Abstract
Activation of T cells via the stimulation of the TCR plays a central role in the adaptive immunological response. Although much is known about TCR-stimulated signaling pathways, there are still gaps in our knowledge about the kinetics and sequence of events during early activation and about the in vivo specificity of kinases involved in these proximal signaling pathways. This information is important not only for understanding the activation of signaling pathways important for T cell function but also for the development of drug targets and computer-based molecular models. In this study, phospho-specific Abs directed toward individual sites on signaling proteins were used to investigate the early phosphorylation kinetics of proteins involved in proximal TCR-induced pathways. These studies indicate that linker for activation of T cells' tyrosines have substantially different phosphorylation kinetics and that Src homology 2 domain-containing leukocyte protein of 76 kDa has rapid, transient phosphorylation kinetics compared to other proteins. In additions, we provide evidence that ZAP-70 is the primary in vivo kinase for LAT tyrosine 191 and that Itk plays a role in the phosphorylation of tyrosine 783 on phospholipase C-γ1. In total, these studies give new insight into the sequence, kinetics and specificity of early TCR-mediated signaling events that are vital for T cell activation.
The activation of T cells via the interaction of the multi-subunit TCR with a peptide-MHC complex expressed on the surface of an APC is vital for proper immunological function and response to infection (1, 2). This association leads to the stimulation of multiple intracellular signaling pathways that are regulated by a delicate balance between phosphorylation and dephosphorylation events (3, 4). These signaling networks are highly complex, with the activation of multiple tyrosine kinases leading to the phosphorylation of numerous effector and adaptor proteins. Each kinase phosphorylates a unique set of sites on effector and adaptor proteins (3, 4), leading to the ordered and highly organized activation of various kinases and adaptor and effector proteins. This activation of various proteins in an ordered, sequential manner is necessary for the induction and propagation of intracellular signaling pathways induced by TCR stimulation (4).
One of the first signaling events that occurs upon the interaction of the TCR with the peptide-MHC complex is the stimulation of two members of the Src family of intracellular tyrosine kinases, Lck and Fyn (4, 5). The activation of these kinases results in the binding of ZAP-70, a member of the Syk family of intracellular kinases, to dually phosphorylated ITAM motifs and the subsequent activation of ZAP-70 by the phosphorylation of several residues including tyrosine 319 (6-8). T cells deficient in ZAP-70 have substantially decreased TCR-induced tyrosine phosphorylation of downstream signaling molecules (9). Upon activation, these kinases then phosphorylate specific sites on a number of downstream substrates.
One protein rapidly phosphorylated upon TCR activation is linker for activation of T cells (LAT),2 a hemopoietic-specific transmembrane adaptor protein with no apparent enzymatic activity (3, 10). LAT has nine conserved tyrosines, with the last four, LAT tyrosines 132, 171, 191, and 226, known to be important for LAT function (11, 12). Although the in vivo kinases for individual LAT tyrosines have not been identified, several in vitro studies have implicated ZAP-70, Itk, and Lck in the phosphorylation of LAT (10, 13, 14). When phosphorylated, these last four conserved LAT tyrosines serve as docking sites for Src homology (SH) 2 domain-containing proteins, including phospholipase C-γl (PLC-γ1), Grb2, Gads, and Grap, and indirectly associate with SH3 domain ligands of these proteins including Src homology 2 domain-containing leukocyte protein of 76 kDa (SLP-76), son of sevenless (SOS), and c-Cbl (10, 15-17). Multiple structure/function studies have examined specifically which LAT tyrosines interact with individual SH2 domain-containing proteins and their SH3 domain ligands. These studies have shown that PLC-γ1 binds to LAT tyrosine 132 (11, 14, 18, 19). Similarly, Grb2, along with its SH3 domain ligands SOS and c-Cbl, associate with LAT tyrosines 171, 191, and 226 (11, 18, 19), whereas, Gads and its SH3 domain ligand, SLP-76, interact with LAT tyrosines 171 and 191 (11, 18). The recruitment of signaling molecules to LAT results in the formation of multiprotein complexes that bind to specific tyrosines on LAT through a combination of affinity preferences and cooperative interactions (20). These LAT-containing multiprotein complexes are vital for T cell differentiation and for the initiation of TCR-mediated intracellular signaling pathways (21-23).
Upon binding to LAT, PLC-γ1 is phosphorylated on multiple tyrosines including tyrosine 783, a site known to be vital for the in vivo function of PLC-γ1 in T cells (3, 24). Similarly, the phosphorylation of three tyrosines of SLP-76, including tyrosine 145, in a ZAP-70- and LAT-dependent manner, is vital for the in vivo function of SLP-76 in both T cell development and TCR-mediated activation of signaling pathways (9, 22, 23, 25, 26). These tyrosines serve as binding sites for SH2 domain-containing proteins including an apparent trimolecular complex among SLP-76, Vav, and Itk, a member of the Tec family of tyrosine kinases (27, 28). This trimolecular complex appears to be important for the localization of Itk to the LAT complex (27). Interestingly, both the tyrosine phosphorylation and lipase activity of PLC-γ1 is dependent on LAT, SLP-76, and Itk, since T cells deficient in these molecules have reduced PLC-γ1 phosphorylation and Ca2+ influx (22, 23, 29-32). These data suggest that LAT and SLP-76 may localize Itk to the LAT complex, thereby mediating the phosphorylation of PLC-γ1 by Itk (32-34).
Even with the substantial amount of information known about the signaling pathways activated upon TCR stimulation, there are still gaps in our understanding of these processes. We have an incomplete understanding of the early activation/phosphorylation kinetics of individual tyrosines on proteins involved in proximal TCR-mediated signaling events or about the exact sequence of these events. Similarly, we do not know exactly which kinases phosphorylate specific tyrosines on effector and adaptor proteins and in what order these phosphorylation events occur. It is important that we understand the sequence, specificity and kinetics of early TCR-mediated signaling pathways, not only to have a better understanding of T cell activation, but also to choose appropriate targets for drug intervention and to begin to develop computer-based models of intracellular signaling pathways. To this end, we have examined the early phosphorylation kinetics of individual tyrosines on proteins involved in early TCR-mediated signaling pathways using phospho-specific Abs for individual tyrosines on ZAP-70, LAT, c-Cbl, SLP-76, and PLC-γ1. These experiments were performed using normal human T cells, wild-type (WT) Jurkat T cell lines, and various mutant Jurkat lines that are deficient in signaling molecules to give insight into the contribution of these proteins to signaling events downstream of TCR stimulation. Together, these studies provide new information on the activation kinetics and in vivo kinase specificities of proteins involved in proximal TCR-mediated signaling pathways.
Materials and Methods
Materials
RPMI 1640, FBS, l-glutamine solution, penicillin/streptomycin solution, and 10× TBS were acquired from BioSource International. The Itk-specific small interference RNA (siRNA) was synthesized by Qiagen-Xeragon. The IL-2 was acquired from R&D Systems. The anti-CD4 Ab, clone RPA-T4, was obtained from BD Biosciences and the anti-mouse IgG was purchased from KPL. Tris, NaCl, and EDTA solutions were purchased from Quality Biological. Brij-97, PHA, BSA, Na3VO4, and other chemicals were obtained from Sigma-Aldrich. n-Octyl-β-d-glucopyranoside was purchased from EMD Biosciences. Complete Inhibitor tablets were obtained from Roche, Criterion Precast Polyacrylamide gels were acquired from Bio-Rad, and polyvinylidene difluoride was purchased from Millipore. The phospho-LAT tyrosine 132, phospho-LAT tyrosine 191, phospho-PLC-γ1 tyrosine 783, and phospho-SLP-76 tyrosine 145 were acquired from BioSource International. The phospho-ZAP-70 tyrosine 319 and phospho-c-Cbl tyrosine 731 Abs were purchased from Cell Signaling Technologies. The GAPDH Ab was obtained from Biodesign International and the LAT Ab was described previously (10).
Cell stimulation and immunoblotting
E6.1, JCaM2.5, P116, J14, and JVav Jurkat cells were grown to a concentration of 2–5 × 105 cells/ml in RPMI 1640 supplemented with 10% FBS, 2 mM l-glutamine, 50 U/ml penicillin, and 50μg/ml streptomycin. Before treatment, the cells were washed once with RPMI 1640 without supplements and resuspended to a concentration of 5 × 107 cells/ml. The cells were then treated with anti-CD3 (OKT3 ascites, 1/200) for various time points and lysed using a 4-fold excess of hot 2× sample buffer (20 mM Tris (pH8.0), 2 mM EDTA, 2 mM Na3VO4, 20 mM DTT, 2% SDS, and 20% glycerol). The samples were then heated at 95°C for 4 min and sonicated to reduce the viscosity of the solution. To analyze the phosphorylation status of various signaling proteins, 2 × 105 cell equivalents were separated by PAGE using 4 –15% Criterion Precast polyacrylamide gels. The separated proteins were then transferred to polyvinylidene difluoride and the membrane was blocked for 1 h at room temperature using TBST (10 mM Tris (pH 8.0), 150 mM NaCl, and 0.05% Tween 20) with 1% BSA. The membranes were incubated for 1 h at room temperature with primary Abs diluted in TBST with 1% BSA, followed by a 30-min incubation at room temperature with the appropriate secondary Ab diluted in TBST with 1% BSA. The blots were then visualized by chemiluminescence. The anti-phospho-ZAP-70 tyrosine 319, anti-phospho-LAT tyrosine 132, anti-phospho-LAT tyrosine 191, anti-phospho-PLC-γ1 tyrosine 783, andanti-GAPDH Abs were highly specific, giving only a single band on the x-ray film (data not shown). The anti-c-Cbl tyrosine 731 and anti-SLP-76 tyrosine 145 Abs were less specific but still gave a predominant band at the correct molecular weight with little background in the general vicinity of that band (data not shown).
