Abstract
Sjögren’s syndrome (SS), an inflammatory disease affecting the lacrimal and salivary glands, is the leading cause of aqueous tear-deficient type of dry eye. We previously showed that IL-1β protein is up regulated in the lacrimal gland of a murine model of SS and that exogenous addition of this cytokine inhibits neurotransmitter release and lacrimal gland protein secretion. In the present study we investigated the role cJun NH2-terminal kinase (JNK) in IL-1β mediated inhibition of lacrimal gland secretion and tear production. In vitro, IL-1β induced a time-dependent activation of JNK with a maximum 7.5-fold at 30 min. SP600125, a JNK inhibitor, inhibited, in a concentration-dependent manner, IL-1β induced activation of JNK with a maximum of 87% at 10-4 M. In vivo, IL-1β stimulated JNK and the expression of the inducible isoform of nitric oxide synthase (iNOS). IL-1β inhibited high KCl and adrenergic agonist induced protein secretion by 85% and 66%, respectively. SP600125 alleviated the inhibitory effect of IL-1β on KCl- and agonist-induced protein secretion by 79% and 47%, respectively and completely blocked the expression of iNOS. Treatment for 7 days with SP600125 increased tear production in a murine model of SS dry eye. We conclude that JNK plays a pivotal role in IL-1β mediated inhibition of lacrimal gland secretion and subsequent dry eye.
Keywords: Inflammation, dry eye, Sjögren’s syndrome, interleukin-1β
The lacrimal gland is the main contributor to the aqueous layer of the tear film. It secretes proteins, electrolytes and water, which help nourish and protect the ocular surface (Dartt 1994). Lacrimal gland secretion is primarily under neural control, which is achieved through a neural reflex arc (Botelho et al. 1966). Stimuli to the ocular surface activate afferent sensory nerves in the cornea and conjunctiva (Botelho et al. 1966). This in turn activates efferent parasympathetic and sympathetic nerves in the lacrimal gland to stimulate secretion (Botelho et al. 1966). Neurotransmitters and neuropeptides released by the lacrimal gland nerves include acetylcholine, vasoactive intestinal peptide, norepinephrine, and neuropeptide Y. Each of these neuromediators interacts with specific receptors present on the surface of lacrimal gland cells to elicit a specific response (Hodges and Dartt 2003). A decrease or lack of lacrimal gland secretion is the leading cause of aqueous tear deficient type of dry eye syndrome (Schaumberg et al. 2002).
In several pathological conditions, the lacrimal gland can become a target of the immune system and show signs of inflammation that ultimately lead to aqueous tear deficient type of dry eye. Lacrimal gland inflammation can be a result of autoimmune diseases (Sjögren’s syndrome, diabetes, thyroid disease, sarcoidosis) (Gilbard and Farris 1983; Drosos et al. 1999; Grus et al. 2002; Punzi and Betterle 2004), following surgical transplants (graft-versus-host disease, GVHD) (Ogawa and Kuwana 2003), acquired immunodeficiency syndrome due to infection by human immunodeficiency virus (HIV) (DeCarlo et al. 1995), hepatitis C (Zegans et al. 2002), or simply as a result of aging (Rios et al. 2005).
Sjögren’s syndrome, a systemic inflammatory disease affecting primarily the lacrimal and salivary glands, is the leading cause of aqueous tear deficient type of dry eye (Fox et al. 1999). This syndrome is associated with an extensive lymphocytic infiltration of the lacrimal and salivary glands and destruction of epithelial cells (Fox et al. 1999). Sjögren’s syndrome may exist as a primary disorder (primary Sjögren’s syndrome) or can be associated with other autoimmune diseases (secondary Sjögren’s syndrome) such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), or systemic sclerosis (Fox et al. 1999). Increased production of autoantibodies and proinflammatory cytokines, both of which are believed to interfere with tears and saliva production are common in Sjögren’s syndrome (Fox et al. 1999).
