Abstract
In the late 1970s/early 1980s, Baddeley and colleagues conducted a series of experiments investigating the role of eye movements in visual working memory. Although only described briefly in a book (Baddeley, 1986), these studies have influenced a remarkable number of empirical and theoretical developments in fields ranging from experimental psychology to human neuropsychology to nonhuman primate electrophysiology. This paper presents, in full detail, three critical studies from this series, together with a recently performed study that includes a level of eye movement measurement and control that was not available for the older studies. Together, the results demonstrate several facts about the sensitivity of visuospatial working memory to eye movements. First, it is eye movement control, not movement per se, that produces the disruptive effects. Second, these effects are limited to working memory for locations, and do not generalize to visual working memory for shapes. Third, they can be isolated to the storage/maintenance components of working memory (e.g., to the delay period of the delayed-recognition task). These facts have important implications for models of visual working memory.
In the past three decades, a considerable amount of effort in cognitive psychology, neuropsychology, and cognitive neuroscience has been devoted to elucidating the theoretical and mechanistic underpinnings of working memory. One fruitful development has been the functional partitioning of working memory for visually perceived stimuli into object (i.e., the appearance of objects and visual arrays) and spatial/sequential components (Della Sala, Gray, Baddeley, Allamano, & Wilson, 1999; Logie, 1995; Smith et al., 1995; Tresch, Sinnamon, & Seamon, 1993), a development that has evolved from the observation by Goldman-Rakic (1987; 1990) that the functional organization of visual working memory may mirror the what/where organization of the visual system (Ungerleider & Mishkin, 1982). Several years prior to these developments, however, a series of studies was carried out by Baddeley and colleagues to explore possible functional links between visuospatial working memory (not yet explicitly partitioned into visual and spatial) and the motor system. Early among these were demonstrations that concurrent tracking – of a pursuit rotor (Baddeley, Grant, Wight, & Thomson, 1975) and of an overhead swinging pendulum (Baddeley & Lieberman, 1980) – selectively interfered with the spatial version of the Brooks (1967) memory span task. These results pointed to a critical spatial component for visual working memory, which led to a consideration of the role of eye movements in establishing and maintaining a spatial frame of reference, which, in turn, led to the performance of the experiments that are presented here as Experiments 1–3. These experiments were performed between 1978 and 1983, while three of the authors, Baddeley, Idzikowski, and Logie, were at the Medical Research Council Applied Psychology Unit in Cambridge, UK.
Until now, the results of these three eye-movement experiments have been known primarily through their discussion in Baddeley’s 1986 book Working Memory. Although they were only briefly summarized over a few pages in the book, they have influenced a considerable amount of subsequent work in many domains. This includes studies of the disruptive effects of secondary motor activity on spatial working memory performance (e.g., concurrent finger tapping (Farmer, Berman, & Fletcher, 1986; Salway & Logie, 1995; Smyth, Pearson, & Pendleton, 1988), pointing (Hale, Myerson, Rhee, Weiss, & Abrams, 1996), eye movements (Baddeley, 1986; Hale et al., 1996; Lawrence, Myerson, Oonk, & Abrams, 2001; Pearson & Sahraie, 2003), and arm movements (Baddeley et al., 1980; Lawrence et al., 2001; Logie & Marchetti, 1991; Quinn & Ralston, 1986), studies of cognitive deficits among various patient groups, particularly Parkinson’s disease (PD, e.g., Cooper, Sagar, Jordan, Harvey, & Sullivan, 1991; Fournet, Moreaud, Roulin, Naegele, & Pellat, 1996; Hodgson, Dittrich, Henderson, & Kennard, 1999; Ketcham, Hodgson, Kennard, & Stelmach, 2003; Postle, Jonides, Smith, Corkin, & Growdon, 1997; Postle, Locascio, Corkin, & Growdon, 1997), and autism (Koczat, Rogers, Pennington, & Ross, 2002) studies of the electrophysiology of delay period activity of the frontal eye fields in nonhuman primates (Balan & Ferrera, 2003a, 2003b), and theoretical developments in working memory (e.g., Baddeley & Logie, 1999; Logie, 1995, 2003; Postle & D’Esposito, 2003). In view of the enduring influence of the eye-movement studies described by Baddeley (1986), we believe it to be important that a thorough description of their methods and results be accessible in the archival literature. One reason that they have not been published in their entirety until now has been a concern that they did not include satisfactory control and measurement of eye movements. The present paper, therefore, supplements the three original studies with a fourth study that acts as a complement to the original work by, among other factors, including a level of eye-position monitoring that was not available for the original studies.
The backdrop for Experiments 1–3 was the well established evidence that imagery plays an important part in human memory (e.g., Paivio, 1969, 1971), and that images result from the activation of perceptual systems in the absence of sensory input. Further, it had been suggested (e.g., Hebb, 1968) that eye movements or their control systems may play an important intermediate role in imagery. The empirical evidence for this idea, however, was inconclusive (Brown, 1968; Hiscock & Bergstrom, 1981). Further complicating the question of a functional role for eye movements in imagery was the possibility that eye movements could have both involuntary and voluntary components, each possibly having a different effect. With these considerations, one could imagine at least three ways in which eye movements could be involved in imagery.
Eye movements themselves may play an intermediate role in the formation, “scanning” or maintenance of images. If so, then disruption by the induction of involuntary eye movements should selectively interfere with tasks involving imagery.
The voluntary control of eye movements and the formation, maintenance or “scanning” of images may share common processing capacities, in which case involuntary eye movements should not disrupt performance on imagery tasks. Concurrent voluntary controlled eye movements should, however, produce disruption.
Eye movements or their control processes may only be involved in parts of the imagery process. For instance if, as Kosslyn and Shwartz (1981) had suggested, images are “scanned” in a similar way to a real visual scene, then it is possible that eye movements are not involved in the formation of images, but only in subsequent processing of imaginal information. Alternatively, eye movements may only be involved in the formation of an image. Neisser’s (1976) view of imagery, that images are the products of anticipatory phases in perception and are representations of potentially perceivable objects in the real world, would seem to imply this.
The experiments presented here were designed to test the role of eye movements in imagery, and to differentiate between some of the possibilities outlined above. The methods adopted for them followed from previous studies that had demonstrated that tasks involving imagery can be selectively disrupted by concurrent tasks that involve spatial information processing (Baddeley, Grant, Wight, & Thomson, 1973; Baddeley et al., 1980; Brooks, 1967, 1968). The approach was to investigate the role of eye movements in visual working memory by disrupting or controlling eye movements during a task involving imagery, and determining whether this influenced performance on that task, without affecting performance on a similar task that involved little or no imagery. One attempt that preceded the present experiments is discussed in some detail in Baddeley’s 1986 book (pp. 116–118). In it, Nancy Vye in the laboratory of John Bransford (then at Vanderbilt University) attempted to disrupt imagery selectively by inducing optokinetic nystagmus. The method that they used — requiring participants to watch a moving pattern on a screen — however, only produced nystagmic eye movements in 40 out of 60 participants, and the results were inconclusive (Vye, 1979).
The first experiment in this report was designed to test whether performance on a task involving imagery could be selectively disrupted by concurrent involuntary eye movements in the absence of any visuospatial sensory input. The involuntary eye movements were a postrotational nystagmus, which is thought to be mediated by a vestibulo-ocular reflex. The nystagmus was induced by spinning participants in an electrically powered revolving chair. If eye movements themselves are involved in imagery then this would be expected to disrupt selectively performance on a memory task involving imagery, whilst having little or no effect on tasks employing mainly verbal encoding.
The second experiment tested the possibility that the control of voluntary eye movements and imagery share common processing capacity by instructing participants to watch a moving spot on a monitor. If eye movement control and imagery share common processing capacity, this would be expected to selectively disrupt performance on a task involving imagery, again having little or no effect on a similar task involving verbal coding. This experiment also tested the possibility that processing of visuospatial information caused by changes in the visual field could selectively disrupt performance on an imagery task, and also tested whether preventing free eye movements (by fixation of a stationary object) could do so.
The third experiment was designed both to replicate and extend the findings of the second experiment. It looked at the possibility of presentation-recall differences in the selective disruption effect found, and used a similar method, with participants again watching a moving spot on a monitor.
The fourth experiment employed a procedure – delayed recognition – and a design – two types of memory material and three levels of distraction – that differed from those used in Experiments 1–3, and so could address several questions that the first three experiments had left unresolved. One of these was clarifying whether maintenance-related processes, independent of encoding and response, are sensitive to disruption by eye movements. A second was that, by employing a level of eye-movement measurement that was not available for Experiments 1–3, Experiment 4 could rule out the possibility that the selective sensitivity of spatial working memory performance to concurrent saccades is confounded by differential distracting saccadic activity during nonspatial vs. spatial trials. A third was to confirm that the sensitivity of spatial working memory performance to concurrent saccades is indeed a selective effect, by including a second type of distraction (word naming) that was expected to disrupt selectively working memory for the second type of memoranda (shapes). Finally, a question that only became fully appreciable several years after Experiments 1–3 had been performed, was whether eye movements have differential effects on the two major classes of visuospatial working memory: memory for objects; and memory for locations and pathways between those locations. Some of these questions have been addressed, individually, in studies published after 1986, but never have they all been accounted for in a single repeated measures design.
Experiment 1
If eye movements themselves play an important intermediate role in visuospatial imagery, then inducing post-rotational nystagmus should disrupt performance on a task involving visuospatial imagery, whilst having little or no effect on a task that involves mainly verbal coding. In this experiment participants performed the short-term memory tasks that had been shown by Brooks (1967) and Baddeley and Lieberman (1980) to emphasize either spatial or verbal coding. Both tasks were performed by participants either with or without post-rotational nystagmus.