PBL isolation and stimulation
Human PBLs were isolated from whole blood of healthy donors. Briefly, mononuclear cells were isolated by Ficoll density gradient centrifugation. After washing, cells were stimulated with 5 μg/ml PHA for 24 h. After two washes, cells were maintained in RPMI 1640 supplemented with 10% FBS, 2 mM l-glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin and 20 ng/ml rIL-2 for 5–6 days. The cells were then washed and incubated overnight in RPMI 1640 supplemented with 10% FBS, 2 mM l-glutamine, 50 U/ml penicillin, and 50 μg/ml streptomycin with no IL-2. Flow cytometric analysis of cell surface markers showed predominantly T cells. Before stimulation, the cells were washed twice with RPMI 1640 and then resuspended to a concentration of 1 × 108 cells/ml. The cells were then treated with anti-CD3 (OKT3 ascites, 1/200) and anti-CD4 (10 μg/ml) for 30 min on ice. The cells were then warmed at 37°C for 10 min and stimulated with anti-mouse IgG (50 μg/ml) for various time periods and lysed using a 2-fold excess of hot 2× sample buffer. The samples were then heated at 95°C for 4 min and sonicated to reduce the viscosity of the solution. Phosphoprotein analysis was performed as described above with 1 × 106 cell equivalents loaded per lane.
siRNA treatment
Treatment of cells with siRNA to Itk was completed as previously described (28). Briefly, Jurkat TAg cells were transfected with control siRNA or siRNA to Itk (1.1 μg/107 cells) using an Electro Square Porator (BTX Harvard Apparatus). Following electroporation, the cells were placed into transfection medium (RPMI 1640, 20 mM HEPES, and 20% FBS) and incubated for 24–36 h. The sequence of the siRNA duplex to Itk was previously described (28). Stimulation and phosphoprotein analysis was performed as described above in Cell Stimulation and Immunoblotting.
Analysis of immunoblotting
To analyze the relative phosphorylation kinetics of various signaling proteins, immunoblotting films were electronically scanned and the relative intensity of the bands were determined using the gel plotting macro of NIH Image. Immunoblotting film exposures in the linear range of the x-ray film were chosen such that the maximal response from each individual experiment had approximately equal intensity. To control for variations during the gel electrophoresis and immunoblotting, GAPDH was used as a loading control for all experiments. By using rigorous normalization procedures, the intrinsic error from the immunoblotting technique was equalized for all of the phospho-specific Abs.
The percentage of activation for each time point was then calculated as described below with the values of 0 and 100% set as the level of normalized phosphorylation at 0 and 120 s, respectively. To allow for the analysis of the percentage of activation of SLP-76, the 60- and 120-s time points were removed from the analysis and the values of 0 and 100% were set as the level of normalized phosphorylation at 0 and 30 s, respectively. Setting the dynamic range of activation values by normalization substantially reduced the variation between experiments and allowed for the comparison of the activation kinetics of various phosphorylation sites by making the effect of differences in Ab affinity on band intensity irrelevant to the analysis.
The percent activation at 120 s for the E6.1 Jurkat cell experiments was calculated using the following formula: percent activation at 120 s = (X s − 0 s)/(120 s − 0 s) × 100%.
For the experiments where the activation of various Jurkat cell lines were compared against the activation of E6.1 Jurkat cells, the percent activation of E6.1 Jurkat cells at 120 s was calculated using the formula as follows: percent Jurkat E6.1 activation at 120 s = (X s − 0 s)/(E6.1 at 120 s − 0 s) × 100%.
After normalization against GAPDH, the percent activation of the maximum phosphorylation in the siItk-treated Jurkat cell experiments was calculated using the following formula: percent maximum phosphorylation = (X s − 0 s)/(maximum phosphorylation time point − 0 s) × 100%.
The values for percent activation for each time point from three separate experiments were then averaged and the mean ± SEM was plotted using Origin (OriginLab). Curves were fit using a four-parameter logistic equation and weighted with the average SEM at each time point for all curves shown in a single graph. The four-parameter logistic equation was selected for the curve fitting due to the ability of this equation to accurately model variable slopes at the inflection point of the curve. The time of 50% maximum stimulation was calculated using Origin and the F test statistical analysis was performed using the Fit Comparison Tool of Origin.
Results
To better understand the early phosphorylation kinetics of proximal signaling events associated with TCR activation, we examined the site-specific phosphorylation kinetics of tyrosine 319 on ZAP-70 along with two sites on LAT, tyrosine 132 and tyrosine 191. We also examined the phosphorylation kinetics of molecules known to directly or indirectly associate with LAT phosphotyrosines, including tyrosine 783 on PLC-γ1, tyrosine 731 of c-Cbl, and tyrosine 145 on SLP-76. The phosphorylation of these proteins was investigated in WT Jurkat E6.1 cells as well as in the ZAP-70-negative Jurkat cell P116, the LAT-deficient Jurkat cell JCaM 2.5, the SLP-76-deficient Jurkat mutant J14, and the Vav-negative Jurkat cell JVav (35).
Specificity of the phospho-LAT Abs
To more fully characterize the specificity of the phospho-LAT tyrosine 132 and phospho-LAT tyrosine 191 Abs, the reactivity of these Abs toward previously described LAT mutants expressed in LAT-deficient JCaM 2.5 cells (18) was examined. JCaM 2.5 cells that had been transfected with WT LAT, LAT mutated at tyrosine 132 (Y132F), at tyrosine 191 (Y191F), or LAT with the three or four C-terminal tyrosines mutated to phenylalanine (3YF or 4YF; Fig. 1A) were stimulated by anti-CD3 Abs and the phosphorylation of the LAT tyrosine 132 and LAT tyrosine 191 was determined by immunoblotting. The phospho-LAT tyrosine 132 Ab detected LAT in stimulated JCaM 2.5 cells expressing WT, 3YF, and Y191F LAT but had no reactivity toward LAT in JCaM 2.5 cells expressing Y132F and 4YF LAT (Fig. 1B), suggesting that this Ab is specific for phosphorylated LAT tyrosine 132. Similarly, the phospho-LAT tyrosine 191 Ab recognized LAT in cells expressing WT and Y132F LAT but not in cells expressing Y191F, 3YF or 4YF LAT (Fig. 1B), indicating that this Ab is specific for phosphorylated LAT tyrosine 191. Interestingly, the phosphorylation of LAT tyrosine 132 did not appear to depend on the presence of LAT tyrosines 171, 191, or 226, since there was a similar level of phosphorylation of LAT tyrosine 132 in JCaM 2.5 cells expressing either LAT WT or the LAT 3YF mutant (Fig. 1B). Similarly, the phosphorylation of LAT tyrosine 191 was not dependent on the presence of LAT tyrosine 132, since the phospho-LAT tyrosine 191 Ab recognized both LAT WT and the LAT Y132F mutant (Fig. 1B).
FIGURE 1.
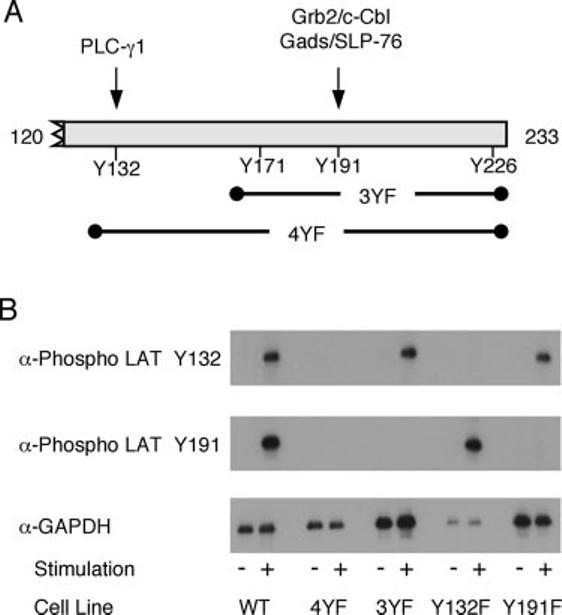
Specificity of the anti-phospho-LAT Abs. A, Schematic of LAT showing the four C-terminal tyrosine phosphorylation sites along with the proteins that interact with LAT tyrosines 132 and 191. Also shown are the tyrosines mutated to phenylalanine in the 3YF and 4YF mutations. B, JCaM 2.5 cells transfected with various forms of LAT were treated with or without anti-CD3 for 2 min. The phosphorylation of LAT tyrosines 132 and 191 and the expression levels of GAPDH were assessed by immunoblotting as described in Materials and Methods.
Phosphorylation kinetics of proximal signaling molecules in E6.1 Jurkat cells
To begin the analysis of the early phosphorylation kinetics of proximal TCR-mediated signaling pathways, we first examined the phosphorylation kinetics of various signaling molecules in WT E6.1 Jurkat cells. E6.1 Jurkat cells were stimulated and the site-specific phosphorylation of ZAP-70, LAT, c-Cbl, PLC-γ1, and SLP-76 were determined at various time points by immunoblotting with phospho-specific Abs. To compare the kinetics of stimulation of the various proteins, the percentage of activation at 120 s for each protein was calculated after normalizing each band to GAPDH expression. These values were then plotted graphically and the time of 50% activation was calculated as a measure of the rapidity of phosphorylation. By using rigorous normalization procedures, the intrinsic error introduced by the immunoblotting technique was equal for all examined Abs. Similarly, by setting the range of the activation between the 0-s time point and the 120-s time point for each analyzed site, the effects of differences in Ab affinity on band intensity were made irrelevant since the calculated values for percentage of maximal activation are independent of Ab affinity. Tyrosine 319 on ZAP-70 was rapidly phosphorylated in stimulated Jurkat E6.1 cells with demonstrable phosphorylation occurring as early as 5 s and maximal stimulation occurring by 30 s (Fig. 2, A and B, black line). The time of 50% maximal stimulation of ZAP-70 was ∼19 s. The activation kinetics of LAT tyrosine 191 (Fig. 2, A and B, green line) and c-Cbl tyrosine 731 (Fig. 2, A and B, purple line) were similar to tyrosine 319 on ZAP-70 (Fig. 2, A and B, black line). These sites were detectably phosphorylated by 10 s, with maximal stimulation by 60 s. The time of 50% maximal stimulation for LAT tyrosine 191 and c-Cbl tyrosine 731 was 19 and 21 s, respectively.