Interleukin-1 (IL-1) consists of two isoforms α and β that bind with equal affinity to the same receptor (Dinarello 1996). There are two receptors for IL-1, a signaling receptor named IL-1RI and a decoy receptor named IL-1 RII (Dinarello 1996). Activation of the IL-1RI triggers a signaling cascade leading to the activation of several transcription factors that initiate the translation of genes involved in the inflammatory response (Dinarello 1996). Depending on the cell types, IL-1 can lead to the activation of the mitogen-activated protein kinase (MAPK, also known as the extracellular signal regulated kinase, ERK), the stress-activated protein kinase (SAPK, also known as the c-Jun NH2-terminal kinase, JNK), and/or the p38 MAPK (Dinarello 2000). Once ERK, JNK, and p38 MAPK are activated, they lead to activation of two major transcription factors, activator protein 1 (AP-1) and nuclear factor-κB (NF-κB) (Paul et al. 1997; Dinarello 2000). In turn, AP-1 and NF-κB induce the transcription of several genes involved in acute and chronic inflammation and connective tissue diseases (Paul et al. 1997; Dinarello 2000). One such a gene involved in inflammation is the one for the inducible isoform of nitric oxide synthase (iNOS) (Dinarello 2000).
In previous studies, using a murine model of Sjögren’s syndrome, we showed that in inflamed lacrimal glands, activation of nerves with high KCl did not lead to the release of neurotransmitter which resulted in impaired protein secretion from this gland (Zoukhri and Kublin 2001). We also reported that the amounts of IL-1β and IL-1RI proteins were elevated in diseased mice and that exogenous addition of this cytokine inhibited neurally mediated lacrimal gland secretion (Zoukhri et al. 2002). The purpose of the present study was to investigate the role of JNK and iNOS in IL-1β mediated inhibition of lacrimal gland secretion.
Materials and Methods
Materials
Phenylephrine, polyethylene glycol 400 (PEG400), and hydrogen peroxide were from Sigma (St. Louis, MO). Amplex Red was from Molecular Probes (Eugene, OR). Recombinant human IL-1β was a generous gift from Dr. Craig W. Reynolds (Biological Resources Branch, National Cancer Institute Preclinical Repository, Rockville, MD). Polyclonal antibodies against phosphorylated (active) JNK, cJun, p38MAPK, Erk and non-discriminatory polyclonal antibodies that recognize the whole pools of JNK, cJun, and Erk were from New England Biolabs (Beverly, MA). An affinity purified polyclonal antibody against iNOS and RAW 264.7 cell lysates were from Santa Cruz Biotechnology (Santa Cruz, CA). SP600125 was a generous gift from Dr. Brydon Bennett (Celgene Corporation, San Diego, CA).
Animals and treatment
Female BALB/c mice (10-12 wk old) were purchased from Taconic (Germantown, NY). Female MRL/Mp-Fas-lpr/lpr and MRL/Mp-+/+ mice were purchased from Jackson Laboratories (Bar Harbor, ME). Animals were maintained in constant temperature rooms with fixed light/dark intervals of 12 hours’ length and were fed ad libitum. All experiments were in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Tufts-New England Medical Center Animal Care and Use Committee.
Animals were anesthetized and the exorbital lacrimal glands were left untreated (control) or were injected with either saline (vehicle) or rhIL-1β (1 μg) in a total volume of 2 μl. Twenty-four hours post-injection, the lacrimal glands were removed; lobules were prepared and separated into two groups. One group was used to measure protein secretion and the other group of lobules was homogenized and the proteins in the cell lysates were processed for western blotting, as described below.
Measurement of peroxidase secretion.