Method
Participants
Eight Cambridge University undergraduate and graduate students served as participants.
Apparatus
The participant was seated in a chair which could be rotated at about 12 revolutions per minute by means of an electric motor. AC electro-oculography (accuracy 3° ±1.5°) was employed to provide a quantitative measure of any nystagmus induced. Silver-silver chloride electrodes were attached at the external canthi of the two eyes. The signal was analyzed by a PDP 11/60 computer and also displayed on an oscilloscope. A tape recorder was used to present the memory material.
Material
This comprised the two types of memory material used by Brooks (1967) and Baddeley and Lieberman (1980, Experiments 1 and 2). The first type of sequence, the “spatial” material, was visualized, and the second type, the “nonsense” material, was formally equivalent but not easily visualized. For the spatial material, the participant was told to imagine a 4x4 matrix, and was taught that one particular square (the second square on the second row) would always constitute the starting square. Each message described the location of the digits 1 to 8 within the matrix, and the digit 1 always appeared in the starting square, with successive digits appearing in adjacent squares. The message was always presented in the sequence 1 to 8, and so it was always possible to remember the message in terms of a path through the matrix. The message described the position of each digit as being located either above, below, to the right or to the left of the previous digit’s location (e.g., In the starting square put a 1…In the next square to the right put a 2…In the next square down put a 3… etc.). The nonsense messages were formally equivalent except that the words “up” and “down” were replaced with “good” and “bad”, and the words “left” and “right” by “slow” and “quick” (e.g., In the starting square put a 1…In the next square to the good put a 2…In the next square to the bad put a 3… etc.). Each sentence in a message lasted approximately 1.5 sec.
Baddeley and Brooks both found that the spatial messages were easier than the nonsense ones, but found that reducing the length of the nonsense messages from eight to six items made the probability of correct reproduction approximately equal for the two sets. Baddeley and Lieberman (1980) had also shown that this reduction in number of items was not responsible for any differential impairment of performance on the spatial task when the tasks were carried out with another concurrent task. For these reasons, eight-digit sequences were used for the spatial material, and six-digit sequences for the nonsense material.
Design
All participants were tested on four conditions comprising the two types of material, tested with or without spinning to induce post-rotational nystagmus. Participants received eight messages in each of the four conditions. Eight students served as participants; one dropped out part way through testing (he was feeling nauseated) and was replaced. Testing was carried out over two days to eliminate any carry-over effects of the spinning due to nausea. Half of the participants began with the “spin” condition, and half with the “no-spin” condition. Spatial and nonsense messages were alternated with half of the participants receiving a spatial message first, the rest receiving a nonsense message first.
The messages were grouped into two blocks of eight and each participant received the equivalent nonsense and spatial messages on different days. The design was balanced for material, order of conditions and for spatial or nonsense message first.
Procedure
Participants were given six practice trials on each type of memory task unless they reached a criterion of two successive perfect runs in less than six trials. The importance of using the correct strategy was emphasized.
“Spin” condition.
The participant was seated in the motor driven chair. The EOG apparatus was calibrated by asking participants to move their eyes between two points separated by a known angle. Participants were then rotated for 45 sec. During this time they were asked to perform a simple arithmetic task (i.e., repeated subtraction of a small number from a large one) in order to control their activity during the interval between successive messages. After the 45 sec of rotation the motor of the chair was stopped and the participant was tested on either a nonsense or a spatial message. The EOG was sampled for 15 sec to determine the magnitude of nystagmus induced during the presentation and recall of the memory task. Immediately after the participant had finished the recall the chair was restarted and the procedure was repeated with alternating spatial and nonsense tasks. After 8 trials participants were allowed a 2 min break. Participants were instructed to keep their eyes closed throughout the experiment apart from the break halfway through.
“No spin” condition.
The procedure was identical except that participants were stationary during the 45 sec of mental arithmetic and no EOG readings were taken.
Results and Discussion
Performance on the memory tasks was scored in terms of the number of sequences in which reproduction was perfect. (Maximum score = 8 in each condition). The nystagmus occurring on each trial in the “spin” condition was rated as being “good” (>10°); “acceptable” (10°–5°); or “poor” (<5°). Across all participants there were 28 trials when nystagmus was “good”, 72 trials when it was “acceptable”, and 28 trials when it was “poor”. One participant failed to produce any nystagmus of greater than 5° on any trial, accounting for 16 of the “poor” nystagmus trials.
The results indicated that spatial task performance was slightly numerically higher in the Spin than the No-spin condition, whereas the opposite trend was seen with the nonsense task (Table 1). Two way analyses of variance on both the complete and modified data revealed no significant differences across conditions, and no interaction between type of memory material and spin condition (F < 1 in all cases for the complete data and F < 1 in all cases for the modified data).
Table 1.
Mean Memory Task Scores (% correct sequences) in Experiment 1. (Figures in brackets indicate scores when trials with nystagmus < 5° are eliminated.)
| Spatial
|
Nonsense
|
|||
|---|---|---|---|---|
| Condition | % correct | S.D. | % correct | S.D. |
| Spin | 73.5 | 21.1 | 57.8 | 28.6 |
| (78.6 | 24.0) | (69.3 | 27.6) | |
| No-spin | 70.4 | 24.1 | 76.6 | 14.6 |
The results thus showed that the induction of post-rotational nystagmus does not significantly impair performance on the memory tasks. There was no trace of the interaction that would occur if the induction of involuntary eye movements interfered selectively with imagery. If anything, performance was more impaired on the nonsense task than on the imagery task by the “spin” condition. The results would therefore seem to imply that eye movements per se do not play any important intermediate role in imagery.
Experiment 2
The results of Experiment 1 did not support the idea that involuntary eye movements can disrupt imagery. However it was possible that although eye movements themselves do not play an intermediate role in visuospatial imagery, processes involved in the voluntary control of eye movements and imagery processes may share common capacity. In this case it would be expected that having to make eye movements under voluntary control would selectively interfere with visuospatial imagery, but that involuntary induced eye movements would not. It is however inevitable that when the eyes move and are open, a change in the input of visuospatial information occurs, and processing of this information, rather than the voluntary control of the movements of the eyes may share capacity with processing of imaginal information. In addition it is possible that having to hold the eyes stationary or fixating may disrupt imagery.
In the second experiment, therefore, a four-way experimental design was used, with the eyes and/or the visual field moving. Participants were required to watch an object on a monitor. The object was either moving or stationary, corresponding to a changing or fixed visual input. There was also a control condition in which participants were allowed free eye movements. If imagery involves some processing capacity which is also involved in the control of voluntary eye movements then there should be an interaction between type of memory task and eye movement condition, with performance on the spatial material being selectively disrupted in conditions when the eyes move. On the other hand, if it is the monitoring of changes in the visual field and subsequent processing of visuospatial information that shares common capacity with processing of spatial imaginal information, then the interaction expected would be that performance on the spatial tasks would be selectively disrupted when there was movement in the visual field, with or without any eye movements. If eye movements themselves are necessary for imagery then it would be expected that a condition where no eye movement is permitted would produce selective disruption for the spatial tasks, when compared with a free eye movement condition.
Method
Participants
Twenty four Cambridge University undergraduate and graduate students served as participants.
Apparatus
Participants were seated about 1 m. in front of a 65 cm monitor upon which a moving display was generated by an Apple II microprocessor. The display contained a small bell shape (1 cm diameter) on a background of dots. The bell could be either stationary in the centre of the screen or moving sinusoidally across the screen, with a period of about two seconds, and from top to bottom. The side to side movement subtended approximately 12° of visual angle. It took approximately 12 sec to move from the top to the bottom of the screen. When it reached the bottom of the screen, it reappeared immediately at the top. The background could either be stationary or moving from left to right with sinusoidal velocity similar to that of the bell. The background and the bell were in phase. The bell shape could be changed by the experimenter to a readily discriminable square. The participant held a button with which to respond when this occurred. The reaction time (RT) to respond to the shape change was measured by the Apple II using interrupts (Idzikowski, 1982). The room was darkened apart from a low level of ambient lighting to enable the experimenters to record results and manipulate apparatus. The monitor and participant were surrounded by a cloth shield to restrict the visual field and to minimize any distracting external stimuli. EOG was again employed to enable the experimenters to check whether a participant’s eyes were moving. As in the first experiment, a tape recorder was used to present the memory material.
Material
The material used was the same as that used in the first experiment. Again, spatial and nonsense messages were alternated, and in a given condition participants received the equivalent spatial and nonsense messages randomly rearranged.
Design
Four experimental conditions plus a control condition were used. Each participant received eight nonsense and eight spatial messages in each condition. The control condition was split into two halves; half occurring before the other conditions, and the other half afterwards. In this condition both the bell and the background were stationary, but participants were not required to fixate on the bell, and were allowed to move their eyes as they wished. The four experimental conditions were as follows:
#1 Both bell and background stationary.
#2 Bell stationary and background moving.
#3 Bell moving and background stationary.
#4 Both bell and background moving.
The order of the conditions was determined by a Latin square.
The material was grouped into lists of eight spatial messages and their equivalent nonsense messages. The four lists used in the experimental conditions were balanced across conditions in a Latin square design in view of the possibility that some of the messages may be easier to remember or visualize than others.
Reaction times were tested on half of the trials, i.e. on eight RTs per condition. Of these eight, two were tested during presentation of spatial messages (Spatial input RT), two during presentation of nonsense messages (Verbal input RT), two during recall of spatial messages (Spatial output RT) and two during recall of nonsense messages (Verbal output RT).