FIGURE 2.
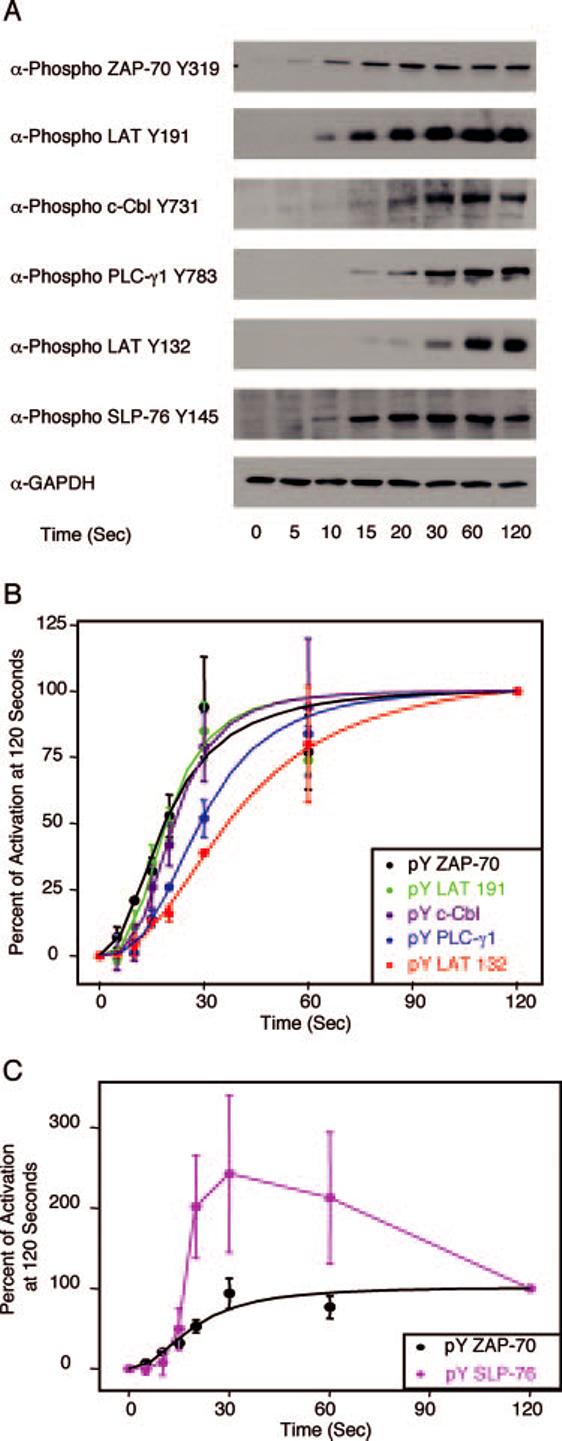
Phosphorylation kinetics of various signaling proteins in E6.1 Jurkat cells. E6.1 Jurkat cells were treated with or without anti-CD3 for various times and the cellular proteins were separated by PAGE. A, The tyrosine phosphorylation of specific sites on ZAP-70, LAT, c-Cbl, PLC-γ1, and SLP-76, along with the expression level of GAPDH, was determined by immunoblotting as described in Materials and Methods. B, The percent activation at 120 s for ZAP-70, LAT, c-Cbl, and PLC-γ1 was determined as described in Materials and Methods. The mean ± SEM of three separate experiments for each time point was plotted and fit using Origin. C, The percent activation at 120 s for ZAP-70 and SLP-76 were determined. The mean ± SEM of three separate experiments for each time point was plotted. A sigmoidal curve for the ZAP-70 data was fit using Origin.
After ZAP-70 tyrosine 319, LAT tyrosine 191, and c-Cbl tyrosine 731 phosphorylation, the activation kinetics of other proteins segregated into two distinct patterns. First, LAT tyrosine 132 (Fig. 2, A and B, red line) and tyrosine 783 on PLC-γ1 (Fig. 2, A and B, blue line) were visibly phosphorylated by 15 s, with maximal phosphorylation occurring by 120 s. The time of 50% maximal stimulation was 29 s for PLC-γ1 and 37 s for LAT tyrosine 132. Second, SLP-76 had a phosphorylation pattern that was different from the other analyzed proteins. SLP-76 (Fig. 2, A and C, magenta line) was phosphorylated within 10 s and had maximal phosphorylation by 30 s. The phosphorylation of this protein then decreased with a loss of ∼50% of maximal phosphorylation by 120 s (Fig. 2, A and C). The time of 50% maximal stimulation was determined to be 17 s, which was the fastest of any of the proteins analyzed, although it appeared that the initial ZAP-70 phosphorylation occurs earlier than the first detectable phosphorylation of SLP-76.
The majority of the error in the curve fitting for ZAP-70 tyrosine 319, c-Cbl tyrosine 731, LAT tyrosine 191, and PLC-γ1 tyrosine 783 came from the 60-s time point, which was substantially lower for these sites than the expected value from the curve fitting. Whether the lower than expected phosphorylation of these sites is physiologically relevant is still unknown but it is interesting that this effect is seen at this time point for many of the analyzed proteins. The large amount of error from the 60-s time point coupled with the relatively small number of points used in the curve fitting resulted in an inconclusive pairwise statistical analysis using the statistical F test. When this time point was not considered in the analysis, the curves for LAT tyrosine 191 and c-Cbl tyrosine 731 were significantly different from the curve for LAT tyrosine 132 with a p < 0.02. However, even after excluding this time point from the analysis, the curve fit for ZAP-70 tyrosine 319 and for PLC-γ1 tyrosine 783 was not statistically different from the curve fit for LAT tyrosine 132 with p = 0.09 and 0.41, respectively. For the comparison of the phosphorylation kinetics of ZAP-70 tyrosine 319 to LAT tyrosine 132, this lack of statistical significance was likely due to the greater variability of the curve fitting compared with other phosphorylation sites. In total, these data suggest that the activation of proximal TCR-induced signaling proteins follows a specific sequence with the activation of ZAP-70 one of the earliest events. Interestingly, LAT tyrosine 132 and LAT tyrosine 191 have different kinetics of phosphorylation upon TCR stimulation, with the time of 50% maximal phosphorylation of LAT tyrosine 191 occurring 15–20 s faster than for LAT tyrosine 132.
Phosphorylation kinetics of LAT in human PBLs
As shown above, TCR stimulation of E6.1 Jurkat cells resulted in a substantial difference in the phosphorylation kinetics of LAT tyrosine 132 and LAT tyrosine 191. To confirm this finding in normal nontransformed T cells, the phosphorylation kinetics of these sites on LAT was examined in stimulated human PBLs. To begin, various Abs that activate PBLs were tested to determine the best stimulation conditions for experimental procedures. As shown in Fig. 3A, the treatment of PBLs for 2 min with anti-CD3 alone or anti-CD3 and anti-CD28 resulted in little increase in the amount of phosphorylation of LAT tyrosines 132 and 191 compared with untreated cells. In contrast, treatment of human PBLs for 2 min with anti-CD3 and anti-CD4 resulted in an increase in the phosphorylation levels of LAT tyrosines 132 and 191 compared with untreated cells (Fig. 3A). The relative levels of LAT phosphorylation were substantially less in the anti-CD3- and anti-CD4-treated PBLs than in E6.1 Jurkat cells activated for 2 min with anti-CD3 (Fig. 3A). These data show that only anti-CD3- and anti-CD4-stimulated PBLs have sufficient levels of LAT phosphorylation required for the analysis of the phosphorylation kinetics of individual LAT tyrosines. To this end, the activation kinetics of LAT tyrosines 132 and 191 were examined in anti-CD3- and anti-CD4-induced PBL cells. The stimulated PBL cells constantly had slower activation kinetics compared with various Jurkat cells. LAT tyrosine 191 had detectable phosphorylation by 30 s in anti-CD3- and anti-CD4-activated PBLs, with maximal phosphorylation occurring between 90 and 120 s (Fig. 3, B and C). In contrast, LAT tyrosine 132 had detectable phosphorylation by 40 s in stimulated PBLs, with maximal phosphorylation occurring by 120 s (Fig. 3, B and C). The time of 50% of maximum stimulation in the activated PBL cells was 50 s for LAT tyrosine 191 and 62 s for LAT tyrosine 132, which is similar to the differences in the phosphorylation kinetics observed in stimulated E6.1 Jurkat cells. These data show that the substantial difference in the phosphorylation kinetics of LAT tyrosine 132 and LAT tyrosine 191 is observed in both transformed and nontransformed T cells, stimulated under different conditions, suggesting that the lag in LAT tyrosine 132 phosphorylation is occurring upon TCR activation.
FIGURE 3.
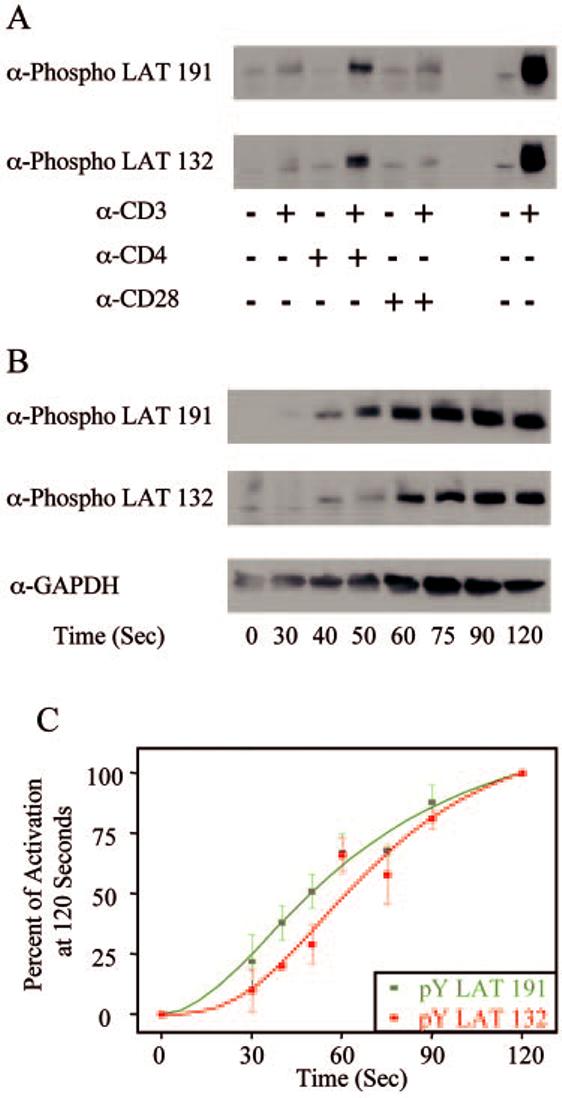
Phosphorylation kinetics of various signaling proteins in human PBLs. A, Human PBLs were treated with anti-CD3 alone, anti-CD3 and anti-CD4, or anti-CD3 and anti-CD28. The tyrosine phosphorylation of specific sites on LAT was then determined by immunoblotting as described in Materials and Methods. B, Human PBLs were treated with or without anti-CD3 and anti-CD4 for various times and the tyrosine phosphorylation of specific sites on LAT, along with the expression level of GAPDH, was determined by immunoblotting as described in Materials and Methods. C, The percent activation at 120 s for specific LAT tyrosines, as examined in B, was determined as described in Materials and Methods. The mean ± SEM of three separate experiments for each time point was plotted and fit using Origin.