Peroxidase secretion was measured as previously described (Zoukhri et al. 2002). Briefly, lacrimal gland lobules were placed in cell strainers, and pre-incubated for 60 minutes at 37°C in Krebs-Ringer bicarbonate buffer (KRB, containing in mM: 120 NaCl, 5 KCl, 1 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, and 25 NaHCO3) supplemented with 10 mM HEPES and 5.5 mM glucose (pH 7.4). The lobules were then incubated for 20 minutes in a total volume of 0.8 ml in normal KRB (referred to as spontaneous secretion) then for another 20 minutes in depolarizing KRB (evoked secretion) solution where the concentration of KCl was increased to 75 mM and that of NaCl was decreased to 50 mM to maintain isotonicity. Lacrimal gland lobules were further incubated for 20 minutes in 0.8 ml of normal KRB containing phenylephrine (an α1-adrenergic agonist, 10-4 M). After incubation, the amount of peroxidase in the media and tissue homogenate was determined using Amplex Red (Zoukhri et al. 2002). After incubation, the fluorescence was determined in a fluorescence microplate reader using 530 nm excitation wavelength and 590 nm emission wavelength. The amount of secreted peroxidase was expressed as percent of total: (peroxidase in media/peroxidase in media + peroxidase in tissue) × 100 (Zoukhri et al. 2002).
Electrophoresis and Western Blotting
Total amount of protein in the cell lysate was determined using the method of Bradford and equal amounts of protein (20 μg) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE, 4-15% gradient gels). For western blotting, the membranes were first blotted with polyclonal antibodies against phosphorylated (active) JNK, p38MAPK, Erk (all at 1:1000 dilution), or phosphorylated cJun (1:500). Following removal of the antibodies by incubating the membranes for 40 min at 50°C in stripping buffer (62.5 mM Tris-HCl, pH 6.8, 2% SDS, 100 mM β-mercaptoethanol), the membranes were blotted with non-discriminatory polyclonal antibodies that recognize the whole pools of these enzymes (all at 1:1000 dilution) or a monoclonal against β-actin (1:5000, Sigma). After western blotting, immunoreactive bands were visualized using the enhanced chemilumenescence method, and quantitated using NIH Image software (Version 1.69). The amounts of phosphorylated JNK and cJun were normalized to those of total JNK and cJun in order to control for gel loading. Expression of iNOS was detected using an affinity purified polyclonal antibody (1:200).
Histopatholgy and Immunohistochemistry.
Lacrimal glands pieces were fixed, overnight at 4°C, in 4% formaldehyde made in phosphate buffered saline (PBS, containing in mM: 145 NaCl, 7.3 Na2HPO4, and 2.7 NaH2PO4 at pH 7.2). After cryopreservation overnight in 30% sucrose in PBS, the tissue was frozen in O.C.T. embedding medium. Cryostat sections (6 μm) were placed on gelatin-coated slides and dried overnight at 37°C. For histopatholgy experiments, sections were either stained with hematoxylineosin or with giemsa to stain. For immunohistochemistry, sections were incubated for 30 minutes at room temperature in PBS containing 1% bovine serum albumin (BSA) and 0.1% Triton X-100. After blocking for 30 min in 5% donkey serum and 1% BSA in PBS, sections were incubated, overnight at 4°C, with the primary antibody diluted in PBS containing 1% BSA. A secondary antibody conjugated to FITC (1:100 in PBS + 1% BSA) was applied for 30 minutes at room temperature. Coverslips were mounted with a Vectashield mounting medium (Vector Laboratories, Burlingame, CA). Sections were viewed using a Nikon UFXII microscope equipped for epi-illumination. Negative control experiments were performed by omitting the primary antibody.
Measurement of Aqueous Tear Production
Tear production was measured on lightly anesthetized mice using phenol red impregnated cotton threads (Zone-Quick, Lacrimedics). The threads were held with jeweler forceps and applied to the ocular surface, on both eyes, in the lateral canthus for 10 seconds. Wetting of the thread (which turns red in contact with tears) was measured in millimeters under a dissecting microscope.
Data Presentation and Statistical Analysis
Data are expressed as means ± SEM. The data were statistically analyzed using Student’s t-test for paired or unpaired values. Values of p < 0.05 were considered to be significant.