The eight reaction times occurred in random positions during a given condition, and in different orders across conditions. The order remained constant within a condition across participants. No reaction times were tested during the control conditions.
Procedure
Participants were seated in front of the monitor. The two types of memory task were explained to them and the importance of using the correct strategy was emphasized. participants were given six practice trials on each type of memory task unless they reached a criterion of two successive perfect runs in less than six trials. EOG electrodes were attached, and the oscilloscope reading checked to see if the experimenter could tell whether the participant’s eyes were moving.
Participants were given two practice trials on the RT task. They were told that during the experimental conditions they should watch the bell at all times and were told that the purpose of the RT test and the EOG was to ensure that they were doing so.
The cloth shield was placed around the participant and the monitor, the lights dimmed, and a #1 display put on the screen (stationary bell and stationary background). The participant was told that in this control condition there would be no RT trials, and that s/he need not look at the bell, and could “do what you like with your eyes.”
After these initial control trials were completed, participants were told that from now on they should watch the bell at all times. Before each of the four experimental conditions they were given two practice trials on each type of message. They were then given the sixteen messages comprising the memory material for that condition.
Between each experimental condition or control condition there was a brief break while the display on the monitor changed.
After the four experimental conditions, participants completed the eight trials comprising the final control trials.
Results
Performance on the memory tasks was scored in terms of the number of sequences in which reproduction was perfect. For spatial material, accuracy was highest in the free eye movement condition, only slightly lower for the two eyes fixed conditions (i.e., #1 and #2), and markedly lower for the two eyes moving conditions (i.e., #1 and #2). A qualitatively similar pattern was seen with nonsense material, but with a much smaller difference between the eyes fixed and eyes moving conditions.
A multifactor analysis of variance, with factors eye movement, background movement, type of memory task, and presence of a reaction time, was used to analyze the data. The analysis showed a highly significant main effect of eye movement (F1,23 = 24.49, p < 0.001) with performance dropping from 69.3% to 56.5% when the eyes moved. This effect was modified by a significant interaction with type of memory material. (F1,23 = 9.68, p < 0.01). The Newman-Keuls test indicated that the interaction was due to a decrease in performance on the spatial task from 70.6% to 51.6% when the eyes moved (q2 = 1.988, df = 46, p < 0.05).
The analysis of variance also showed that there was no significant difference in the overall level of performance on the spatial and nonsense tasks (F < 1). There was also no significant effect of background movement (F < 1), nor did this interact with eye movement (F < 1). The presence of a reaction time trial had a marginally significant effect on performance on the memory tasks (F1,23 = 3.90, 0.1 > p > 0.05) but this did not interact with type of memory material. There was also a significant three way interaction between the presence of a reaction time, background movement and type of memory material (F1,23 = 6.15, p < 0.05) although the implication of this interaction is unclear.
Another 2 x 2 analysis of variance between the free eye movement condition and condition #1 (bell and background stationary) revealed no significant main effects or interactions (F< 1 in all cases).
An analysis of variance for the reaction time data showed a highly significant main effect of eye movement (F1,23 = 83.96, p < 0.001) with mean reaction time increasing from 611 ms to 881 ms when the eyes moved. There was also a significant effect of background movement (F1,23 = 6.28, p < 0.05) with mean reaction time increasing from 717 ms to 775 ms when the background moved. The interaction between eye and background movement also reached significance (F1,23 = 4.82, p < 0.05).
There was however no effect of type of memory task on reaction times (F1,23 = < 1) with mean reaction times of 749 ms and 743 ms for tests during spatial and nonsense tasks respectively. In addition there was no difference in reaction times tested during presentation or recall of the memory material (F1,23 = 2.12, p > 0.1) with mean reaction times of 732 ms and 760 ms for presentation and recall respectively.
No other main effects or interactions in either the memory data or the reaction time data approached significance.
Overall the results show that making voluntary eye movements gives a significant impairment of performance on the memory task involving imagery, but no such effect occurs for a task that involves mainly verbal coding. No such differential impairment was found when movements, comparable to the movements of the retinal image during the eye movement, occurred in the visual field. In addition, having to hold the eyes stationary had no effect on performance when compared with a free eye movement condition. Having to perform a reaction time task failed to have a significant effect on the performance of the memory tasks, and reaction times themselves did not depend on which type of memory task was being undertaken concurrently.
Discussion
The results of the first two experiments combine to rule out any direct intermediate role of eye movements per se in imagery, because selective impairment of performance on the spatial tasks did not occur when involuntary eye movements are induced, nor when the eyes were held stationary, but did occur when voluntary eye movements had to be made. The reaction time data in the second experiment suggested that participants were controlling their eye movements equally well during the spatial and nonsense tasks. However, for the imagery material, they appeared to be controlling their eye movements at the expense of performance on the memory task. The increase in RTs when the eyes or background moved may have been due, at least in part, to the increased difficulty of the shape change discrimination when the bell or background on the screen moved. Because no disruption of performance occurred for the verbally coded material, the results implied that having to control voluntary movements of the eyes somehow disrupted the process of imagery.
There were several possible explanations of these results. The selective disruption of imagery performance would seem to imply that the control of voluntary eye movements shares processing capacity with imaginal information processing. It would seem plausible however that this shared capacity is only involved in part of the imagery process. If images are scanned in a way similar to the scanning of the real visual world then it is possible that part of a system involved in the control of voluntary eye movements is not involved in the formation of an image, but only in subsequent processing of imaginal information. Similarly it is possible that these processes are only involved in the formation of an image, or in both formation and scanning, or in maintenance of the image.
Experiment 3
The third experiment was designed to determine more precisely the involvement of the processes used to control voluntary eye movements in imagery. One possibility, that eye movement control processes are involved in the retrieval or “scanning” of imaginal information, would lead to the prediction of selective disruption of performance on the spatial version of the Brooks task only when participants move their eyes during recall of the material. A second possibility, that eye movement control processes are involved during encoding or formation of images, would lead to the prediction that disruption would only occur for eye movements during the presentation of material. A third possibility, that maintenance of imaginal information engages eye movement control processes, would lead to the prediction of disruption when eye movements occur both during presentation and recall of the material. This last prediction would also hold if the processes involved in the voluntary control of eye movements were involved in both encoding and retrieval of imaginal information.
Method
Participants
Twenty four members of the Applied Psychology Unit participant panel, aged between 18 yrs and 50 yrs, served as participants and were paid for participating.
Apparatus
Apparatus was similar to that used in Experiment 2, the only differences being that no reaction times were tested, the background of the display was always stationary, and the moving bell could be instantly stopped and centered, and then started again by the experimenter.
Material
The material used was the same as in Experiment 2.
Design
The design was similar to Experiment 2 except that no reaction times were collected and the control conditions each consisted of two spatial and two nonsense messages. The four experimental conditions used were:
#1 Bell stationary
#2 Bell moving during presentation, stationary during recall
#3 Bell stationary during presentation, moving during recall
#4 Bell moving during presentation and recall
Procedure
Participants were seated in front of the monitor. The two types of memory task were explained to them and the importance of using the correct strategy was emphasized. Participants were given six practice trials on each type of memory task unless they reached a criterion of two successive perfect runs in less than six trials. If they failed to reach the criterion then their results were discarded and they were replaced. EOG electrodes were attached and the oscilloscope reading checked to see if the experimenter could tell whether the participant’s eyes were moving. Participants were told that the purpose of the EOG was to enable the experimenter to ensure that they were watching the bell at all times.
The lights were dimmed and a stationary display put on the screen. Participants were told that they need not look at the screen but could do what they liked with their eyes. After this control condition the participant was told that from now on s/he should watch the bell at all times. Before each of the four experimental conditions participants were given two practice trials on each type of message. They were then given the sixteen messages comprising the memory material for that condition.
Between each experimental or control condition there was a 2 min break.
After the four experimental conditions, participants completed the final control trials.
Results
Performance on the memory tasks was scored in terms of the number of sequences in which reproduction was perfect. The results from the spatial task showed a marked drop in performance between the stationary condition (#1) and the other three, whereas performance on the nonsense task was comparable between the stationary and moving at recall (#3) conditions, and slightly lower for the moving at presentation (#2) and moving at presentation and recall (#4) conditions.
Our first question was whether we had succeeded in replicating the main result of Experiment 2, that eye movements differentially disrupt performance on the spatial memory task. A preliminary analysis of variance involving only Conditions 1 (eyes stationary) and 4 (eyes moving at all times), showed that we had. There was a significant overall effect of eye movement (F1,23 = 11.83, p < 0.005) modified by a significant interaction with type of memory material (F1,23 = 8.58, p < 0.01). This interaction was due to a decrease in performance on the spatial task when the eyes moved (q2 = 4.49, df = 46, p < 0.01).
A further 2 x 2 x 2 analysis of variance was then performed, the factors being eye movement at presentation, eye movement at recall, and type of memory material. This showed main effects of eye movement at both presentation (F1,23 = 9.44, p < 0.01) and at recall (F1,23 = 6.47, p < 0.025). There was also a significant effect of type of memory material (F1,23 = 4.78, p < 0.05). The effect of eye movement during recall was modified by a significant interaction with type of memory material (F1,23 = 6.54, p < 0.025). The interaction between eye movement at presentation and type of memory material failed to reach significance (F1,23 = 1.865, p > 0.1). Examination of performance in the individual conditions however, indicates that this does not stem from an absence of eye-movement during input on the spatial memory task. Performance is just as severely disrupted by eye movement during input as during output, or indeed both; all three conditions lead to significantly poorer performance than occurs with eyes stationary (p < .01 in each case), and do not differ between themselves (p > .1). However, examination of the nonsense condition suggests that eye movement during input may have a small disrupting effect relative to movement during output, although differences between conditions on the nonsense task do not reach statistical significance (p > .1 in all cases).