Phosphorylation kinetics of proximal signaling molecules in ZAP-70-deficient Jurkat cells
The data presented above suggest that the activation of ZAP-70 is one of the first events induced in E6.1 Jurkat cells by TCR activation. Likewise, previous studies have shown reductions in LAT, PLC-γ1, and SLP-76 tyrosine phosphorylation upon TCR stimulation in the ZAP-70-deficient Jurkat cell P116 (9, 36), again suggesting that ZAP-70 activation is an early event in proximal TCR-mediated signaling. To determine more fully the role of ZAP-70 in the phosphorylation kinetics of proximal signaling molecules, we examined the kinetics of early site-specific activation of LAT, cCbl, SLP-76, and PLC-γ1 in the ZAP-70-deficient Jurkat cell P116. Consistent with earlier studies, there was little detectable phosphorylation at any time points of LAT tyrosine 132, LAT tyrosine 191, c-Cbl tyrosine 731, SLP-76 tyrosine 145, or PLC-γ1 tyrosine 783 in stimulated P116 Jurkat cells compared with stimulated E6.1 Jurkat cells (Fig. 4). These data suggest that ZAP-70 is required for the early TCR-mediated phosphorylation of c-Cbl, SLP-76, PLC-γ1, and both LAT tyrosine 132 and tyrosine 191.
FIGURE 4.
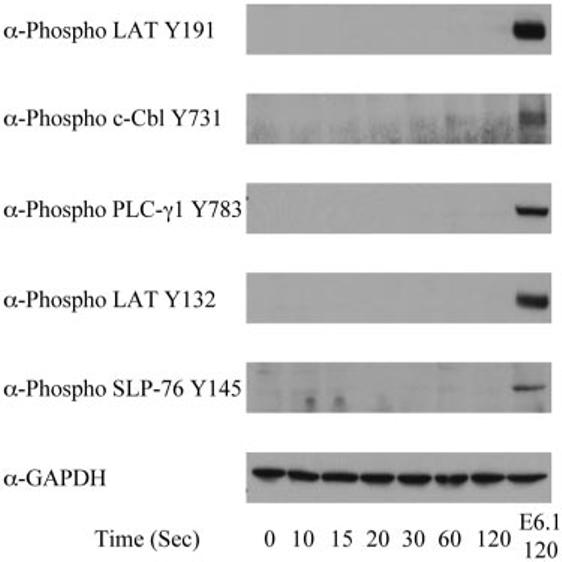
Phosphorylation kinetics of various signaling proteins in P116 Jurkat cells. P116 Jurkat cells were treated with or without anti-CD3 for various times and the cellular proteins were separated by PAGE. The tyrosine phosphorylation of specific sites on LAT, PLC-γ1, and SLP-76, along with the expression level of GAPDH, was determined by immunoblotting. For comparison, the phosphorylation of these proteins and the expression level of GAPDH in E6.1 Jurkat cells treated with anti-CD3 for 120 s are shown.
Phosphorylation kinetics of proximal signaling molecules in LAT-deficient Jurkat cells
Previous studies using the LAT-deficient Jurkat cells, JCaM 2.5, have shown that LAT is required for the phosphorylation and activation of PLC-γ1 and SLP-76 but does not appear to be required for c-Cbl phosphorylation (22). This led us to more fully examine the role of LAT in the activation of proximal signaling proteins by determining the kinetics of site-specific phosphorylation of ZAP-70, c-Cbl, PLC-γ1, and SLP-76 in the LAT-deficient Jurkat cell JCaM 2.5. Stimulated JCaM 2.5 Jurkat cells had similar levels of ZAP-70 tyrosine 319 and c-Cbl tyrosine 731 phosphorylation (Fig. 5A) compared with E6.1 Jurkat cells, which is consistent with previous studies that have shown that ZAP-70 and c-Cbl phosphorylations are not dependent on the recruitment of c-Cbl to LAT (22). In contrast, the phosphorylation of PLC-γ1 on tyrosine 783 was absent in stimulated JCaM2.5 cells (Fig. 5, B and C), suggesting that LAT is required for the early phosphorylation of PLC-γ1. Interestingly, the site-specific phosphorylation of tyrosine 145 of SLP-76 was reduced but not absent in stimulated JCaM 2.5 cells (Fig. 5, B and C). Compared with the phosphorylation in E6.1 Jurkat cells, the levels of maximal phosphorylation of SLP-76 were reduced by ∼90%. Together, these data indicate that LAT is required for the early phosphorylation of PLC-γ1 and suggest that SLP-76 has both LAT-dependent and LAT-independent modes of activation.
FIGURE 5.
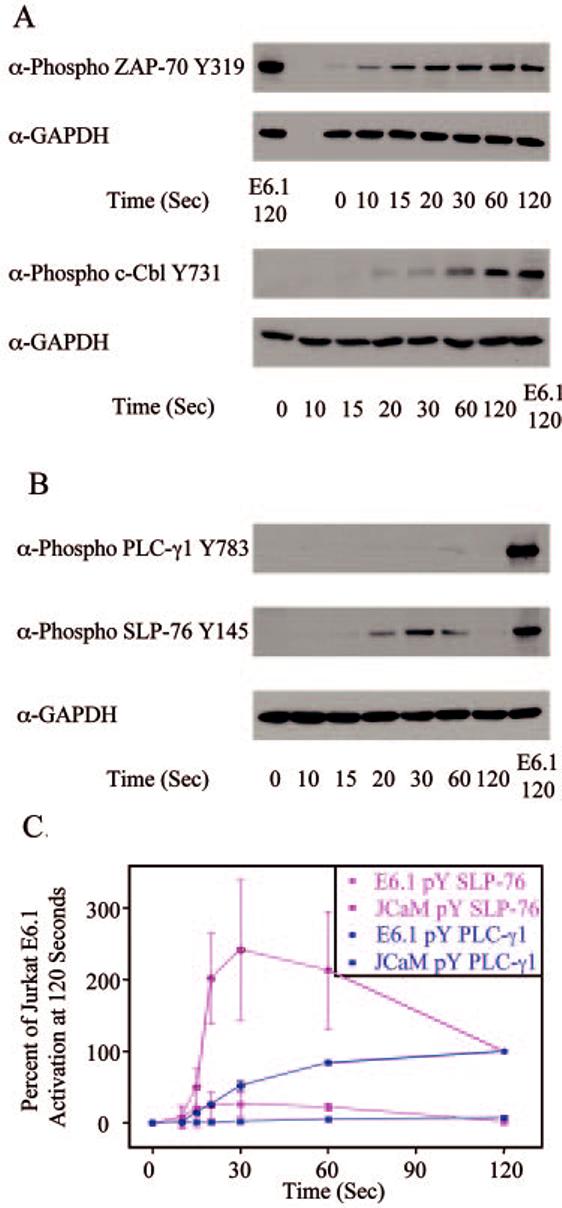
Phosphorylation kinetics of various signaling proteins in JCaM 2.5 Jurkat cells. JCaM2.5 Jurkat cells were treated with or without anti-CD3 for various times and the cellular proteins were separated by PAGE. A, The tyrosine phosphorylation of specific sites on ZAP-70 and c-Cbl, along with the expression level of GAPDH, was determined by immunoblotting. For comparison, the phosphorylation of these proteins and the expression level of GAPDH in E6.1 Jurkat cells treated with anti-CD3 for 120 s are shown. B, The tyrosine phosphorylation of specific sites on PLC-γ1 and SLP-76, along with the expression level of GAPDH, was determined by immunoblotting. For comparison, the phosphorylation of these proteins and the expression level of GAPDH in E6.1 Jurkat cells treated with anti-CD3 for 120 s are shown. C, The percent activation of E6.1 Jurkat cells at 120 s for PLC-γ1 and SLP-76 in E6.1 and JCaM 2.5 Jurkat cells was determined. The mean ± SEM of three separate experiments for each time point was plotted and fit using Origin.