Results
Effect of IL-1β on JNK activity, in vitro
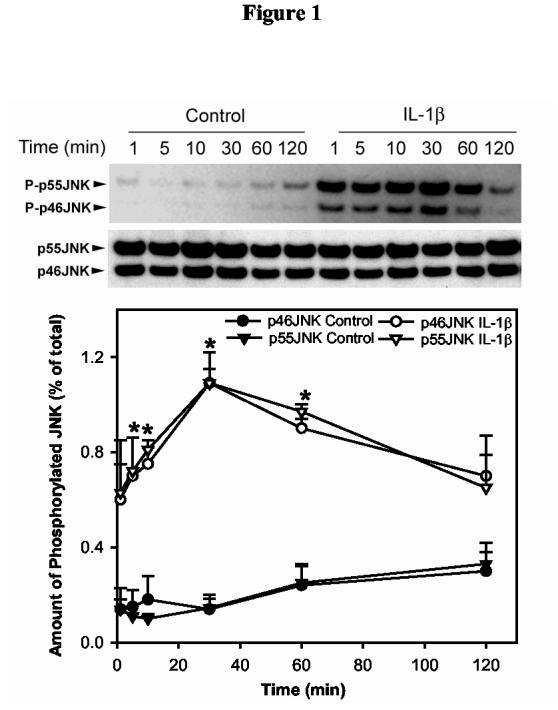
Lobules were prepared from lacrimal glands that were removed from female BALB/c mice and incubated for 0-120 minutes without or with IL-1β (10 ng/ml). JNK activity was monitored by western blotting using an antibody that specifically recognizes the phosphorylated (active) isoforms of JNK. The membranes were also blotted with a non-discriminatory antibody that recognizes the whole pools of JNK in order to normalize for equal gel loading. As shown in Figure 1, IL-1β induced a time-dependent activation of p46JNK and p55JNK with a maximum 7.5-fold at 30 minutes. JNK activity declined slightly with time but was still above basal level at 120 minutes (Fig. 1).
Figure 1.
Kinetics of IL-1β induced activation of JNK in the lacrimal gland, in vitro.Lacrimal gland lobules were incubated in the presence or absence of IL-1β (10 ng/ml) for 0-120 minutes then homogenized and the proteins in the cell lysate separated by SDS-PAGE followed by western blotting using antibodies against phosphorylated JNK or a non-discriminatory antibody against the whole pool of JNK, as described in the Methods section. Data are the means ± SEM from 3 independent experiments. *Denotes statistically significant difference from control.
These results show that IL-1β activated p46JNK and p55JNK in the lacrimal gland in a time-dependent manner.
Effect of IL-1β on JNK and iNOS, in vivo
To determine the effect of IL-1β on JNK activity in vivo, female BALB/c mice exorbital lacrimal glands were left untreated (control) or were injected with either saline (vehicle) or rhIL-1β. Twenty-four hours post-injection, the lacrimal glands were removed; lobules were prepared and separated into two groups. One group was used to measure basal secretion of peroxidase (spontaneous) or secretion in response to a depolarizing KCl (evoked, 75 mM) solution and to a α1-adrenergic agonist (phenylephrine, 10-4 M). The other group of lobules was homogenized and the proteins in the cell lysates were processed for western blotting.
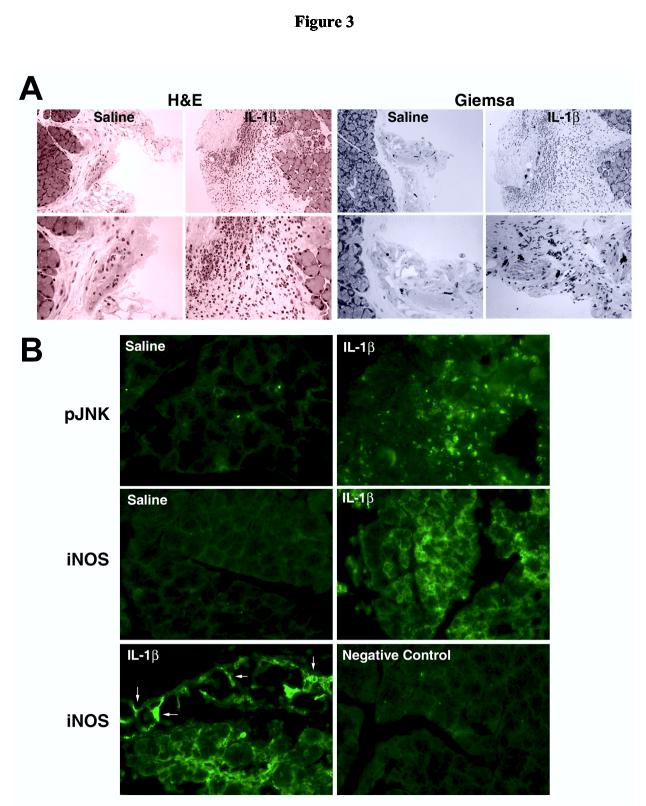
In agreement with our previous findings, rhIL-1β significantly inhibited KCl- as well as phenylephrine-induced peroxidase secretion by 90% and 78%, respectively (Figure 2A). When lobules from the same group of animals were processed for western blotting, it was found that IL-1β treatment resulted in a 4.5- and 6.8-fold increase in p46JNK and p55JNK activity, respectively (Figure 2B). Furthermore, whereas iNOS protein was undetectable in control and vehicle injected lacrimal glands, this protein was abundant in samples prepared from IL-1β injected glands (Figure 2C). The identity of iNOS was confirmed by running samples prepared from RAW 264.7 cells stimulated or not with a phorbol ester (Figure 2C).