A further reason for caution in interpreting the nonsignificant interaction as evidence that eye movements are not disruptive during input comes from a further analysis of a subset of the experiment. If no input effect was observed, then one might expect no difference between the stationary control condition and the condition in which movement occurred only during input. A separate analysis of variance of these two conditions showed a highly significant overall effect of eye movement (F1,23 = 12.00, p < .005) modified by a significant interaction with type of material (F1,23 = 4.60, p < .05). On the basis of the available evidence, it would seem unwise to place too much emphasis on the distinction between eye movements during input and during recall.
Discussion
The impairment of performance on the spatial task occurred for voluntary eye movements during both presentation and recall of the memory material. The impairment during recall was limited to the spatial task, and although the results of eye movement during input were less clearly specific, it never the less seems likely that a system involved in the control of voluntary eye movements must either share capacity with both the encoding of imaginal information and the retrieval or scanning of it, or with processing involved in the maintenance of the image.
If the formation and scanning of the image are both affected independently by making voluntary eye movements, then the effect of eye movements in each case should be additive, and performance on the spatial task in condition #4 where the eyes moved at all times should have been worse than in conditions #2 and #3 where the eyes moved only at presentation or recall. Because this was not the case, the second alternative would appear more plausible. This interpretation must be treated with caution however, because it is possible that a floor effect is starting to occur, the observed level of performance perhaps being maintained by encoding some of the spatial information verbally.
The equivocal nature of this conclusion reflects the fact that the Brooks task is poorly suited for an analysis of whether the maintenance of imaginal information depends on eyemovement control processes. This is because the task does not isolate maintenance- from encoding- and retrieval-related processes. For Experiment 4, therefore, we employed a different experimental procedure that would permit the isolation of maintenance-related processes.
Experiment 4
Experiment 4 was administered as a delayed-recognition task that required memory for the location or the shape of a target stimulus, crossed with three levels of delay-period distraction: saccades; word reading; and no distraction. We selected this task because it could produce data that would be complementary with those produced by Experiments 1-3. In particular, because the delayed-recognition task features a delay period that is temporally separated from stimulus-encoding and response periods of the task, it would afford unequivocal assessment of whether maintenance-related processes depend on those also used for eyemovement control. Specifically, the “maintenance” hypothesis tentatively supported by the results of Experiment 3 predicted that delayed-recognition of locations would be sensitive to eye movements that occurred only during the delay period. The design of Experiment 4 would also complement the results of the first three experiments in other ways. For one, by employing a level of eye-movement measurement that wasn’t available for Experiments 1-3, Experiment 4 could rule out the possibility that the selective sensitivity of spatial working memory performance to concurrent saccades is confounded by different levels of secondary saccadic activity during nonspatial vs. spatial trials. Further, by including a second distraction task – word reading – Experiment 4 could rule out the alternative (left open by the first three experiments) that spatial working memory is somehow more susceptible to distraction by any secondary task, and thus that the sensitivity to concurrent eye movements needn’t imply a shared resource between the two. Finally, Experiment 4 also permitted the evaluation of the specificity of the saccadic disruption effect on spatial working memory, because it afforded the hypothesis that working memory for shapes would not be sensitive to saccadic interference, but would instead be sensitive to word-reading interference. (Word reading was the secondary task that was expected to selectively disrupt working memory for shapes because previous work has demonstrated that a secondary verbal task is effective at selectively disrupting working memory for shape stimuli (Postle, D’Esposito, & Corkin, in press).
Methods
Participants
The twelve participants were undergraduates enrolled at the University of Wisconsin-Madison (mean age = 21, 9 males), and were compensated with course credit or with money.
Apparatus
Testing was performed in a dark room in which all sources of light (with the exception of the screen of the computer monitor that displayed stimuli) were occluded. Additionally, the “brightness” setting on the monitor (ViewSonic, Optiquest Q95) was set to 0 in order to prevent the gray-black glow that typically emanates from a computer monitor that is displaying a uniformly blank screen. The effect for the participant was to be sitting in a room in which nothing was visible except for the periodic appearance of instructions and stimuli. Although the eye-tracker (Eye Mark V, NAC Inc.) was designed to be head-mounted, we mounted it onto a custom-built frame in order to minimize head movements and to control distance of the eyes from the computer screen. The participant was positioned by first sitting in the testing chair, then leaning the head and trunk forward until the forehead was snugly against the forehead pad on the eye tracker apparatus. Finally, the back and seat positions of the chair were adjusted such that this “leaning forward” position could be maintained comfortably for an extended period of time. The end result was a position from which comfortably looking “straight ahead” resulted in fixating the centre of the computer screen. Data from the eye-tracker were analyzed with ILAB software (Gitelman, 2002) to confirm fixation during appropriate trial types, and to quantify eye movements (number of saccades and total distance traveled by eyes) during the distraction periods.
Materials
Delayed-recognition tasks.
The stimulus on location memory trials was a white circle whose diameter subtended approximately 4 deg of visual angle. Stimuli for the shape delayedrecognition task were drawn from a pool of 64 stimuli. Sixteen of these were drawn from the subset of stimuli developed by Attneave and Arnoult (1956) that were determined normatively to have the lowest association values (Vanderplas & Garvin, 1959). From each of these, three variants had been created by modifying one of its salient features (Postle & D’Esposito, 1999). Thus, each shape target stimulus was associated with three visually similar stimuli drawn from the same “prototype group” that served as foils on negative shape trials. Shape targets were selected randomly with the constraints that an item from each prototype group was selected before any group repeated, and no item repeated as a target. (A total of 48 items served as target stimuli across a 96-trial experiment.) All shape stimuli were scaled such that their widest and/or tallest portion matched the diameter of the location-memory circle stimulus. A backward mask was introduced after pilot testing revealed that dark-adapted participants could detect a faint residual glow of phosphorescence at the screen location where a target stimulus had appeared. The mask was uniformly white except at its edges, where it decreased in intensity so as to disappear before reaching the edge of the screen. It covered all areas of the screen at which stimuli might appear.
Saccadic distraction.
The onset and offset of saccadic distraction (see Procedure, Saccadic distraction, below) were each signaled with a tone emanating from a loudspeaker positioned directly below the computer monitor.
Word-reading distraction.
Distraction words were drawn from a pool of 196 common, concrete nouns, each comprising 1-4 syllables, all referring to manipulable objects. They were displayed centrally in lowercase Courier New 40-point font.
Procedure
Delayed-recognition tasks.
Each trial began with a message instructing participants to attend to the target’s “location” or “shape” (1000 msec). After a 1000 msec ISI, the target stimulus appeared for 1000 msec at a pseudorandomly determined location on the screen, the two constraints being that no two appeared at precisely the same location (across all 96 trials of the experiment) and that six appeared in each of the four quadrants of the screen within each 24- trial block. These procedures applied to both location and shape targets. A 14 sec delay period followed target offset. 1000 msec after the offset of the target the backward mask was presented for 500 msec. 2.5 sec after the offset of the mask the 9 sec-long distraction period began. The onset of the distractor period was selected as a time by which any residual trace of the mask was no longer detectable on the screen, even by dark-adapted participants. A 1 sec ISI separated the offset of the distractor period from the onset of the memory probe stimulus. The probe was displayed for 1 sec, and responses were collected during the display of the probe and for an additional 2 sec (Fig. 2). On location trials, invalid probes appeared at a location abutting the target location, but at which no part of the probe stimulus overlapped an area that had been occupied by the target. (During training, participants were informed that there would be no partially overlapping memory probes.) On shape trials the memory probe always appeared in a location that was unpredictably different from that of the target. Invalid shape probes were selected from the three belonging to the same prototype group as the target. The intertrial interval (ITI) was 3 sec. Trials were blocked in groups of 24, and a total of four blocks were administered. Domain of memorandum and distraction type were pseudorandomized such that each possible combination occurred four times during each block. The experimenter sat in an adjacent room during testing, so that he could monitor the eye-tracking equipment. He entered the room after each block, however, to ensure calibration of the head-mounted eye-tracker, and to turn on the overhead lights in the room so as to reverse the process of dark adaptation of the participants’ eyes.
Figure 2.