Phosphorylation kinetics of proximal signaling molecules in SLP-76-deficient Jurkat cells
The data presented above show that LAT is not needed for the rapid phosphorylation of tyrosine 319 on ZAP-70 or tyrosine 731 on c-Cbl but is required for the early phosphorylation of PLC-γ1 tyrosine 783. One possible explanation for this latter observation is that LAT nucleates PLC-γ1 with the kinases that directly phosphorylate tyrosine 783. This hypothesis is supported by studies that have shown that SLP-76 is required for the phosphorylation of PLC-γ1 (29) and that have suggested that Itk, a kinase brought to the LAT complex by SLP-76, is involved in the phosphorylation of PLC-γ1 (33). Also, a previous study has shown that Itk may phosphorylate specific sites on LAT (13). However, these studies are in contrast to the observation that SLP-76 does not appear to be required for total LAT tyrosine phosphorylation (29). To better understand the role of SLP-76 in the phosphorylation of proximal signaling molecules, the kinetics of phosphorylation of LAT and PLC-γ1 were examined in the SLP-76-deficient Jurkat cell line J14. There appeared to be a small but consistent reduction in the phosphorylation levels of ZAP-70 tyrosine 319, LAT tyrosine 132, or LAT tyrosine 191 in stimulated J14 Jurkat cells compared with E6.1 Jurkat cells (Fig. 6A). However, there were no differences in the phosphorylation kinetics of these sites in the stimulated J14 cells compared with E6.1 Jurkat cells (Fig. 6A, and data not shown). This suggests that J14 cells have a general reduction in the levels of protein phosphorylation compared with E6.1 Jurkat cells but indicates that SLP-76 does not appear to affect the phosphorylation kinetics of LAT. Consistent with previous studies (29), there was little tyrosine phosphorylation of tyrosine 783 on PLC-γ1 at any time point in stimulated J14 Jurkat cells (Fig. 6B) compared with E6.1 Jurkat cells. These data show that SLP-76 or the proteins translocated to the LAT complex by SLP-76, such as Itk, do not appear to be involved in the phosphorylation of LAT but are required for phosphorylation of tyrosine 783 on PLC-γ1.
FIGURE 6.
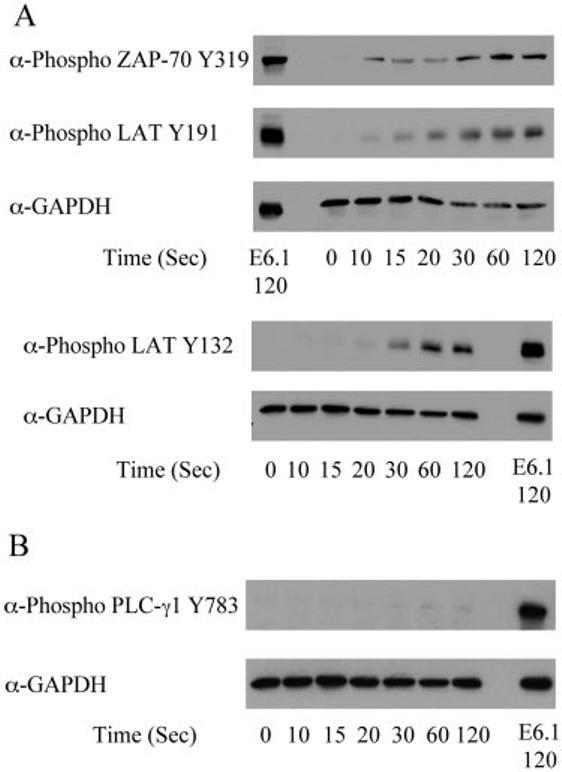
Phosphorylation of various signaling proteins in J14 Jurkat cells. J14 Jurkat cells were treated with or without anti-CD3 for various times and the cellular proteins were separated by PAGE. A, The tyrosine phosphorylation of ZAP-70 Y319, LAT tyrosine 132, and LAT tyrosine 191, along with the expression level of GAPDH, was determined by immunoblotting. For comparison, the phosphorylation of these proteins and the expression level of GAPDH in E6.1 Jurkat cells treated with anti-CD3 for 120 s are shown. B, The phosphorylation of PLC-γ1, along with the expression level of GAPDH, was determined by immunoblotting. For comparison, the phosphorylation of PLC-γ1 and the expression level of GAPDH in E6.1 Jurkat cells treated with anti-CD3 for 120 s are shown. C, The percent activation of E6.1 Jurkat cells at 120 s for PLC-γ1 in E6.1 and J14 Jurkat cells was determined as described in Materials and Methods. The mean ± SEM of three separate experiments for each time point was plotted and fit using Origin.
Phosphorylation kinetics of LAT and PLC-γ1 in Vav1-deficient Jurkat cells
A molecule that has been suggested to be important for the interaction of Itk with SLP-76, via the formation of a cooperative trimolecular complex, is Vav (27, 28). Therefore, to more fully investigate the role of the trimolecular complex among SLP-76, Vav, and Itk on the phosphorylation of LAT and PLC-γ1, the role of Vav on the kinetics of early activation of these proteins was examined in the Vav1-deficient Jurkat cell line JVav. There does not appear to be any effect on the kinetics or intensity of phosphorylation of LAT tyrosine 132 or LAT tyrosine 191 in stimulated JVav Jurkat cells compared with E6.1 Jurkat cells (Fig. 7A). However, there was a significant difference in the phosphorylation of tyrosine PLC-γ1 tyrosine 783 in JVav Jurkat cells compared with E6.1 Jurkat cells. Stimulated JVav cells had both slower activation kinetics and reduced overall levels of PLC-γ1 tyrosine 783 phosphorylation (Fig. 7, B and C). The 75% reduction of PLC-γ1 tyrosine 783 phosphorylation observed in the JVav Jurkat cells is similar to the reduction in phosphorylation of this site on PLC-γ1 in Vav1-deficient mouse T cells (27). These data show that Vav, like SLP-76, is required for the phosphorylation of PLC-γ1, suggesting that a trimolecular complex among SLP-76, Itk, and Vav may be involved in the phosphorylation of tyrosine 783 on PLC-γ1.
FIGURE 7.
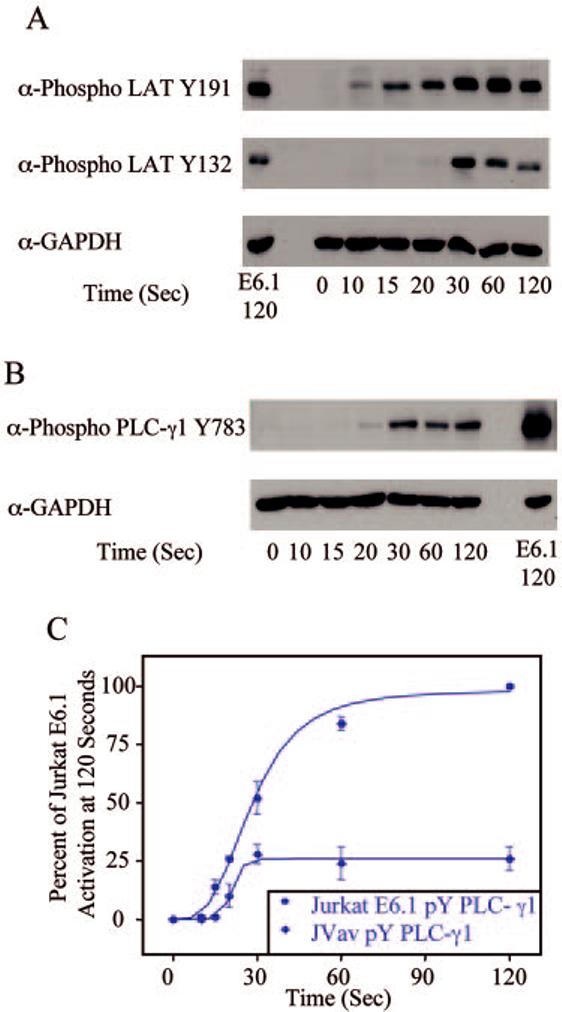
Phosphorylation kinetics of various signaling proteins in JVav Jurkat cells. JVav Jurkat cells were treated with or without anti-CD3 for various times and the cellular proteins were separated by PAGE. A, The tyrosine phosphorylation of specific sites on LAT, along with the expression level of GAPDH, was determined by immunoblotting. For comparison, the phosphorylation of these proteins and the expression level of GAPDH in E6.1 Jurkat cells treated with anti-CD3 for 120 s are shown. B, The phosphorylation of PLC-γ1, along with the expression level of GAPDH, was determined by immunoblotting. For comparison, the phosphorylation of PLC-γ1 and the expression level of GAPDH in E6.1 Jurkat cells treated with anti-CD3 for 120 s are shown. C, The percent activation of E6.1 Jurkat cells at 120 s for PLC-γ1 in E6.1 and JVav Jurkat cells was determined. The mean ± SEM of three separate experiments for each time point was plotted and fit using Origin.
Effects of siItk on the phosphorylation kinetics of LAT and PLC-γ1
The experiments presented above have suggested that Itk is not required for the phosphorylation of LAT but appears to be necessary for the phosphorylation of tyrosine 783 on PLC-γ1. To more fully investigate the role of Itk in phosphorylation of these proteins, the expression of Itk was reduced by Itk-specific siRNA treatment of Jurkat cells. The treatment of Jurkat cells with Itk-specific siRNA resulted in an ∼70% reduction in Itk expression but had no effect on the expression of LAT or PLC-γ1 (Fig. 8D). Treatment of Jurkat cells with Itk-specific siRNA did not have an effect upon the kinetics or intensity of the phosphorylation of LAT at either tyrosine 132 or tyrosine 191 (Fig. 8, A and B, and data not shown), confirming that Itk is not involved in the phosphorylation of LAT. In contrast, in Jurkat cells treated with Itk-specific siRNA, tyrosine 783 on PLC-γ1 had slower initial phosphorylation kinetics and was more transiently phosphorylated compared with untreated cells (Fig. 8, A and C) or cells transfected with nonspecific siRNA (data not shown). The lack of complete inhibition of PLC-γ1 tyrosine 783 phosphorylation was likely due to the presence of residual Itk and normal levels of Rlk and Tec, two other members of the Tec family of kinases that are thought to phosphorylate PLC-γ1 (33, 37). These data show that reduced Itk expression leads to slower, more transient phosphorylation kinetics for tyrosine 783 on PLC-γ1, suggesting that Itk is required for the phosphorylation of this site.
FIGURE 8.