Figure 2.
Effect of IL-1β on lacrimal gland secretion, JNK activity, and iNOS expression, in vivo.Lacrimal gland lobules from control, saline (vehicle), or IL-1β injected animals were prepared and used to measure protein secretion (A) or to measure JNK activity (B) and iNOS expression (C). A. Lobules were incubated for 20 minutes each in normal KRB buffer (spontaneous), in depolarizing KRB (evoked) solution where the concentration of KCl was increased to 75 mM, then in KRB containing phenylephrine (an α1-adrenergic agonist, 10-4 M). After incubation, the amount of peroxidase in the media and tissue homogenate was determined using Amplex Red, as described in the Methods section. Data are the means ± SEM from 3 independent experiments. *Denotes statistically significant difference from spontaneous. #Denotes statistically significant difference from IL-1β.B and C. Lacrimal gland lobules were homogenized and the proteins separated by SDS-PAGE followed by western blotting using an antibody against phosphorylated JNK, a non-discriminatory antibody that recognizes the whole pools of JNK (B), or an antibody against iNOS (C). Lysates prepared from unstimulated (-) or phorbol ester stimulated (+) RAW 264.7 cells were used as a positive control for iNOS expression (C). Each lane in the blots represents samples prepared from a separate animal. Data in B are expressed as means ± SEM (n = 3). *Denotes statistically significant difference from control and vehicle.
In summary, these data show that the proinflammatory cytokine IL-1β impairs lacrimal gland secretion while also increasing JNK activity and inducing iNOS
Immunolocalization of JNK and iNOS
The results described above showed that exogenous addition of IL-1β to the lacrimal gland activated JNK and induced the expression of iNOS. Since the lacrimal gland is composed of several cells types: acini, ducts, nerves, myoepithelial, mast, and plasma cells, it was important to determine the cell type in which IL-1β induced its effects. To that purpose, we performed histological and immunohistochemical studies.
