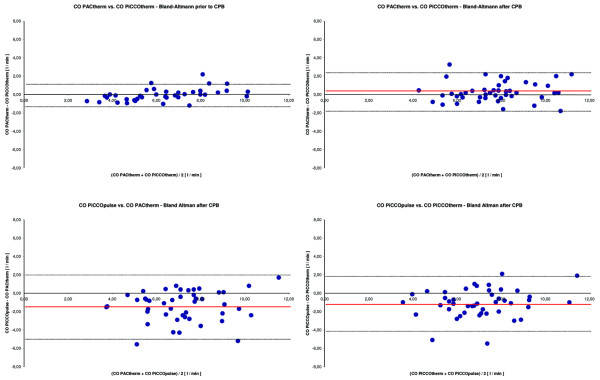
Bland-Altman plot of pulmonary artery catheter thermodilution cardiac output (COPACtherm) versus aortic transpulmonary thermodilution cardiac output (COPiCCOtherm) prior to and after cardiopulmonary bypass (CPB), and Bland-Altman plot of continuous arterial pulse contour analysis cardiac output (COPiCCOpulse) versus COPiCCOtherm and versus COPACtherm after CPB. CO, cardiac output.
Acknowledgments
Acknowledgements
The authors appreciate the diligent linguistic revision of this manuscript by Mrs Sirka Sander (certified and approved translator of the English language) and thank their colleagues Mrs Lisa Adam, Mrs Anja Heinemann and Alexander Döpke (all from the Department of Anesthesiology and Intensive Care Medicine, Charité University Medicine Berlin, Charité Campus Mitte, Germany) for helping with the acquisition of the data, as well as Mrs Gerda Siebert, Dipl.-Math. (Department of Medical Biometry, Charité University Medicine Berlin, Germany) for the detailed statistical advice for analyzing the data. This study was financially supported by departmental funding and institutional research grants of the Charité Medical School (University Hospital Berlin).
References
- Connors AF, Jr, Speroff T, Dawson NV, Thomas C, Harrell FE, Jr, Wagner D, Desbiens N, Goldman L, Wu AW, Califf RM, et al. The effectiveness of right heart catheterization in the initial care of critically ill patients. SUPPORT Investigators. JAMA. 1996;276:889–897. doi: 10.1001/jama.276.11.889. [DOI] [PubMed] [Google Scholar]
- Richard C, Warszawski J, Anguel N, Deye N, Combes A, Barnoud D, Boulain T, Lefort Y, Fartoukh M, Baud F, et al. Early use of the pulmonary artery catheter and outcomes in patients with shock and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2003;290:2713–2720. doi: 10.1001/jama.290.20.2713. [DOI] [PubMed] [Google Scholar]
- Harvey S, Harrison DA, Singer M, Ashcroft J, Jones CM, Elbourne D, Brampton W, Williams D, Young D, Rowan K, PAC-Man study collaboration Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): a randomised controlled trial. Lancet. 2005;366:472–477. doi: 10.1016/S0140-6736(05)67061-4. [DOI] [PubMed] [Google Scholar]
- Rauch H, Muller M, Fleischer F, Bauer H, Martin E, Bottiger BW. Pulse contour analysis versus thermodilution in cardiac surgery patients. Acta Anaesthesiol Scand. 2002;46:424–429. doi: 10.1034/j.1399-6576.2002.460416.x. [DOI] [PubMed] [Google Scholar]
- Godje O, Hoke K, Goetz AE, Felbinger TW, Reuter DA, Reichart B, Friedl R, Hannekum A, Pfeiffer UJ. Reliability of a new algorithm for continuous cardiac output determination by pulse-contour analysis during hemodynamic instability. Crit Care Med. 2002;30:52–58. doi: 10.1097/00003246-200201000-00008. [DOI] [PubMed] [Google Scholar]
- Sakka SG, Reinhart K, Meier-Hellmann A. Comparison of pulmonary artery and arterial thermodilution cardiac output in critically ill patients. Intensive Care Med. 1999;25:843–846. doi: 10.1007/s001340050962. [DOI] [PubMed] [Google Scholar]
- Godje O, Hoke K, Lamm P, Schmitz C, Thiel C, Weinert M, Reichart B. Continuous, less invasive, hemodynamic monitoring in intensive care after cardiac surgery. Thorac Cardiovasc Surg. 1998;46:242–249. doi: 10.1055/s-2007-1010233. [DOI] [PubMed] [Google Scholar]