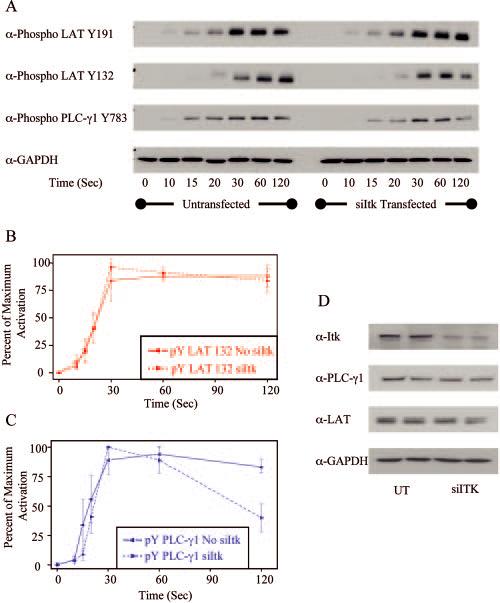
Effects of Itk siRNA on the phosphorylation of various signaling molecules in Jurkat cells. Jurkat cells untransfected or transfected with Itk-specific siRNA were treated with or without anti-CD3 for various times and the cellular proteins were separated by PAGE. A, The tyrosine phosphorylation of specific sites on LAT and PLC-γ1, along with the expression level of GAPDH, was determined by immunoblotting. B, The percentage of maximum phosphorylation for LAT tyrosine 132 in Jurkat cells was determined. The mean ± SEM of three separate experiments for each time point was plotted and fit using Origin. C, The percentage of maximum phosphorylation for PLC-γ1 tyrosine 783 in Jurkat cells was determined. The mean ± SEM of three separate experiments for each time point was plotted and fit using Origin. D, The expression levels of Itk and GAPDH were determined by immunoblotting in Jurkat cells untreated or treated with Itk-specific siRNA. Duplicate samples for both treatments are shown.
Discussion
In these studies, we have taken advantage of two useful sets of reagents: 1) various Jurkat cell lines, both WT cells and mutants that are lacking specific signaling proteins and 2) a number of Abs that recognize site-specific phosphorylations. Recently, the reliability of Jurkat cells as a model for T cell biology has been called into question. However, Jurkat T cells lines have proven to be an incredibly powerful tool in understanding TCR-mediated early signaling events (35). One criticism of Jurkat T cells is their lack of the lipid phosphatase phosphate and tensin homology deleted on chromosome 10. However, recent studies have shown that phosphate and tensin homology deleted on chromosome 10 expression has no effect on proximal signaling but instead appears to control basal levels of phospholipids (38). Also, as seen in this study and others, the proximal TCR-mediated signaling pathways investigated using Ab-stimulated Jurkat T cells have been largely recapitulated in studies using normal human and mouse T cells (35). Similarly, the discreet early signaling complexes that contain ZAP-70, LAT, PLC-γ1, and other signaling molecules seen in Jurkat T cells responding to stimulatory Ab-coated coverslips (39) are remarkably similar to the punctate localization of both phosphorylated tyrosine residues and ZAP-70 observed during the early times of normal peptide-MHC-TCR interactions (40, 41). These data suggest that Ab stimulation can recapitulate many of the early signaling events induced by peptide-MHC-TCR interactions. In fact, Ab stimulation of T cells has several advantages over Ab-coated coverslips/beads and/or Ag-pulsed APCs such as enabling the rapid, synchronized induction of the TCR, which allowed for the recognition of the differential phosphorylation of LAT tyrosines.
Along with the Jurkat cell lines, this study could not be completed without the availability of several phospho-specific Abs. However, these Abs and the immunoblotting technique used for these studies have a number of potential pitfalls that must be taken into account. The immunoblotting technique itself, specifically the potential nonlinear nature of the detection method using chemiluminescence and x-ray film, can lead to the introduction of substantial error into the final results if not properly controlled. To this end, both the number of cell equivalents examined and the exposures that were quantitated were chosen to fall within the linear range of the detection methods for the immunoblotting technique (data not shown). Another potential pitfall is that alterations in the affinities of the various phospho-specific Abs make it difficult to compare results between Abs. This would be true if the absolute values for the band intensity in a specific exposure were being compared. However, we eliminated this problem by normalizing the signal from the phospho-specific Ab to the level of protein expression of a specific housekeeping gene and then calculated a percentage of activation at each specific time point. This analysis method was used because the main effect of differences in the affinity of various Abs are changes in the percentage of ligand saturation, which, for immunoblotting, results in alterations in band intensities for a specific exposure time. By analyzing exposures with approximately similar intensities inside the linear range of the detection method and calculating a normalized percentage of activation at each time point in an individual experiment, the effects of Ab affinity are markedly reduced. These normalization methods have been used in numerous immunoblotting studies to compare the expression of different proteins and have recently been used in a protein microarray study that examined the kinetics of phosphorylation of proteins involved in proximal TCR-mediated signaling (42). In the end, this analysis method allows for the semiquantitative comparison of the phosphorylation kinetics of proteins involved in proximal TCR-mediated signaling.
One of the most interesting observations derived from this study is that the phosphorylation kinetics of two important LAT tyrosines was significantly different. We observed that LAT tyrosine 191 was phosphorylated rapidly with a time of 50% maximal stimulation of 19 s (Fig. 2, A and B). In contrast, the kinetics of LAT tyrosine 132 phosphorylation was much slower with a time of 50% maximal stimulation of 37 s (Fig. 2, A and B). In support of this observation in Jurkat cells, a similar lag in the phosphorylation kinetics of LAT tyrosines 191 and 132 was also observed in human PBLs (Fig. 3). Although a difference of ∼15–20 s appears trivial, this is actually a large change in kinetics for signaling pathways activated by TCR stimulation. All of the proteins examined in this study were maximally phosphorylated by 60 s in Jurkat cells, suggesting that changes in protein phosphorylation levels that occur in the initial burst of signaling induced by TCR activation occurs in a window of 30–45 s. In light of the rapid kinetics of early TCR-mediated signaling, a difference of 15–20 s is substantial.
The observation that LAT tyrosines have different phosphorylation kinetics leads to the next question, what are the kinases that directly phosphorylate specific LAT tyrosines? Previous studies have shown that ZAP-70, Itk, and Lck have the potential to phosphorylate LAT (13, 14). However, these studies used purified, recombinant kinases to phosphorylate purified LAT peptides, thereby suggesting only potential cellular LAT kinases (13, 14). This current study gives more insight into the kinases involved in the direct in vivo phosphorylation of specific LAT tyrosines. The kinetics of phosphorylation of LAT tyrosine 191 is similar to the activation of ZAP-70 and is nearly indistinguishable from the phosphorylation kinetics of another known ZAP-70 substrate, cCbl (Fig. 2, A and B) (43, 44). Also, LAT is not phosphorylated in P116 cells in which Lck, Fyn, and Pyk2 are still active (data not shown) but is phosphorylated normally in the J14 and JVav cells where Itk is not recruited to the LAT complex (33). Together, these data indicate that LAT tyrosine 191 is likely a direct in vivo substrate for ZAP-70 (Fig. 9) and is not appreciably phosphorylated by Lck or Itk. In contrast, the kinase that directly phosphorylates LAT tyrosine 132 is still not determined (Fig. 9). ZAP-70 does not appear to rapidly phosphorylate LAT tyrosine 132 since the kinetics of activation of this tyrosine does not closely follow the activation kinetics of this kinase (Fig. 2. B and C). Furthermore, the kinase for LAT tyrosine 132 does not appear to be Lck, Fyn, Pyk2, or Itk since the phosphorylation pattern of this site is similar to LAT tyrosine 191.
FIGURE 9.
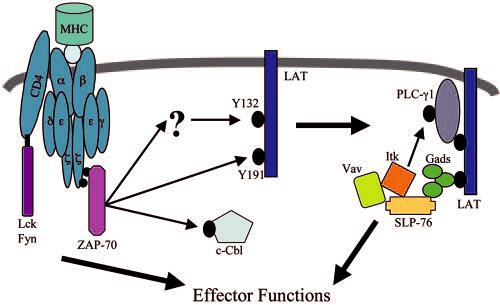
Model of early signal transduction in T cells. Upon TCR stimulation, activated ZAP-70 directly phosphorylates of LAT tyrosine 191 and indirectly mediates the phosphorylation of LAT tyrosine 132 via a mechanism that occurs with slower kinetics than LAT tyrosine 191. Once phosphorylated, LAT associates with several proteins, including PLC-γ1 and the SLP-76-Vav-Itk complex. The recruitment of these proteins to LAT results in the phosphorylation of tyrosine 783 on PLC-γ1, leading to the activation of this protein.
This leaves three possibilities that 1) LAT tyrosine 132 is phosphorylated by a yet undefined kinase, 2) LAT tyrosine 132 is differentially regulated by an unidentified phosphatase, or 3) prior modifications of LAT are required for LAT tyrosine 132 phosphorylation. The only other known tyrosine kinase that is rapidly activated upon TCR stimulation, other than those described above, is c-Abl (3, 4, 14, 45). However, a recent study has suggested that the TCR-stimulated activity of c-Abl is not dependent upon ZAP-70 (45), indicating that c-Abl is not involved in the phosphorylation of LAT tyrosine 132.
A second possibility is that LAT tyrosine 132 may be differentially regulated by an undefined tyrosine phosphatase. This event would not be detected by immunoblotting with phospho-specific Abs since this technique examines steady-state phosphorylation at an individual time point and does not take into account the turnover of tyrosine phosphorylation at these sites. There are several pieces of evidence that would indicate that the proteins investigated in this study are regulated by phosphatases during TCR-mediated signaling. Numerous tyrosine phosphatases are known be activated upon TCR stimulation (4, 46), including CD148, which specifically dephosphorylates LAT and PLC-γ1 compared with other proteins. Interestingly, our study has shown that SLP-76 is differentially regulated by dephosphorylation, a point that is discussed more fully below. Future experiments will have to address the role of tyrosine phosphatases in the regulation of LAT tyrosine 132 phosphorylation.