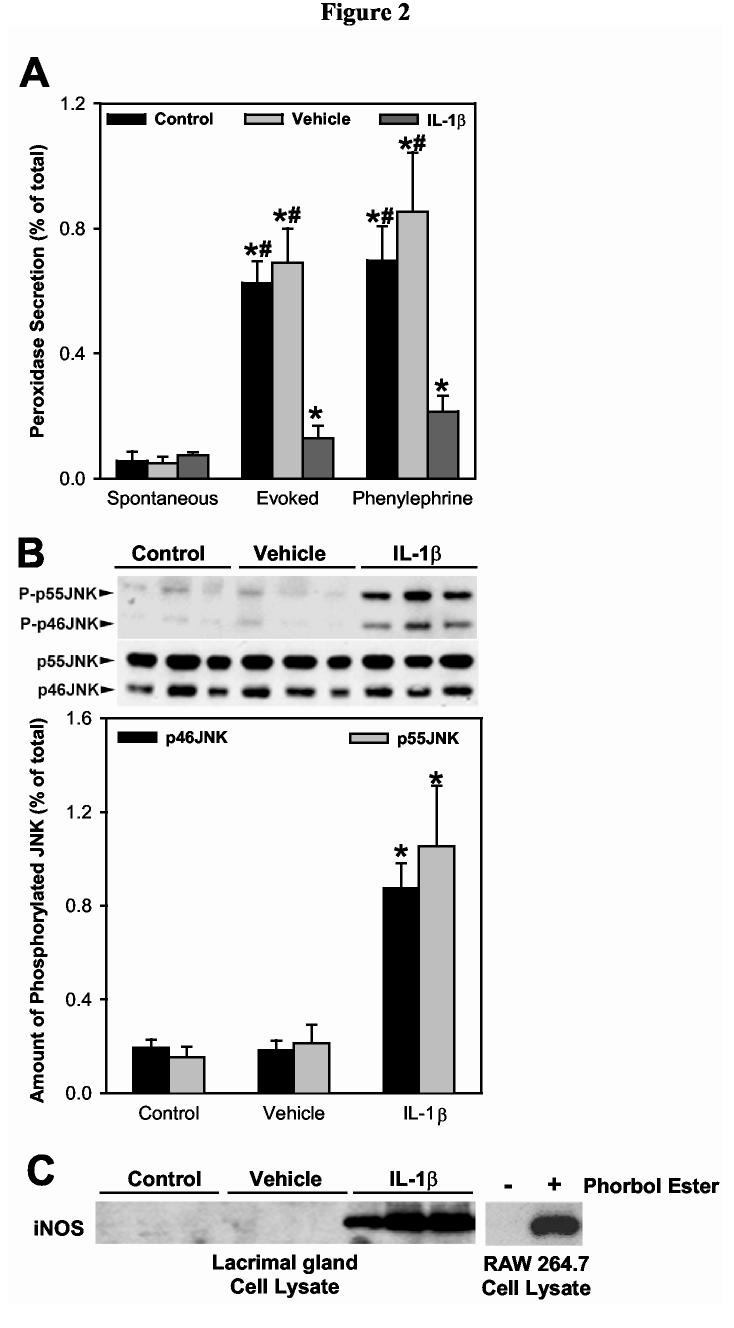
Lacrimal gland sections were stained with hematoxylin-eosin for histology examination, or with giemsa to stain for mast cells. Compared to saline, IL-1β injection induced a severe inflammatory response in the lacrimal gland which was manifested by an increase in the number of granulocytes and an increase in the number of degranulating mast cells (Figure 3A).
Figure 3.
Effect of IL-1β on lacrimal gland histology, JNK activation, and iNOS expression. Lacrimal glands from saline or IL-1β treated animals were fixed and processed for histology (A) or immunofluorescence (B). A. Lacrimal gland sections were stained with hematoxylin and eosin (H&E) or giemsa to stain for mast cells. Magnification: upper photographs 200X, lower photographs 400X. Similar results were obtained in at least 2 other experiments. B. Lacrimal gland sections were incubated in the absence (negative control) or presence of the specified antibodies followed by a FITC-conjugated antibody. Arrows indicate iNOS immunoreactivity associated with blood vessels. Magnification 400X. Similar results were obtained in at least 2 other experiments.
As shown in Figure 3B, phospho-JNK immunoreactivity was scarce in saline-injected lacrimal glands. In contrast, strong immunoreactivity for phospho-JNK was detected in IL-1β injected lacrimal glands (Figure 3B). Phospho-JNK immunoreactivity seems to be detected only in the infiltrating immune cells, although we need to perform double labeling experiments (with markers of immune cells) to confirm this conclusion. Figure 3B also shows that, whereas iNOS immunoreactivity was absent in saline-injected lacrimal glands, it was easily detectable in IL-1βinjected lacrimal glands. In contrast to phospho-JNK, iNOS immunoreactivity was associated with both the infiltrating immune cells as well as with the epithelial cells. Also, strong immunoreactivity was associated with the blood vessels in IL-1β injected lacrimal glands (Figure 3B, arrows). Omission of the primary antibodies resulted in a loss of immunoreactivity (Figure 3B, Control).