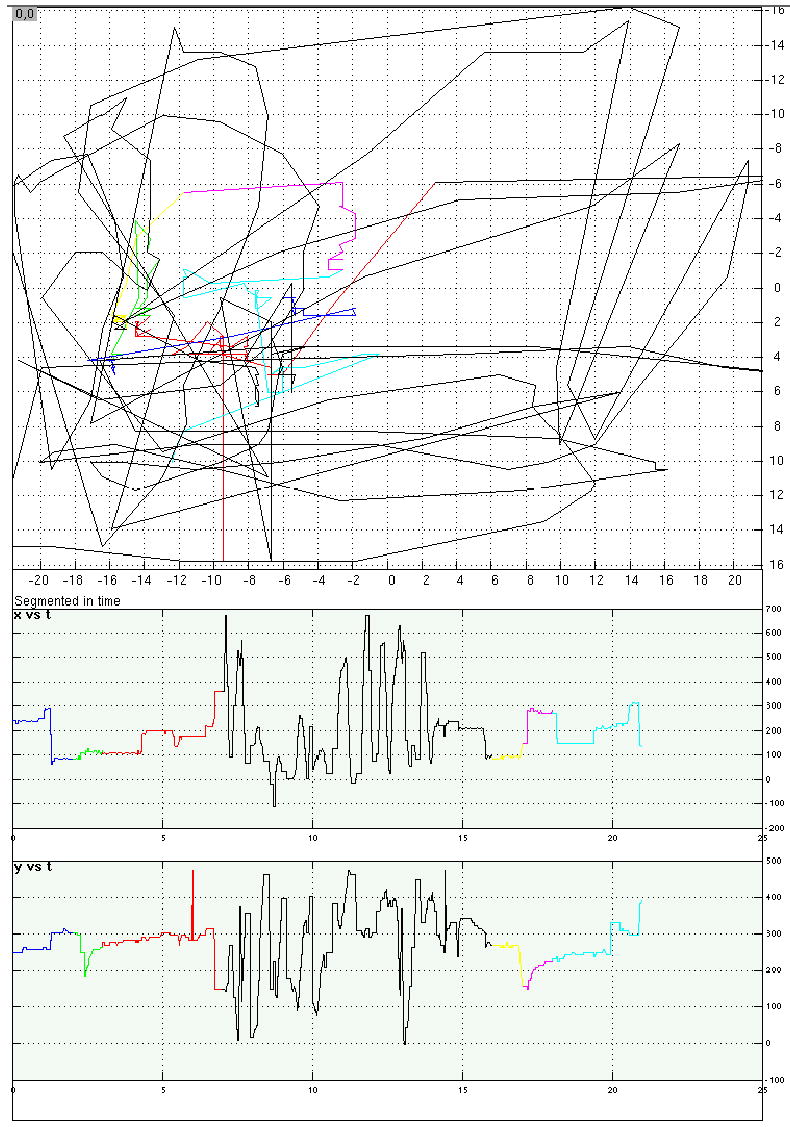
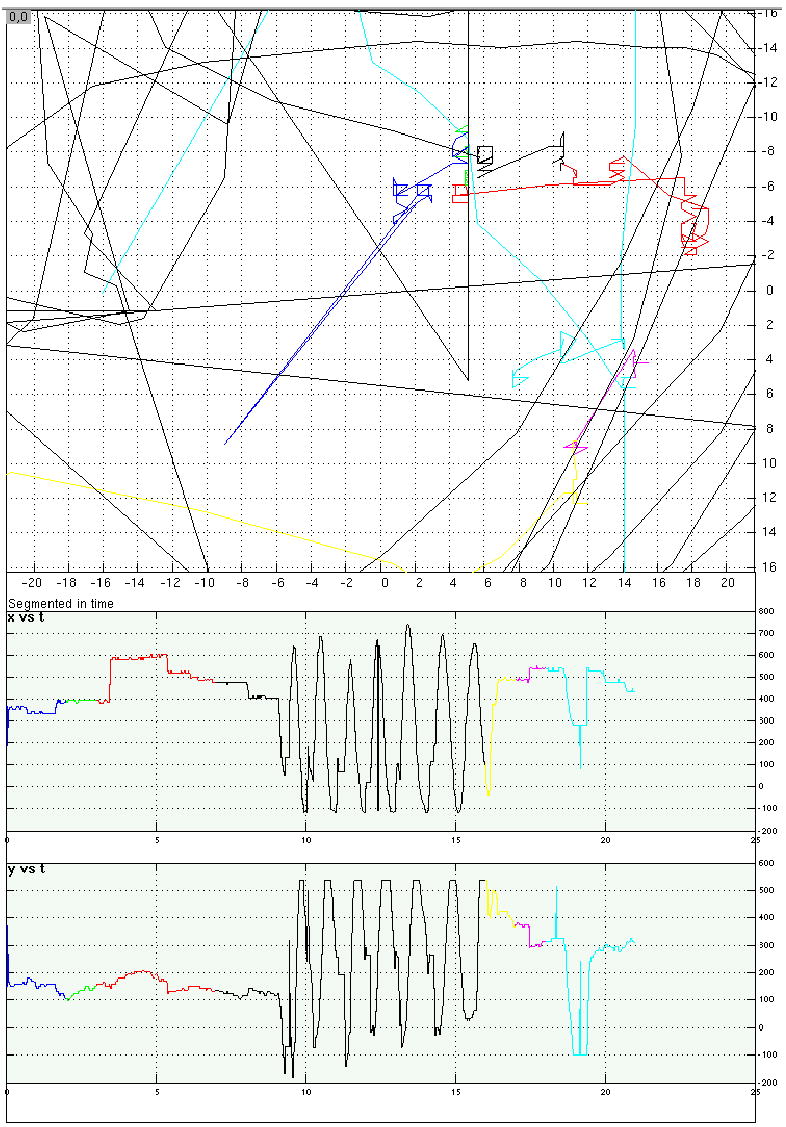
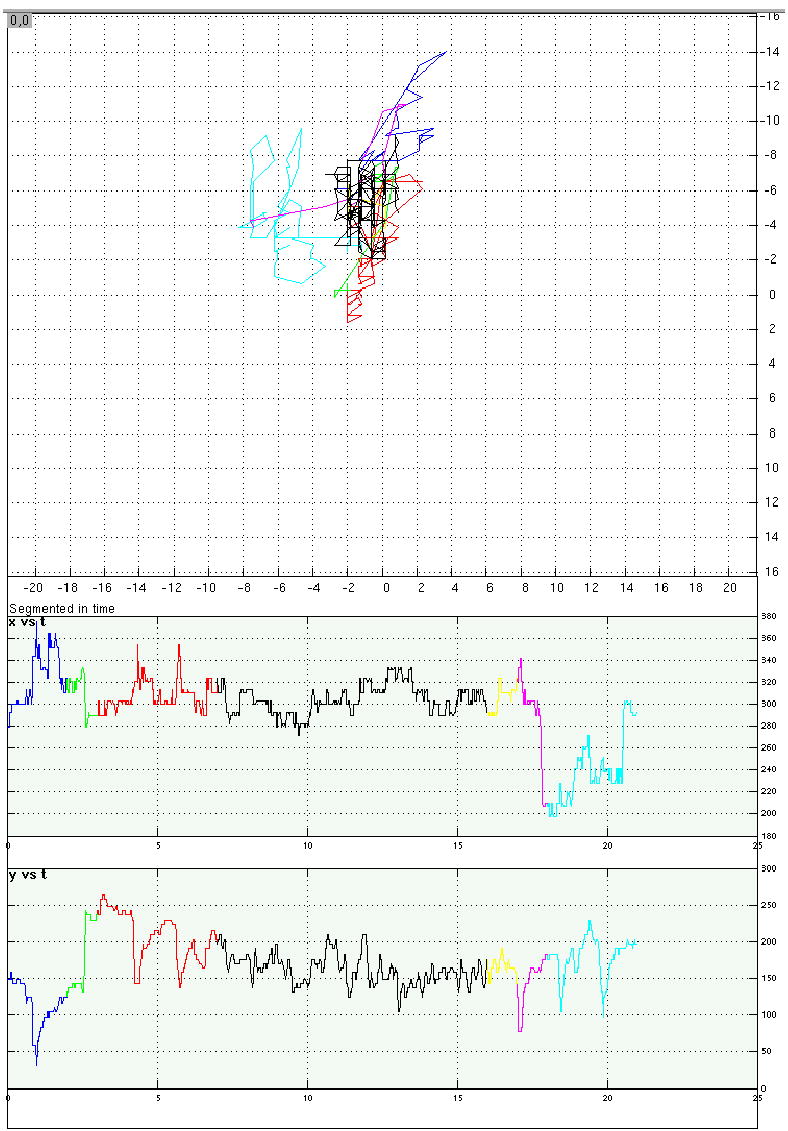
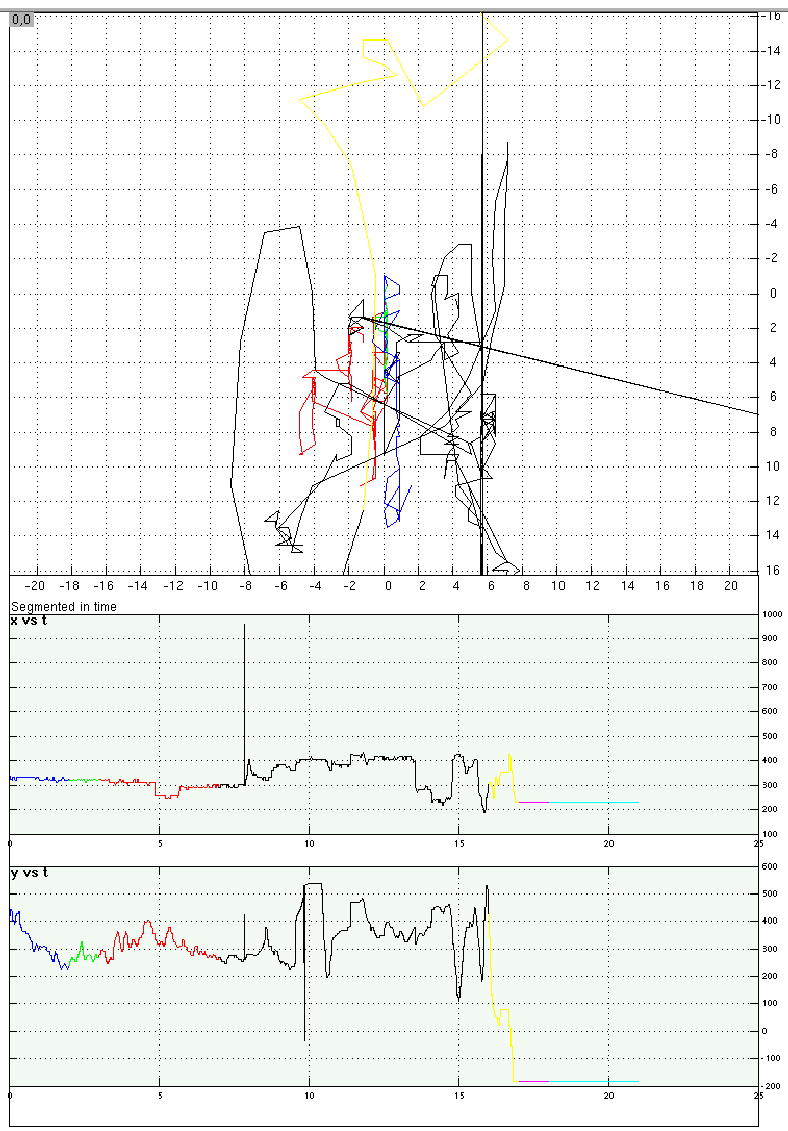
Eye-position traces from four representative trials from Experiment 4.