Finally, there is the possibility that prior modifications of LAT are required for the phosphorylation of LAT tyrosine 132. In support of this hypothesis, previous studies have shown that LAT tyrosine 132 is not phosphorylated on LAT proteins that contain only this tyrosine (11), suggesting that LAT tyrosine 132 requires a prior event to be phosphorylated. It is possible that aggregation and/or prior phosphorylation of LAT induces a conformational change that allows for the phosphorylation of LAT tyrosine 132 by ZAP-70 or an undefined kinase. Interestingly, the present study shows that LAT tyrosine 132 was still phosphorylated on a LAT 3YF mutant (Fig. 1B), indicating that the presence of these tyrosines is not required for LAT tyrosine 132 phosphorylation. This suggests that undefined sites on LAT, other than the last three C-terminal tyrosines, may be sufficient for the phosphorylation of LAT tyrosine 132, likely by inducing a conformational change in LAT that allows the kinase for LAT tyrosine 132 access to this site.
Physiologically, the processive phosphorylation of LAT may have substantial implications for TCR-mediated signaling. By linking the phosphorylation of LAT tyrosine 132 to a previous stimulatory event, likely the phosphorylation of another LAT tyrosine, PLC-γ1 activity will be tightly controlled both in unstimulated and stimulated T cells. In unstimulated T cells, the requirement for prior activation events for the phosphorylation of LAT tyrosine 132 would result in extremely low levels of basal phosphorylation of this site compared with other LAT tyrosines. Similarly, the processive phosphorylation of LAT would lead to the phosphorylation of LAT tyrosine 132 only in situations with sustained TCR activation, such as the association of the TCR with agonist peptide-bound MHC, and not during events with transient TCR stimulation, such as the interaction of the TCR with null or antagonist-bound MHC. In both cases, the delayed phosphorylation of LAT tyrosine 132 would tightly control the PLC-γ1 activity, a crucial function due to the importance of PLC-γ1 for the stimulation of downstream signaling pathways in T cells (3).
Along with examining the phosphorylation of LAT, this study investigated what adaptor proteins are required for the phosphorylation of tyrosine 783 on PLC-γ1 and suggests a possible in vivo kinase for this site. This study and others have shown that both LAT and ZAP-70 expression are required for total PLC-γ1 phosphorylation (9, 22, 23, 36), although this current study is the first to examine the requirement of these proteins for site-specific phosphorylation of PLC-γ1. Interestingly, SLP-76 was also required for the phosphorylation of tyrosine 783 on PLC-γ1 (Fig. 6B). The lack of SLP-76 does not appear to destabilize the interaction between PLC-γ1 and LAT since there is still association between these proteins in J14 cells (29). This suggests that the role of SLP-76 in the phosphorylation of tyrosine 783 of PLC-γ1 is to bring a kinase, such as Itk, to the LAT complex, data that are consistent with previous studies that have indicated that Itk is involved in the phosphorylation of tyrosine 783 on PLC-γ1 (32, 34). This hypothesis is supported by the observation that there is a substantial reduction in the phosphorylation of tyrosine 783 on PLC-γ1 in Jurkat cells deficient in Vav1 (Fig. 7, B and C), a protein that has recently been shown to be vital for the binding of Itk and SLP-76 (27). Similarly, reducing the level of Itk by ∼70% using siRNA specific for Itk resulted in slower, more transient phosphorylation kinetics of PLC-γ1 tyrosine 783 in cells treated with Itk-specific siRNA compared with untreated cells (Fig. 8, A and C). The actual role that Itk plays in the phosphorylation of tyrosine 783 on PLC-γ1 is still unknown. It is likely that Itk directly phosphorylates this site on PLC-γ1 but may also be involved in stabilizing the binding of PLC-γ1 to LAT. Itk has been shown to stabilize the binding of Vav and SLP-76 in a kinase-independent manner (28), suggesting that Itk can function both as a tyrosine kinase and an adaptor protein. In total, these data indicate that recruitment of both PLC-γ1 and the Itk/Vav1/SLP-76 complex to LAT is required for the phosphorylation of tyrosine 783 of PLC-γ1 (Fig. 9).
Interestingly, the examination in this current study of the early phosphorylation kinetics of SLP-76 suggests that this protein is differentially regulated compared with other proteins activated by TCR induction. We observed an ∼50% reduction in SLP-76 tyrosine 145 phosphorylation at 120 s compared with the maximal phosphorylation at 30 s (Fig. 2, A and C). This suggests that dephosphorylation of SLP-76 occurs more rapidly compared with other signaling molecules induced by TCR stimulation. However, there are still several unanswered questions. It is an open question as to whether other phosphorylation sites on SLP-76 are regulated with similar kinetics, which can only be answered when more site-specific Abs for SLP-76 become available. Also, it is interesting to speculate on what phosphatases differentially regulate SLP-76 compared with other signaling proteins and what the physiological function of the rapid dephosphorylation kinetics of SLP-76 is.
The use of phosphosite-specific Abs and Jurkat cells deficient in various signaling molecules allows for the investigation of the differential kinetics of activation of signaling proteins in human T cells along with the examination of the in vivo kinases for specific tyrosines on these proteins. The techniques used in this study have proven to be very robust, with multiple Jurkat cell lines showing similar activation kinetics for proteins such as LAT, ZAP-70, and PLC-γ1. These investigations have given new insights into the sequence and kinetics, along with the kinase specificity, of early signaling events that occur in human T cells after TCR stimulation, but have left open several interesting questions about the early activation kinetics of proteins important for T cell activation.
Acknowledgments
We thank Dr. Peter Munson for helpful advice on data analysis, Dr. Dana Mahadeo for technical assistance, and Dr. Pamela Schwartzberg for helpful discussions. We thank Dr. Arthur Weiss for providing the JCaM 2.5 and J14 cell lines and Dr. Robert Abraham for providing the P116 and JVav cell lines.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Abbreviations used in this paper: LAT, linker for activation of T cells; SH, Src homology; PLC, phospholipase C; SLP-76, Src homology 2 domain-containing leukocyte protein of 76 kDa; SOS, son of sevenless; siRNA, small interference RNA; WT, wild type.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Krogsgaard M, Huppa JB, Purbhoo MA, Davis MM. Linking molecular and cellular events in T-cell activation and synapse formation. Semin. Immunol. 2003;15:307–315. doi: 10.1016/j.smim.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Gao GF, Rao Z, Bell JI. Molecular coordination of αβ T-cell receptors and coreceptors CD8 and CD4 in their recognition of peptide-MHC ligands. Trends Immunol. 2002;23:408–413. doi: 10.1016/s1471-4906(02)02282-2. [DOI] [PubMed] [Google Scholar]
- 3.Samelson LE. Signal transduction mediated by the T cell antigen receptor: the role of adapter proteins. Annu. Rev. Immunol. 2002;20:371–394. doi: 10.1146/annurev.immunol.20.092601.111357. [DOI] [PubMed] [Google Scholar]
- 4.Mustelin T, Tasken K. Positive and negative regulation of T-cell activation through kinases and phosphatases. Biochem. J. 2003;371:15–21. doi: 10.1042/BJ20021637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nel AE. T-cell activation through the antigen receptor. Part 1: signaling components, signaling pathways, and signal integration at the T-cell antigen receptor synapse. J. Allergy Clin. Immunol. 2002;109:758–770. doi: 10.1067/mai.2002.124259. [DOI] [PubMed] [Google Scholar]
- 6.Wange RL, Guitian R, Isakov N, Watts JD, Aebersold R, Samelson LE. Activating and inhibitory mutations in adjacent tyrosines in the kinase domain of ZAP-70. J. Biol. Chem. 1995;270:18730–18733. doi: 10.1074/jbc.270.32.18730. [DOI] [PubMed] [Google Scholar]
- 7.Chan AC, Dalton M, Johnson R, Kong GH, Wang T, Thoma R, Kurosaki T. Activation of ZAP-70 kinase activity by phosphorylation of tyrosine 493 is required for lymphocyte antigen receptor function. EMBO J. 1995;14:2499–2508. doi: 10.1002/j.1460-2075.1995.tb07247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Bartolo V, Mege D, Germain V, Pelosi M, Dufour E, Michel F, Magistrelli G, Isacchi A, Acuto O. Tyrosine 319, a newly identified phosphorylation site of ZAP-70, plays a critical role in T cell antigen receptor signaling. J. Biol. Chem. 1999;274:6285–6894. doi: 10.1074/jbc.274.10.6285. [DOI] [PubMed] [Google Scholar]
- 9.Williams BL, Schreiber KL, Zhang W, Wange RL, Samelson LE, Leibson PJ, Abraham RT. Genetic evidence for differential coupling of Syk family kinases to the T-cell receptor: reconstitution studies in a ZAP-70-deficient Jurkat T-cell line. Mol. Cell. Biol. 1998;18:1388–1399. doi: 10.1128/mcb.18.3.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 11.Zhu M, Janssen E, Zhang W. Minimal requirement of tyrosine residues on linker for activation of T cells in T cell activation and thymocyte development. J. Immunol. 2003;170:325–333. doi: 10.4049/jimmunol.170.1.325. [DOI] [PubMed] [Google Scholar]