These results show that IL-1β induced a severe inflammatory response in the lacrimal gland that was manifested by an increase in the number of granulocytes and an increase in the number of degranulating mast cells. Concomitantly, IL-1β activated JNK in the infiltrating immune cells and induced the expression of iNOS by both the infiltrating cells as well as the lacrimal gland epithelial cells.
Effect of inhibiting JNK activity on lacrimal gland secretion
SP600125 is a newly described and relatively selective inhibitor of JNK (Han et al. 2001). In a first series of experiments, we determined the efficiency of this inhibitor, in vitro, on IL-1β induced JNK activity and phosphorylation of cJun (a cellular substrate of JNK). As shown in Figure 4, SP600125 inhibited, in concentration-dependent manner, IL-1β induced activation of JNK (Fig. 4A) and phosphorylation of cJun (Fig. 4B). Half maximal inhibition was achieved with 25 μM SP600125 whereas maximal inhibition was observed with a 100 μM SP600125. To investigate the specificity of SP600125 towards JNK, we tested its effect on IL-1β induced activation of p38 MAPK and ERK isoforms. As shown in Fig. 4C,D SP600125 did not have any effect, even at the highest concentration tested, on the activity of these enzymes in response to IL-1β.
Figure 4.
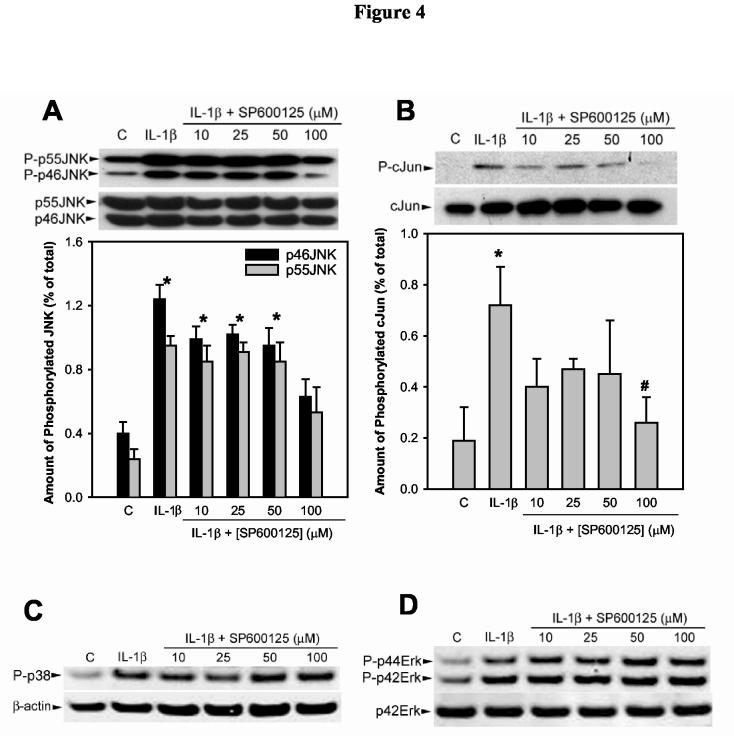
Effect of SP600125 on IL-1β induced JNK, p38, and Erk activation and phosphorylation of cJun.Lacrimal gland lobules were preincubated for 30 minutes in the absence or presence of increasing concentrations of SP600125. They were then incubated for 30 minutes in the absence (control, C) or presence of IL-1β (10 ng/ml). Proteins in the cell lysate were separated by SDS-PAGE followed by western blotting using antibodies against phosphorylated JNK or total JNK (A), phosphorylated cJun or total cJun (B), phosphorylated p38 or β-actin (C), phosphorylated ERK or p42ERK (D). Data in the plots are means ± SEM of three independent experiments. *Denotes statistically significant difference from control. #Denotes statistically significant difference from IL-1β. The blots shown in C and D are representative of data obtained in 2 other experiments.
