Short abstract
Recent work has identified genes implicated in the tissue tropism of breast-cancer metastasis.
Abstract
Transplantation of human breast cancer cells into immunodeficient mice together with gene-expression microarray studies has recently identified genes implicated in the tissue tropism of breast-cancer metastasis. Such signatures of site-specific metastatic capabilities might allow the targeting of therapy to likely sites of metastasis.
Metastases are the primary cause of death of cancer patients, and improving the means of foretelling their development is a major goal of current clinical research. Already, new genomic-based tests predicting the likelihood of tumor recurrence are making the first tentative steps into the clinic [1]. Recent work [2-4], most notably studies of breast cancer by Minn and colleagues [3,4], suggests that it is possible to refine these tests so that the site of distant recurrence can also be predicted. Such an enhancement of cancer diagnostics to address the tissue specificity of metastasis would aid the oncologist and patient by allowing additional treatment after surgery to be targeted to the tissue.
The metastatic potential of tumors
The establishment of metastases at sites distant from the origin of the tumor is non-random, and thus potentially predictable. Breast tumors, for example, primarily spread to lung and bone marrow, while colorectal cancer commonly metastasizes to liver. Some limits to this tumor tropism are clearly anatomical, relating to the distribution and dimensions of the vascular or lymphatic system that disseminates circulating tumor cells. As long ago as 1889, however, Paget [5] suggested the well-known 'seed and soil' hypothesis [6], which proposes that migrating tumor cells (the seeds) are only able to establish themselves in certain receptive environments (the soil). A striking illustration of this intrinsic specificity of circulating tumor cells was exemplified by a study of ovarian cancer [7,8], looking at patients with implanted venous shunts. Ovarian cancer rarely metastasizes outside of the peritoneum, and in this study, although the shunts allowed vast numbers of ovarian tumor cells to enter the venous circulation, no increase in distant metastasis was observed [7,8]. Clearly, the cells of a primary tumor are functionally restricted in terms of the tissues in which they can establish themselves and proliferate.
How the tissue specificity of metastasis is determined is unknown, reflecting continued uncertainty about the general nature of metastatic origins. Several independent lines of evidence suggest that a tumor is a heterogeneous collection of cells with varying growth and metastatic potentials (for reviews see [6,9]). Metastases appear to arise from single progenitor cells that escape the primary tumor and find fertile ground for growth elsewhere. Consistent with these observations, metastasis has long been viewed as a progressive disorder in which the diversity of cells within the tumor provides a basis for selection of aggressive growth, with the ultimate escape of a metastatic cell from the primary neoplasm and its subsequent establishment in other tissues. Multi-step tumor progression could imply that a primary tumor at its earliest stages would, on the whole, provide little information as to the likelihood or nature of future metastasis. While not invalidating the multi-step model, recent microarray-based analyses of the molecular physiology of tumors at diagnosis and of later occurring metastases demonstrated that metastases can be very similar molecularly to the primary tumor [10,11]. This suggests that some aspects of primary tumors, be they genetic or epigenetic, are stable enough to be inherited by disseminated tumors. Furthermore, by distinguishing a variety of shared features within the primary tumors, such microarray studies have proposed a number of signatures that predict aggressive disease [1,10,12-16]. These results suggest that the heterogeneity of tumors at diagnosis presents phenotypes that influence their metastatic properties, although they do not make it clear whether such features represent the product of mutation and selection or whether they distinguish properties of the transformed cell type.
Several molecular origins have been proposed for the tissue tropism of tumor metastases. Compatible adherent molecules between tumor cell and host tissue, and the presence of necessary growth factors at the metastatic site, have all been implicated, and several specific genes have been suggested to play a role in the distant establishment of a metastasis (for review see [9]). For example, the chemokine receptor CXCR4 has been functionally implicated in the lung specificity of breast tumor metastases, where the tissue-specific activity of its ligand CXCL12 allows chemokine-mediated signal activation [17,18]. Similarly, the chemokine receptor CCR10 found in melanomas has a chemokine ligand CCL27 that is expressed in skin, consistent with the high incidence of skin metastases [17]. Gene-expression studies that profiled metastases established in mouse models after injection with human breast or small-cell lung cancer cells have shown striking differences between the populations of cells that colonize distinct organs, suggesting that many potential factors may determine organ-specific metastasis and modulate the phenotype of tumor cells located at distant sites [19-21].