a. A location memory/saccadic distraction trial. Top panel is a 2D representation of eye position on the computer screen over the course of the trial; axis labels correspond to degrees of visual angle. Bottom panels display horizontal (“x”) and vertical (“y”) eye position as a function of time, with time in sec on the horizontal axes and position in pixels on the vertical axes (each pixel measured .58 mm2). For all three panels, trial epoch is color coded: blue = instruction message; green = target stimulus; red = backward mask portion of the delay period; black = distraction period; yellow = postdistraction blank period; pink = probe stimulus; teal = ITI.
b. A shape memory/saccadic distraction trial.
c. A shape memory/word-reading distraction trial in which the participant complied with instructions to fixate the center of the screen during RSVP presentation of distracting words.
d. A shape memory/word-reading distraction trial that was excluded from analyses because the participant’s eyes were averted from the center of the screen during portions of the distraction period.
Saccadic distraction.
The beginning and end of saccadic distraction periods were signaled with a tone generated by the computer (500 msec duration). Participants had been instructed that, upon hearing the first tone, they were to move their eyes in any manner that they chose until the second tone signaled the end of this distraction period. The intent of this procedure was to minimize the likelihood that nonmotoric factors could explain the selective disruption that saccadic distraction was expected to produce. Because participants were seated in absolute darkness during the distraction portion of the delay period, and because the source of the auditory start and stop signals was 0° with respect to trunk and head position, there were no possible sources of perceptual distraction. And because saccades were endogenously generated, and participants had never been trained to guide them with, for example, a visual array of targets, the likelihood that participants guided their saccades with internally generated mental images was minimized to the greatest extent possible.
Word-reading distraction.
Words were presented serially (exposure duration = 750 msec, ISI = 0 msec, yielding 12 words per distraction period) in the centre of the screen. Items were drawn at random, with no replacement, until the pool of words was exhausted, at which point the selection procedure was reinitiated. Participants were instructed to read each word aloud as it appeared on the screen. The experimenter monitored reading performance via an intercom that connected the control room and the testing room, and noted any trial on which a reading error (including omission of one or more items) occurred. The importance of fixating the distractors during the entire period that they were displayed was emphasized during training.
Results
Saccade quantification
Eye-movement data were analyzed and trials in which participants failed to comply with instructions were discarded. For trials on which participants complied with instructions, we calculated the number of saccades made during the distraction period, and the distance (in pixels) that the eye traveled during the distraction period. The results indicated that, as expected, participants engaged in markedly more saccadic activity during Saccadic distraction periods than during Word-reading and No distraction periods (Table 4, Figure 1). Additionally, and unexpectedly, participants also engaged in more saccadic activity in the shape than in the location memory task (Table 4). These trends were confirmed with an ANOVA assessing the average number of saccades, which indicated that there was a main effect of distraction (F(2,22) = 123.94; p < .0001), a main effect of stimulus domain (F(1,11) = 9.73; p < .01), and no interaction (F(2,22) = .08; n.s.), and an ANOVA assessing the average distance traveled by the eye, which indicated that there was a main effect of distraction (F(2,22) = 53.93; p < .0001), a main effect of stimulus domain (F(1,11) = 7.41; p < .05), and no interaction (F(2,22) = .06; n.s.). It is worthy of note that the imbalance in the amount of saccadic activity across the two memory domains would be expected to work against our prediction that saccadic distraction would disproportionately affect location working memory.
Table 4.
Experiment 4: Quantification of saccades during distraction epoch, by trial type
| Domain of memoranda | Location | Shape | ||||
|---|---|---|---|---|---|---|
| Distraction | None | Word reading | Saccadic | None | Word reading | Saccadic |
| Number of saccades (mean [SE]) | 4.41 [0.71] | 3.81 [.59] | 16.52 [.79] | 5.30 [0.67] | 4.91 [.71] | 17.37 [.76] |
| Distance traveled by eyes (mean number of pixels* [SE]) | 1886.36 [148.86] | 2051.82 [124.4] | 6807.51 [600.19] | 2149.59 [181.17] | 2356.54 [138.03] | 7165.25 [666.34] |
Each pixel measured .58 mm2.
Figure 1.
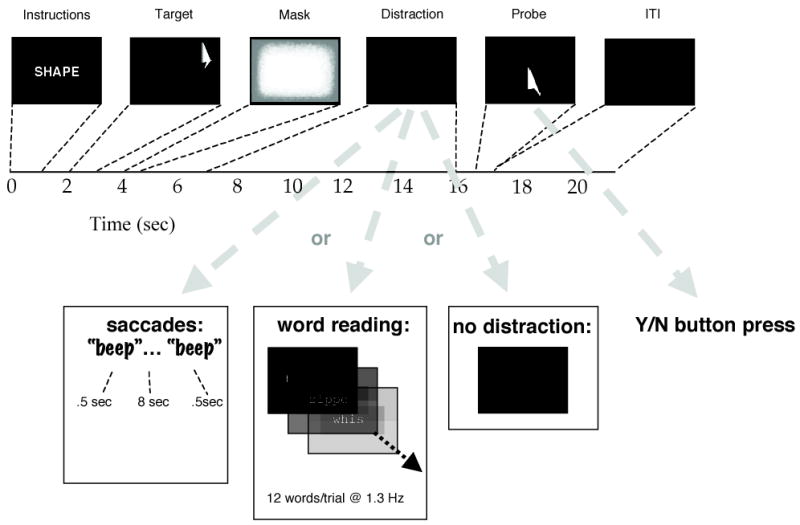
Diagram of the experimental procedure for Experiment 4. See text for details.
Working memory
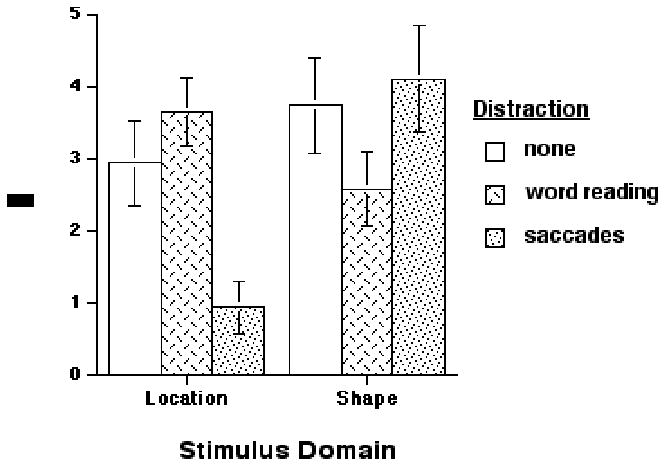
Accuracy data were transformed into measures of d’ and plotted in Figure 3, which reveals a double dissociation (in the predicted direction) of the effect of distraction on delayedrecognition performance. These data were also submitted to an omnibus 2 (domain of memoranda) x 3 (distraction) ANOVA, which revealed no main effects of stimulus domain (F(1,11)=2.58; n.s.), or distraction (F(2,22)=.93; n.s.), and an interaction (F(2,22)=11.48; p < .001). Because the interaction confirmed that there were differential effects of distraction on domain of memoranda, we followed-up with a planned 2 x 2 ANOVA that assessed directly the effects of distraction (saccades, word reading) on the two levels of primary memory task. This 2 x 2 ANOVA also revealed no main effects of stimulus domain (F(1,11)=2.74; n.s.), or of distraction (F(1,11)=.97; n.s.), and an interaction (F(1,11)= 16.45; p < .005). Planned pairwise comparisons confirmed that saccadic distraction had a significant effect on location memory performance (t(11) = 3.16; p < .01), and word-reading distraction had a significant effect on shape memory performance (t(11) = 2.32; p < .05).
Figure 3.
Accuracy results from Experiment 4. See text for details.
ANOVA of the RT data (Table 5) confirmed results consistent with those from the accuracy data: main effects of stimulus domain (F(1,11)=30.84; p < .0005) and of distraction (F(2,22)=6.45; p < .01), and an interaction (F(2,22)=3.74; p < .05). Because of this interaction, the 2 x 3 ANOVA was followed up with a 2 x 2 ANOVA that only included conditions with distracted delay periods. It revealed a main effect of stimulus domain (F(1,11)=22.87; p < .001), no main effect of distraction (F(1,11)=1.95; n.s.) and a significant interaction (F(1,11)=6.50; p < .05). This 2 x 2 interaction reflected the fact memory probe discrimination RTs were differentially slower on location/saccades and shape/word-reading trials.