- 12.Sommers CL, Menon RK, Grinberg A, Zhang W, Samelson LE, Love PE. Knock-in mutation of the distal four tyrosines of linker for activation of T cells blocks murine T cell development. J. Exp. Med. 2001;194:135–142. doi: 10.1084/jem.194.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez-Villar JJ, Whitney GS, Sitnick MT, Dunn RJ, Venkatesan S, O'Day K, Schieven GL, Lin TA, Kanner SB. Phosphorylation of the linker for activation of T-cells by Itk promotes recruitment of Vav. Biochemistry. 2002;41:10732–10740. doi: 10.1021/bi025554o. [DOI] [PubMed] [Google Scholar]
- 14.Paz PE, Wang S, Clarke H, Lu X, Stokoe D, Abo A. Mapping the ZAP-70 phosphorylation sites on LAT (linker for activation of T cells) required for recruitment and activation of signalling proteins in T cells. Biochem. J. 2001;356:461–471. doi: 10.1042/0264-6021:3560461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu SK, Fang N, Koretzky GA, McGlade CJ. The hematopoietic-specific adaptor protein gads functions in T-cell signaling via interactions with the SLP-76 and LAT adaptors. Curr. Biol. 1999;9:67–75. doi: 10.1016/s0960-9822(99)80017-7. [DOI] [PubMed] [Google Scholar]
- 16.Asada H, Ishii N, Sasaki Y, Endo K, Kasai H, Tanaka N, Takeshita T, Tsuchiya S, Konno T, Sugamura K. Grf40, A novel Grb2 family member, is involved in T cell signaling through interaction with SLP-76 and LAT. J. Exp. Med. 1999;189:1383–1390. doi: 10.1084/jem.189.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Law CL, Ewings MK, Chaudhary PM, Solow SA, Yun TJ, Marshall AJ, Hood L, Clark EA. GrpL, a Grb2-related adaptor protein, interacts with SLP-76 to regulate nuclear factor of activated T cell activation. J. Exp. Med. 1999;189:1243–1354. doi: 10.1084/jem.189.8.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang W, Trible RP, Zhu M, Liu SK, McGlade CJ, Samelson LE. Association of Grb2, Gads, and phospholipase C-γ1 with phosphorylated LAT tyrosine residues: effect of LAT tyrosine mutations on T cell antigen receptor-mediated signaling. J. Biol. Chem. 2000;275:23355–23361. doi: 10.1074/jbc.M000404200. [DOI] [PubMed] [Google Scholar]
- 19.Lin J, Weiss A. Identification of the minimal tyrosine residues required for linker for activation of T cell function. J. Biol. Chem. 2001;276:29588–29595. doi: 10.1074/jbc.M102221200. [DOI] [PubMed] [Google Scholar]
- 20.Houtman JC, Higashimoto Y, Dimasi N, Cho S, Yamaguchi H, Bowden B, Regan C, Malchiodi EL, Mariuzza R, Schuck P, et al. Binding specificity of multiprotein signaling complexes is determined by both cooperative interactions and affinity preferences. Biochemistry. 2004;43:4170–4178. doi: 10.1021/bi0357311. [DOI] [PubMed] [Google Scholar]
- 21.Zhang W, Sommers CL, Burshtyn DN, Stebbins CC, DeJarnette JB, Trible RP, Grinberg A, Tsay HC, Jacobs HM, Kessler CM, et al. Essential role of LAT in T cell development. Immunity. 1999;10:323–326. doi: 10.1016/s1074-7613(00)80032-1. [DOI] [PubMed] [Google Scholar]
- 22.Finco TS, Kadlecek T, Zhang W, Samelson LE, Weiss A. LAT is required for TCR-mediated activation of PLCγ1 and the Ras pathway. Immunity. 1998;9:617–626. doi: 10.1016/s1074-7613(00)80659-7. [DOI] [PubMed] [Google Scholar]
- 23.Zhang W, Irvin BJ, Trible RP, Abraham RT, Samelson LE. Functional analysis of LAT in TCR-mediated signaling pathways using a LAT-deficient Jurkat cell line. Int. Immunol. 1999;11:943–950. doi: 10.1093/intimm/11.6.943. [DOI] [PubMed] [Google Scholar]
- 24.Irvin BJ, Williams BL, Nilson AE, Maynor HO, Abraham RT. Pleiotropic contributions of phospholipase C-γ1 (PLC-γ1) to T-cell antigen receptor-mediated signaling: reconstitution studies of a PLC-γ1-deficient Jurkat T-cell line. Mol. Cell. Biol. 2000;20:9149–9161. doi: 10.1128/mcb.20.24.9149-9161.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myung PS, Derimanov GS, Jordan MS, Punt JA, Liu QH, Judd BA, Meyers EE, Sigmund CD, Freedman BD, Koretzky GA. Differential requirement for SLP-76 domains in T cell development and function. Immunity. 2001;15:1011–1026. doi: 10.1016/s1074-7613(01)00253-9. [DOI] [PubMed] [Google Scholar]
- 26.Kumar L, Pivniouk V, de la Fuente MA, Laouini D, Geha RS. Differential role of SLP-76 domains in T cell development and function. Proc. Natl. Acad. Sci. USA. 2002;99:884–889. doi: 10.1073/pnas.022619199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reynolds LF, Smyth LA, Norton T, Freshney N, Downward J, Kioussis D, Tybulewicz VL. Vav1 transduces T cell receptor signals to the activation of phospholipase C-γ1 via phosphoinositide 3-kinase-dependent and -independent pathways. J. Exp. Med. 2002;195:1103–1114. doi: 10.1084/jem.20011663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dombroski D, Houghtling RA, Labno CM, Precht P, Takesono A, Caplen NJ, Billadeau DD, Wange RL, Burkhardt JK, Schwartzberg PL. Kinase-independent functions for Itk in TCR-induced regulation of Vav and the actin cytoskeleton. J. Immunol. 2005;174:1385–1392. doi: 10.4049/jimmunol.174.3.1385. [DOI] [PubMed] [Google Scholar]
- 29.Yablonski D, Kuhne MR, Kadlecek T, Weiss A. Uncoupling of nonreceptor tyrosine kinases from PLC-γ1 in an SLP-76-deficient T cell. Science. 1998;281:413–416. doi: 10.1126/science.281.5375.413. [DOI] [PubMed] [Google Scholar]
- 30.Schaeffer EM, Debnath J, Yap G, McVicar D, Liao XC, Littman DR, Sher A, Varmus HE, Lenardo MJ, Schwartzberg PL. Requirement for Tec kinases Rlk and Itk in T cell receptor signaling and immunity. Science. 1999;284:638–641. doi: 10.1126/science.284.5414.638. [DOI] [PubMed] [Google Scholar]
- 31.Liu KQ, Bunnell SC, Gurniak CB, Berg LJ. T cell receptor-initiated calcium release is uncoupled from capacitative calcium entry in Itk-deficient T cells. J. Exp. Med. 1998;187:1721–1727. doi: 10.1084/jem.187.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller AT, Wilcox HM, Lai Z, Berg LJ. Signaling through Itk promotes T helper 2 differentiation via negative regulation of T-bet. Immunity. 2004;21:67–80. doi: 10.1016/j.immuni.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 33.Lucas JA, Miller AT, Atherly LO, Berg LJ. The role of Tec family kinases in T cell development and function. Immunol. Rev. 2003;191:119–138. doi: 10.1034/j.1600-065x.2003.00029.x. [DOI] [PubMed] [Google Scholar]
- 34.Wilcox HM, Berg LJ. Itk phosphorylation sites are required for functional activity in primary T cells. J. Biol. Chem. 2003;278:37112–37121. doi: 10.1074/jbc.M304811200. [DOI] [PubMed] [Google Scholar]
- 35.Abraham RT, Weiss A. Jurkat T cells and development of the T-cell receptor signalling paradigm. Nat. Rev. Immunol. 2004;4:301–308. doi: 10.1038/nri1330. [DOI] [PubMed] [Google Scholar]
- 36.Williams BL, Irvin BJ, Sutor SL, Chini CC, Yacyshyn E, Bubeck Wardenburg J, Dalton M, Chan AC, Abraham RT. Phosphorylation of Tyr319 in ZAP-70 is required for T-cell antigen receptor-dependent phospholipase C-γ1 and Ras activation. EMBO J. 1999;18:1832–1844. doi: 10.1093/emboj/18.7.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomlinson MG, Kane LP, Su J, Kadlecek TA, Mollenauer MN, Weiss A. Expression and function of Tec, Itk, and Btk in lymphocytes: evidence for a unique role for Tec. Mol. Cell. Biol. 2004;24:2455–2466. doi: 10.1128/MCB.24.6.2455-2466.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seminario M-C, Precht P, Bunnell SC, Warren SE, Morris CM, Taub D, Wange RL. PTEN permits acute increases in D3-phosphoinositide levels following TCR stimulation but inhibits distal signaling events by reducing the basal activity of Akt. Eur. J. Immunol. 2004;34:3165–3175. doi: 10.1002/eji.200425206. [DOI] [PubMed] [Google Scholar]
- 39.Bunnell SC, Hong DI, Kardon JR, Yamazaki T, McGlade CJ, Barr VA, Samelson LE. T cell receptor ligation induces the formation of dynamically regulated signaling assemblies. J. Cell Biol. 2002;158:1263–1275. doi: 10.1083/jcb.200203043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee KH, Dinner AR, Tu C, Campi G, Raychaudhuri S, Varma R, Sims TN, Burack WR, Wu H, Wang J, et al. The immunological synapse balances T cell receptor signaling and degradation. Science. 2003;302:1218–1222. doi: 10.1126/science.1086507. [DOI] [PubMed] [Google Scholar]
- 41.Freiberg BA, Kupfer H, Maslanik W, Delli J, Kappler J, Zaller DM, Kupfer A. Staging and resetting T cell activation in SMACs. Nat. Immunol. 2002;3:911–917. doi: 10.1038/ni836. [DOI] [PubMed] [Google Scholar]
- 42.Chan SM, Ermann J, Su L, Fathman CG, Utz PJ. Protein microarrays for multiplex analysis of signal transduction pathways. Nat. Med. 2004;10:1390–1396. doi: 10.1038/nm1139. [DOI] [PubMed] [Google Scholar]
- 43.Fournel M, Davidson D, Weil R, Veillette A. Association of tyrosine protein kinase Zap-70 with the protooncogene product p120c-cbl in T lymphocytes. J. Exp. Med. 1996;183:301–306. doi: 10.1084/jem.183.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deckert M, Elly C, Altman A, Liu YC. Coordinated regulation of the tyrosine phosphorylation of Cbl by Fyn and Syk tyrosine kinases. J. Biol. Chem. 1998;273:8867–8874. doi: 10.1074/jbc.273.15.8867. [DOI] [PubMed] [Google Scholar]
- 45.Zipfel PA, Zhang W, Quiroz M, Pendergast AM. Requirement for Abl kinases in T cell receptor signaling. Curr. Biol. 2004;14:1222–1231. doi: 10.1016/j.cub.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 46.Mustelin T, Alonso A, Bottini N, Huynh H, Rahmouni S, Nika K, Louis-dit-Sully C, Tautz L, Togo SH, Bruckner S, et al. Protein tyrosine phosphatases in T cell physiology. Mol. Immunol. 2004;41:687–700. doi: 10.1016/j.molimm.2004.04.015. [DOI] [PubMed] [Google Scholar]