- Buhre W, Weyland A, Kazmaier S, Hanekop GG, Baryalei MM, Sydow M, Sonntag H. Comparison of cardiac output assessed by pulse-contour analysis and thermodilution in patients undergoing minimally invasive direct coronary artery bypass grafting. J Cardiothorac Vasc Anesth. 1999;13:437–440. doi: 10.1016/S1053-0770(99)90216-1. [DOI] [PubMed] [Google Scholar]
- Jansen JR, Wesseling KH, Settels JJ, Schreuder JJ. Continuous cardiac output monitoring by pulse contour during cardiac surgery. Eur Heart J. 1990;11 Suppl I:26–32. doi: 10.1093/eurheartj/11.suppl_i.26. [DOI] [PubMed] [Google Scholar]
- Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol. 1993;74:2566–2573. doi: 10.1152/jappl.1993.74.5.2566. [DOI] [PubMed] [Google Scholar]
- Zollner C, Haller M, Weis M, Morstedt K, Lamm P, Kilger E, Goetz AE. Beat-to-beat measurement of cardiac output by intravascular pulse contour analysis: a prospective criterion standard study in patients after cardiac surgery. J Cardiothorac Vasc Anesth. 2000;14:125–129. doi: 10.1016/S1053-0770(00)90003-X. [DOI] [PubMed] [Google Scholar]
- Della RoccaG, Costa MG, Pompei L, Coccia C, Pietropaoli P. Continuous and intermittent cardiac output measurement: pulmonary artery catheter versus aortic transpulmonary technique. Br J Anaesth. 2002;88:350–356. doi: 10.1093/bja/88.3.350. [DOI] [PubMed] [Google Scholar]
- Rodig G, Prasser C, Keyl C, Liebold A, Hobbhahn J. Continuous cardiac output measurement: pulse contour analysis vs thermodilution technique in cardiac surgical patients. Br J Anaesth. 1999;82:525–530. doi: 10.1093/bja/82.4.525. [DOI] [PubMed] [Google Scholar]
- Calafiore AM, Teodori G, Mezzetti A, Bosco G, Verna AM, Di Giammarco G, Lapenna D. Intermittent antegrade warm blood cardioplegia. Ann Thorac Surg. 1995;59:398–402. doi: 10.1016/0003-4975(94)00843-V. [DOI] [PubMed] [Google Scholar]
- von Heymann C. Therapie mit Blut oder Blutbestandteilen. In: Spies CD, Kox WJ, editor. Check-up Anästhesiologie. Berlin: Springer; 2004. pp. 400–402. [Google Scholar]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- Godje O, Friedl R, Hannekum A. Accuracy of beat-to-beat cardiac output monitoring by pulse contour analysis in hemodynamical unstable patients. Med Sci Monit. 2001;7:1344–1350. [PubMed] [Google Scholar]
- Latson TW, Whitten CW, O'Flaherty D. Ventilation, thermal noise, and errors in cardiac output measurements after cardiopulmonary bypass. Anesthesiology. 1993;79:1233–1243. doi: 10.1097/00000542-199312000-00014. [DOI] [PubMed] [Google Scholar]
- Pearse RM, Ikram K, Barry J. Equipment review: an appraisal of the LiDCO plus method of measuring cardiac output. Crit Care. 2004;8:190–195. doi: 10.1186/cc2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrino AC, Jr, Harris SN, Luther MA. Intraoperative determination of cardiac output using multiplane transesophageal echocardiography: a comparison to thermodilution. Anesthesiology. 1998;89:350–357. doi: 10.1097/00000542-199808000-00010. [DOI] [PubMed] [Google Scholar]
- Shanewise JS, Cheung AT, Aronson S, Stewart WJ, Weiss RL, Mark JB, Savage RM, Sears-Rogan P, Mathew JP, Quinones MA, et al. ASE/SCA guidelines for performing a comprehensive intraoperative multiplane transesophageal echocardiography examination: recommendations of the American Society of Echocardiography Council for Intraoperative Echocardiography and the Society of Cardiovascular Anesthesiologists Task Force for Certification in Perioperative Transesophageal Echocardiography. Anesth Analg. 1999;89:870–884. doi: 10.1097/00000539-199910000-00010. [DOI] [PubMed] [Google Scholar]