Identifying genes governing metastatic tissue specificity
Recent papers from the Massagué lab [3,4] report exciting progress in the identification of genes that are functionally important for tissue-specific metastasis. Of key importance to the patient and oncologist, this work also provides early indications that the findings can translate into clinically useful tools. To assess metastasis, Minn et al. [3] used a model of breast cancer in which human cancer cells are transplanted into another species (a xenograft), in this case mice (Figure 1). Using the human breast carcinoma cell line MDA-MB-231 and microarrays to search for genes expressed in cell lines that preferentially metastasized to lung as opposed to bone, they assessed the potential of using these genes in predicting organ-specific metastasis by injection of cells into immunodeficient mice. The parental line, when tested as single cells, displayed heterogeneous metastatic capacities and tissue tropisms. Serial selection and subcloning allowed the establishment of human cell lines that efficiently and preferentially metastasized either to lung or to bone marrow in immunodeficient mice [3,4].
Figure 1.
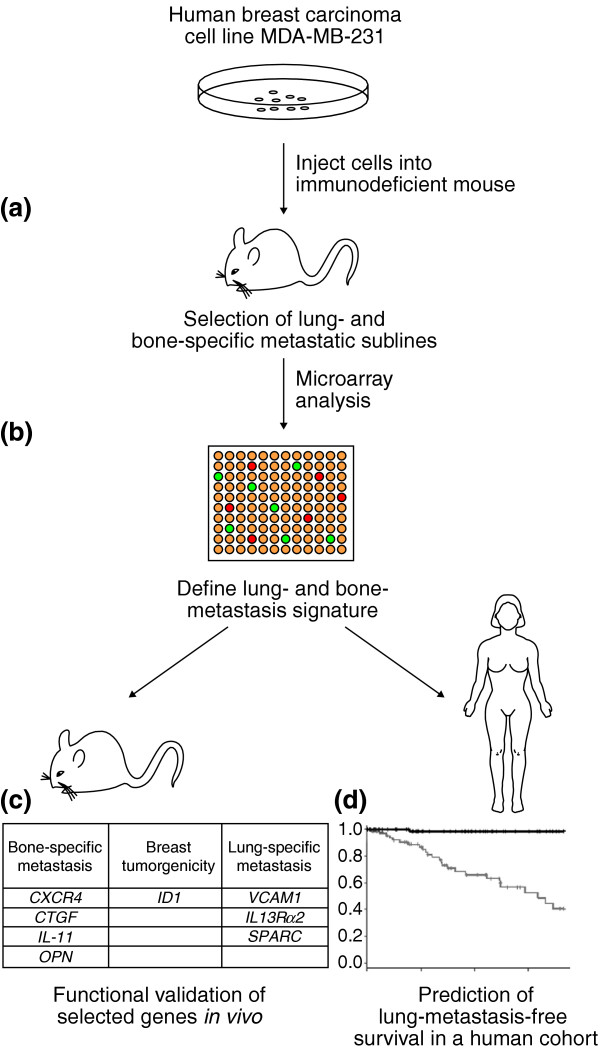
Identification of gene-expression signatures associated with organ site-specific metastasis. The procedure followed by Massagué and colleagues [3,4] was as follows. (a) Selection of stable lung- and bone-metastatic sublines of MDA-MB-231 cells in vivo. (b) Comparison of their gene-expression patterns with parental cell lines. (c) Validation of the role of signature genes in metastatic activity by confirming that selected genes when overexpressed or underexpressed alone or in combination altered the site-specific metastatic activity of the parental line. (d) The lung signature defined from the above study was predictive of metastatic activity in an independent clinical cohort of breast cancer patients.