Table 5.
Experiment 4: Reaction time performance in msec [SE]
| Domain of memoranda | Location | Shape | ||||
|---|---|---|---|---|---|---|
| Distraction | None | Word reading | Saccadic | None | Word reading | Saccadic |
| 1387.5 [125.8] | 1218.8 [126.5] | 1342.0 [129.6] | 1566.8 [138.8] | 1490.57 [119.5] | 1467.6 [134.7] | |
Discussion
The results indicate that saccadic distraction disrupts spatial working memory performance, but not performance on a comparably difficult nonspatial task, whereas wordreading disrupts working memory for shapes, but not for locations. The crossover interactions produced in the accuracy and the RT data permit us to conclude that the two primary tasks draw, at least in part, on separate resources: delayed location recognition on resources shared with endogenously generated saccades; and delayed shape recognition on resources shared with word reading). Thus, the results of Experiment 4 complement those from Experiments 1–3 in many important ways. First, they confirm that the maintenance component of spatial working memory, independent of encoding and/or response, is sensitive to concurrent saccadic activity. Second, they rule out the possibility that differential levels of performance of the secondary eyemovement task might account for the differential effects of saccadic distraction of spatial vs. nonspatial working memory. Third, they rule out the possibility that spatial working memory performance might be disproportionately sensitive to any type of concurrent distraction, by demonstrating its insensitivity to word-reading distraction. And finally, they confirm that the distracting effects of eye movements are limited to working memory for locations, and do not affect working memory for nonspatial visual characteristics that contribute to object identification (i.e., they disrupt working memory for where, but not for what).
So as not to distract from the principal theoretical point of this collection of experiments, we will not devote much space to interpreting the sensitivity of shape working memory to wordreading distraction. One plausible account is that, along with reading each word, participants encoded each word’s shape (Wilson & Baddeley, 1986). A second is that word reading disrupted a verbal code that subjects recruited, along with ventral-stream visual codes, to represent shape memoranda during the delay period. This latter interpretation is consistent with theoretical claims that verbal recoding makes an important contribution to working memory for the identity of visually presented stimuli (Paivio, 1971; Postle et al., in press; Simons, 1996).
GENERAL DISCUSSION
Taken together, the four experiments presented here provide a solid empirical base for the theory that Baddeley discussed in his 1986 book, that, by analogy with the importance of subvocal rehearsal to the phonological loop, the control of visual imagery and visual working memory may derive from the same cognitive resources that support eye movement control. Additionally, as happens with any good idea in science, the preliminary report of Experiments 1–3 spurred a cascade of subsequent studies whose results require an updating of the original idea. In particular, many experiments that followed the initial reports of the results of Experiments 1–3 indicated that comparable levels of selective disruption can be produced by other effector systems, most notably the hands and fingers, hands, and arms (e.g., Baddeley et al., 1980; Della Sala et al., 1999; Farmer et al., 1986; Hale et al., 1996; Lawrence et al., 2001; Logie et al., 1991; Quinn et al., 1986; Salway et al., 1995; Smyth et al., 1988), and several of these studies permit the ruling out of the potential confound of yoked eye movements. These more recent results, therefore, demand modifications to the original idea. One is that the common mechanism underlying visuospatial working memory and eye movement may be more central, perhaps corresponding to an effector-independent motor plan. A second is to allow for fractionation of visuospatial working memory according to the motor effector engaged by the particular task. Recent work demonstrating the sensitivity of working memory-related performance (Cheffi, Allport, & Woodin, 1999; Woodin & Allport, 1999) and brain activity (Postle et al., 2003) to manipulations of spatial frames of reference are consistent with this latter idea.
Another important question for our understanding of spatial working memory is that of the relative contributions of eye movement control vs. the control of visual attention (e.g., Hazlett & Woldorf, 2004; Posner, Snyder, & Davidson, 1980). The importance of this distinction was acknowledged at the time that Experiments 1–3 were performed, although it remains unaddressed by any of the four experiments presented in this report. Promising developments in this regard come from two recent demonstrations of quantitatively smaller disruptive effects produced by shifts of attention alone in comparison to those produced by target-acquiring saccades (Lawrence, Myerson, & Abrams, 2004; Pearson et al., 2003). Further empirical advances along these lines will permit refinement of theoretical and mechanistic of models of spatial working memory.
It is always unsatisfactory when potentially influential experiments are reported only in outline. We trust that the fuller account of Experiments 1 to 3 justify the interest previously shown in them, and that their replication and extension with more adequate eye movement monitoring in Experiment 4 reinforces their conclusions and casts new light on the role of eye movements in visual working memory.
Table 2.
Mean Memory Task Scores (% correct sequences) in Experiment 2
| Memory material | |||||
|---|---|---|---|---|---|
| Condition | Spatial
|
Nonsense
|
|||
| Eye Movement | Background Movement | % correct | S.D. | % correct | S.D. |
| FREE EYE MOVEMENT | 72.4 | 22.1 | 68.5 | 15.3 | |
| #1 FIXED | FIXED | 71.4 | 21.7 | 69.3 | 28.7 |
| #2 FIXED | MOVING | 69.8 | 23.2 | 66.7 | 20.6 |
| #3 MOVING | FIXED | 51.0 | 25.0 | 61.5 | 24.0 |
| #4 MOVING | MOVING | 52.1 | 25.7 | 61.5 | 26.1 |
Table 3.
Mean Memory Task Scores (% correct sequences) in Experiment 3
| Memory material | ||||
|---|---|---|---|---|
| Spatial
|
Nonsense
|
|||
| Condition | % correct | S.D. | % correct | S.D. |
| #1 Stationary | 76.6 | 17.0 | 69.8 | 17.4 |
| #2 Moving at presentation | 56.8 | 12.8 | 64.6 | 21.5 |
| #3 Moving at recall | 55.2 | 19.5 | 69.3 | 18.0 |
| #4 Moving at presentation and recall | 52.6 | 30.1 | 63.0 | 25.2 |
Acknowledgments
We thank Rob Dimbleby and Sohee Park for data collection on Experiments 1-3, and Bradley Fairchild, Olufunsho Faseyitan, Christopher Jordan, George McPhail and Alexander M. Taich for assistance with development, programming, data collection, and analysis on Experiment 4. Experiment 4 supported by NIH grant MH064498 to B.R.P.
Contributor Information
Bradley R. Postle, Department of Psychology, University of Wisconsin-Madison, USA
Christopher Idzikowski, HPRU Medical Centre, University of Surrey, UK.
Sergio Della Sala, Human Cognitive Neuroscience -- Department of Psychology, University of Edinburgh, UK.
Robert H. Logie, Human Cognitive Neuroscience -- Department of Psychology, University of Edinburgh, UK
Alan D. Baddeley, Department of Psychology, University of York, UK
References
- Attneave F, Arnoult MD. Methodological considerations in the quantitative study of shape and pattern perception. Psychological Bulletin. 1956;53:221–227. doi: 10.1037/h0044049. [DOI] [PubMed] [Google Scholar]
- Baddeley, A. D. (1986). Working Memory London: Oxford University Press.
- Baddeley, A. D., Grant, S., Wight, E., & Thomson, N. (1973). Imagery and visual working memory. In P. M. A. Rabbitt & S. Dornic (Eds.), Attention and Performance V (pp. 47–89). London: Academic Press.
- Baddeley, A. D., Grant, W., Wight, E., & Thomson, N. (1975). Imagery and visual working memory. In P. M. A. Rabbitt & S. Dornic (Eds.), Attention and Performance V (pp. 205–217). London: Academic Press.
- Baddeley, A. D., & Lieberman, K. (1980). Spatial working memory. In R. S. Nickerson (Ed.), Attention and Performance VIII (pp. 521–539). Hillsdale, NJ: Erlbaum.
- Baddeley, A. D., & Logie, R. H. (1999). Working memory: the multiple-component model. In A. Miyake & P. Shah (Eds.), Models of Working Memory (pp. 28–61). Cambridge, U.K.: Cambridge University Press.
- Balan PF, Ferrera VP. Effects of gaze shifts on maintenance of spatial memory in macaque frontal eye field. The Journal of Neuroscience. 2003a;23:5446–5454. doi: 10.1523/JNEUROSCI.23-13-05446.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balan PF, Ferrera VP. Effects of spontaneous eye movements on spatial memory in macaque periarcuate cortex. The Journal of Neuroscience. 2003b;23:11392–11401. doi: 10.1523/JNEUROSCI.23-36-11392.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks LR. The suppression of visualization by reading. Quarterly Journal of Experimental Psychology. 1967;19:289–299. doi: 10.1080/14640746708400105. [DOI] [PubMed] [Google Scholar]
- Brooks LR. Spatial and verbal components in the act of recall. Canadian Journal of Psychology. 1968;22:349–368. [Google Scholar]
- Brown BB. Visual recall ability and eye movements. Psychophysiology. 1968;4:300–306. doi: 10.1111/j.1469-8986.1968.tb02771.x. [DOI] [PubMed] [Google Scholar]
- Cheffi S, Allport DA, Woodin M. Hand-centered coding of target location in visuo-spatial working memory. Neuropsychologia. 1999;37:495–502. doi: 10.1016/s0028-3932(98)00082-7. [DOI] [PubMed] [Google Scholar]
- Cooper J, Sagar H, Jordan N, Harvey N, Sullivan E. Cognitive impairment in early, untreated Parkinson’s disease and its relationship to motor disability. Brain. 1991;114:2095–2122. doi: 10.1093/brain/114.5.2095. [DOI] [PubMed] [Google Scholar]
- Della Sala S, Gray C, Baddeley A, Allamano N, Wilson L. Pattern span: a tool for unwelding visuo-spatial memory. Neuropsychologia. 1999;37:1189–1199. doi: 10.1016/s0028-3932(98)00159-6. [DOI] [PubMed] [Google Scholar]
- Farmer EW, Berman JVF, Fletcher YL. Evidence for a visuospatial scratchpad in working memory. Quarterly Journal of Experimental Psychology. 1986;38A:675–688. [Google Scholar]
- Fournet N, Moreaud O, Roulin JL, Naegele B, Pellat J. Working memory in medicated patients with Parkinson’s disease: the central executive seems to work. Journal of Neurology, Neurosurgery, and Psychiatry. 1996;60:313–317. doi: 10.1136/jnnp.60.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman DR. ILAB: a program for post experimental eye movement analysis. Behavioral Research Methods, Instruments, and Computers. 2002;34:605–612. doi: 10.3758/bf03195488. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic, P. S. (1987). Circuitry of the prefrontal cortex and the regulation of behavior by representational memory. In V. B. Mountcastle, F. Plum & S. R. Geiger (Eds.), Handbook of Neurobiology (pp. 373–417). Bethesda: American Physiological Society.