Gene-expression studies comparing a lung metastatic subline with the parental line identified 95 genes correlated with lung-metastatic activity. This gene list was pared down to 54 candidate genes by also requiring that these genes be differentially expressed across multiple independent lung-metastatic sublines. This gene set was largely distinct from a gene set that was previously identified in bone metastatic sublines [4]. Interestingly, the chemokine receptor CXCR4, noted as a potential effector of lung tropism in other studies, was among the genes identified in the current investigation [3]. Efforts to validate a subset of nine of the candidate genes individually through overexpression in the parent cell line demonstrated little change in lung metastatic activity in mouse xenograft experiments, with only overexpression of inhibitor of DNA binding 1 (ID1) showing a small increase in activity compared to controls. When certain sets of genes were coexpressed in combinations of three in the parental line, however, they recapitulated the lung-specific metastatic activity seen in the original selected cell lines. Bone metastatic activity was not altered by the presence of these constructs. Furthermore, knockdown studies performed in a selected subline revealed that a decrease in the expression of several genes (ID1, the gene for the cell adhesion molecule VCAM1, and the IL13Rα2 gene for the interleukin (IL) 13 receptor) decreased lung metastatic activity tenfold [3]. These functional studies suggest that these genes are not only markers of lung-specific metastasis, but potential mediators of the process as well.
It has been proposed that for metastases to be established the effectors necessary for metastasis must probably also provide a selective benefit at the primary tumor site [22]. Consistent with this model, injection of the lung metastatic subline into mouse mammary fat pads showed that, in addition to its increased lung metastatic activity, it also grew more rapidly at the primary site compared to the parental line [3]. To explore the potential role of the individual candidate genes, Minn et al. [3] tested stable knockdown lines and found that decreased expression of ID1 reduced both growth in the mammary fat pads and lung-metastatic ability. The other knockdowns tested (SPARC - an extracellular matrix-associated protein, VCAM1, and IL13Rα2) decreased only the lung-metastatic activity but not primary tumor growth. This implies that genes involved in metastasis can operate both through a general increase in primary growth and the ability to populate specific tissues.
Importantly, this study [3] also explored whether the set of genes identified in the mouse model system might be able to distinguish a subclass of breast cancer patients with a tendency to lung metastasis. The association of the expression of these genes with outcome was measured in a cohort of 82 patients whose lung and bone metastasis-free survival had been assessed in a 10-year follow-up study. By using gene-expression microarray data from diagnostic specimens, 12 of the 54 implicated genes showed a significant association with lung metastasis-free survival. A lung metastasis classifier defined by these genes and weighted by the univariate scores was significantly associated with the risk of lung metastasis, but not with the risk of bone metastases. To assess the generalization of this model to independent datasets, Minn et al. [3] derived a new classifier with this set of genes, training it on an independent breast cancer cohort to distinguish tumors that shared features with the lung-metastatic selected subline. When this new classifier was tested on the original 82-patient cohort, it identified patients who succumbed to metastasis to lung but not to bone. Minn et al. [4] had previously shown that a bone-metastasis signature was similarly able to distinguish patients who suffered bone metastases from those who suffered lung metastases, although this signature was not associated with poor outcome.
An understanding of the mechanism of the specificity of tumor tropism, or at least the ability to predict for an individual patient their likely site of metastasis, is of more than academic importance. Lung cancer, for example, frequently metastasizes to the brain and it has been shown that prophylactic cranial irradiation can be beneficial for patients with small-cell lung cancer [23]. The potential toxicity of this therapy has prevented its widespread use, but the ability to focus the treatment on patients most at risk would be of great utility. Altogether, the emerging ability to identify tissue-tropic biomarkers and the maturing of the field of prognosis predictors promise eventually to allow oncologists to direct treatment plans to those patients and tissues most at risk.
Contributor Information
Brian Z Ring, Email: bzring@applied-genomics.com.
Douglas T Ross, Email: dtross@applied-genomics.com.
References
- Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- D'Amico TA, Aloia TA, Moore MB, Conlon DH, Herndon JE, 2nd, Kinch MS, Harpole DH., Jr Predicting the sites of metastases from lung cancer using molecular biologic markers. Ann Thorac Surg. 2001;72:1144–1148. doi: 10.1016/S0003-4975(01)02979-4. [DOI] [PubMed] [Google Scholar]
- Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massagué J. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minn AJ, Kang Y, Serganova I, Gupta GP, Giri DD, Doubrovin M, Ponomarev V, Gerald WL, Blasberg R, Massagué J. Distinct organ-specific metastatic potential of individual breast cancer cells and primary tumors. J Clin Invest. 2005;115:44–55. doi: 10.1172/JCI200522320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paget S. The distribution of secondary growths in cancer of the breast. (1889). Cancer Metastasis Rev. 1989;8:98–101. [PubMed] [Google Scholar]
- Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- Tarin D, Price JE, Kettlewell MG, Souter RG, Vass AC, Crossley B. Mechanisms of human tumor metastasis studied in patients with peritoneovenous shunts. Cancer Res. 1984;44:3584–3592. [PubMed] [Google Scholar]
- Tarin D, Price JE, Kettlewell MG, Souter RG, Vass AC, Crossley B. Clinicopathological observations on metastasis in man studied in patients treated with peritoneovenous shunts. Br Med J (Clin Res Ed) 1984;288:749–751. doi: 10.1136/bmj.288.6419.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Ross KN, Lander ES, Golub TR. A molecular signature of metastasis in primary solid tumors. Nat Genet. 2003;33:49–54. doi: 10.1038/ng1060. [DOI] [PubMed] [Google Scholar]
- Weigelt B, Glas AM, Wessels LF, Witteveen AT, Peterse JL, van't Veer LJ. Gene expression profiles of primary breast tumors maintained in distant metastases. Proc Natl Acad Sci USA. 2003;100:15901–15905. doi: 10.1073/pnas.2634067100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Nuyten DS, Sneddon JB, Hastie T, Tibshirani R, Sorlie T, Dai H, He YD, van't Veer LJ, Bartelink H, et al. Robustness, scalability, and integration of a wound-response gene expression signature in predicting breast cancer survival. Proc Natl Acad Sci USA. 2005;102:3738–3743. doi: 10.1073/pnas.0409462102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ, Witteveen AT, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F, Talantov D, Timmermans M, Meijer-van Gelder ME, Yu J, et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365:671–679. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- van de Vijver MJ, He YD, van't Veer LJ, Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- Staller P, Sulitkova J, Lisztwan J, Moch H, Oakeley EJ, Krek W. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature. 2003;425:307–311. doi: 10.1038/nature01874. [DOI] [PubMed] [Google Scholar]
- Kakiuchi S, Daigo Y, Tsunoda T, Yano S, Sone S, Nakamura Y. Genome-wide analysis of organ-preferential metastasis of human small cell lung cancer in mice. Mol Cancer Res. 2003;1:485–499. [PubMed] [Google Scholar]
- Lee H, Lin EC, Liu L, Smith JW. Gene expression profiling of tumor xenografts: In vivo analysis of organ-specific metastasis. Int J Cancer. 2003;107:528–534. doi: 10.1002/ijc.11428. [DOI] [PubMed] [Google Scholar]
- Montel V, Huang TY, Mose E, Pestonjamasp K, Tarin D. Expression profiling of primary tumors and matched lymphatic and lung metastases in a xenogeneic breast cancer model. Am J Pathol. 2005;166:1565–1579. doi: 10.1016/S0002-9440(10)62372-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernards R, Weinberg RA. A progression puzzle. Nature. 2002;418:823. doi: 10.1038/418823a. [DOI] [PubMed] [Google Scholar]
- Auperin A, Arriagada R, Pignon JP, Le Pechoux C, Gregor A, Stephens RJ, Kristjansen PE, Johnson BE, Ueoka H, Wagner H, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med. 1999;341:476–484. doi: 10.1056/NEJM199908123410703. [DOI] [PubMed] [Google Scholar]