- Goldman-Rakic, P. S. (1990). Cellular and circuit basis of working memory in prefrontal cortex of nonhuman primates. In H. B. M. Uylings, C. G. V. Eden, J. P. C. DeBruin, M. A. Corner & M. G. P. Feenstra (Eds.), Progress in Brain Research (Vol. 85, pp. 325–336): Elsevier Science Publishers. [DOI] [PubMed]
- Hale S, Myerson J, Rhee SH, Weiss CS, Abrams RA. Selective interference with the maintenance of location information in working memory. Neuropsychology. 1996;10:228–240. [Google Scholar]
- Hazlett CJ, Woldorf MG. Mechanisms of moving the mind’s eye: planning and execution of spatial shifts of attention. Journal of Cognitive Neuroscience. 2004;16:742–750. doi: 10.1162/089892904970735. [DOI] [PubMed] [Google Scholar]
- Hebb DO. Concerning Imagery. Psychological Review. 1968;75:466–477. doi: 10.1037/h0026771. [DOI] [PubMed] [Google Scholar]
- Hiscock M, Bergstrom KJ. Ocularmotility as an indicator of verbal and visuospatial processing. Memory & Cognition. 1981;9:332–338. [Google Scholar]
- Hodgson TL, Dittrich WH, Henderson L, Kennard C. Eye movements and spatial working memory in Parkinson’s disease. Neuropsychologia. 1999;37:927–938. doi: 10.1016/s0028-3932(98)00151-1. [DOI] [PubMed] [Google Scholar]
- Idzikowski C. Interrupts: an interrupts routine for Apple II Pascal 1.1. Behaviour Research Methods and Instrumentation. 1982;14:491. [Google Scholar]
- Ketcham CJ, Hodgson TL, Kennard C, Stelmach GE. Memory-motor transformations are impaired in Parkinson’s disease. Experimental Brain Research. 2003;149:30–39. doi: 10.1007/s00221-002-1332-1. [DOI] [PubMed] [Google Scholar]
- Koczat DL, Rogers SJ, Pennington BF, Ross RG. Eye movement abnormality suggestive of a spatial working memory deficit is present in parents of autistic probands. Journal of Autism Developmental Disorders. 2002;32:513–518. doi: 10.1023/a:1021246712459. [DOI] [PubMed] [Google Scholar]
- Kosslyn, S. M., & Shwartz, S. P. (1981). Empirical constraints on theories of visual mental imagery. In A. D. Baddeley & J. Long (Eds.), Attention and Performance IX Hillsdale, N.J.: Lawrence Erlbaum.
- Lawrence BM, Myerson J, Abrams RA. Interference with spatial working memory: An eye movement is more than a shift of attention. Psychonomic Bulletin & Review. 2004;11:488–494. doi: 10.3758/bf03196600. [DOI] [PubMed] [Google Scholar]
- Lawrence BM, Myerson J, Oonk HM, Abrams RA. The effects of eye and limb movements on working memory. Memory. 2001;9:433–444. doi: 10.1080/09658210143000047. [DOI] [PubMed] [Google Scholar]
- Logie, R. H. (1995). Visuo-Spatial Working Memory Hove, U.K.: Erlbaum.
- Logie, R. H. (2003). Spatial and Visual Working Memory: A Mental Workspace. In D. Irwin & B. Ross (Eds.), Cognitive Vision: The Psychology of Learning and Motivation (Vol. 42, pp. 37–78). USA: Elsevier Science.
- Logie, R. H., & Marchetti, C. (1991). Visuo-spatial working memory: visual, spatial or central executive? In R. H. Logie & M. Denis (Eds.), Mental Images in Human Cognition (pp. 105–115). Amsterdam: Elsevier.
- Neisser, U. (1976). Cognition and Reality San Francisco: Freeman.
- Paivio A. Mental imagery in associative learning and memory. Psychological Review. 1969;76:241–263. [Google Scholar]
- Paivio, A. (1971). Imagery and Verbal Processes London: Holt Rinehart and Winston.
- Pearson DG, Sahraie A. Oculomotor control and the maintenance of spatially and temporally distributed events in visuospatial working memory. Quarterly Journal of Experimental Psychology. 2003;56A:1089–1111. doi: 10.1080/02724980343000044. [DOI] [PubMed] [Google Scholar]
- Posner MI, Snyder CRR, Davidson BJ. Attention and detection of signals. Journal of Experimental Psychology: General. 1980;109:160–174. [PubMed] [Google Scholar]
- Postle BR, D’Esposito M. “What” - then - “where” in visual working memory: an event-related fMRI study”. Journal of Cognitive Neuroscience. 1999;11:585–597. doi: 10.1162/089892999563652. [DOI] [PubMed] [Google Scholar]
- Postle BR, D’Esposito M. Spatial working memory activity of the caudate nucleus is sensitive to frame of reference. Cognitive, Affective, and Behavioral Neuroscience. 2003;3:133–144. doi: 10.3758/cabn.3.2.133. [DOI] [PubMed] [Google Scholar]
- Postle, B. R., D’Esposito, M., & Corkin, S. (in press). Effects of verbal and nonverbal interference on spatial and object visual working memory. Memory & Cognition [DOI] [PMC free article] [PubMed]
- Postle BR, Jonides J, Smith E, Corkin S, Growdon JH. Spatial, but not object, delayed response is impaired in early Parkinson’s disease. Neuropsychology. 1997;11:1–9. doi: 10.1037//0894-4105.11.2.171. [DOI] [PubMed] [Google Scholar]
- Postle BR, Locascio JJ, Corkin S, Growdon JH. The time course of spatial and object visual learning in early Parkinson’s disease. Neuropsychologia. 1997;35:1413–1422. doi: 10.1016/s0028-3932(97)00054-7. [DOI] [PubMed] [Google Scholar]
- Quinn JG, Ralston GE. Movement and attention in visual working memory. Quarterly Journal of Experimental Psychology. 1986;38A:689–703. doi: 10.1080/14640748608401621. [DOI] [PubMed] [Google Scholar]
- Salway AFS, Logie RH. Visuospatial working memory, movement control and executive demands. British Journal of Psychology. 1995;86:253–269. doi: 10.1111/j.2044-8295.1995.tb02560.x. [DOI] [PubMed] [Google Scholar]
- Simons DJ. In sight, out of mind: when object representations fail. Psychological Science. 1996;7:301–305. [Google Scholar]
- Smith EE, Jonides J, Koeppe RA, Awh E, Schumacher EH, Minoshima S. Spatial vs. object working memory: PET investigations. Journal of Cognitive Neuroscience. 1995;7:337–356. doi: 10.1162/jocn.1995.7.3.337. [DOI] [PubMed] [Google Scholar]
- Smyth MM, Pearson NA, Pendleton LR. Movement and working memory: patterns and positions in space. Quarterly Journal of Experimental Psychology. 1988;40A:497–514. doi: 10.1080/02724988843000041. [DOI] [PubMed] [Google Scholar]
- Tresch MC, Sinnamon HM, Seamon JG. Double dissociation of spatial and object visual memory: Evidence from selective interference in intact human subjects. Neuropsychologia. 1993;31:211–219. doi: 10.1016/0028-3932(93)90085-e. [DOI] [PubMed] [Google Scholar]
- Ungerleider, L. G., & Mishkin, M. (1982). Two cortical visual systems. In D. J. Ingle, M. A. Goodale & R. J. W. Mansfield (Eds.), Analysis of Visual Behavior (pp. 549–586). Cambridge, MA: MIT Press.
- Vanderplas JM, Garvin EA. The association value of random shapes. Journal of Experimental Psychology. 1959;57:147–163. doi: 10.1037/h0048723. [DOI] [PubMed] [Google Scholar]
- Vye, N. J. (1979). Motor components of visual imagery. Unpublished Master’s Thesis, Acadia University, Wolfville, Nova Scotia
- Wilson, B., & Baddeley, A. D. (1986). Single case methodology and the remediation of dyslexia. In G. T. Pavlidis & D. F. Fisher (Eds.), Dyslexia: Its Neuropsychology and Treatment (pp. 263–277). London: John Wiley & Sons.
- Woodin ME, Allport A. Independent reference frames in human spatial memory: body-centered and environment-centered coding in near and far space. Memory & Cognition. 1999;26:1109–1116. doi: 10.3758/bf03201188. [DOI] [PubMed] [Google Scholar]