In a second series of experiments, lightly anesthetized female BALB/c mice received an s.c. injection (100 μl) of vehicle (DMSO+PEG400) or SP600125 (0.5 mg). Twenty-four hours later, the mice received a second s.c. injection of SP600125 (or vehicle) followed by injection (2μl) of IL-1β (1 μg) or saline (vehicle for IL-1β) into the exorbital lacrimal glands. Twenty-four hours later, lacrimal glands were removed and lobules were prepared. One set of lobules was used to measure protein secretion and the other set was used for western blotting experiments. As shown in Figure 5A, IL-1β inhibited KCl- and phenylephrine-induced protein secretion by 85% and 66%, respectively. SP600125 alleviated the inhibitory effect of IL-1β on KCl- and phenylephrine-induced protein secretion by 79% and 47%, respectively (Fig. 5A).
Figure 5.
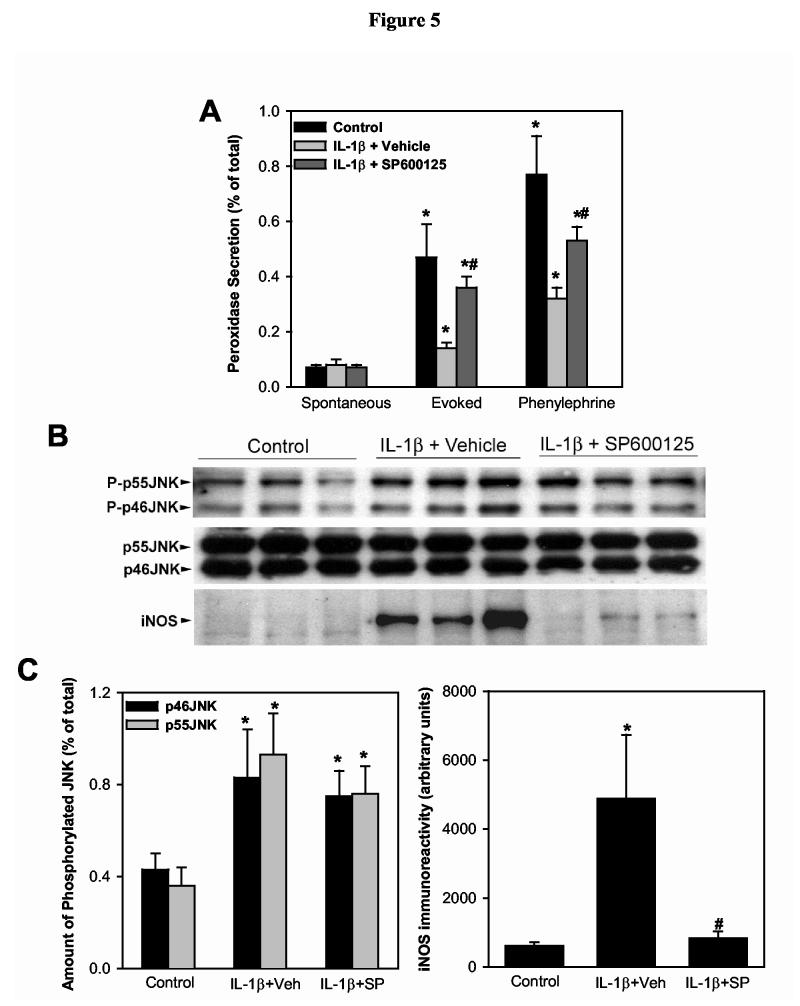
Effect of SP600125 on IL-1β induced inhibition of lacrimal gland secretion, JNK activation, and induction of iNOS expression.Animals were left untreated (control) or received s.c injection of vehicle (DMSO+PEG400) or SP600125 (0.5 mg). Twenty-four hours after injection of IL-1β (1 μg), the lacrimal glands were removed, lobules prepared and used to measure protein secretion (A) or for western blotting (B and C). A. Lobules were incubated for 20 minutes each in normal KRB buffer (spontaneous), in depolarizing KRB (evoked) solution where the concentration of KCl was increased to 75 mM, then in KRB containing phenylephrine (an α1-adrenergic agonist, 10-4 M). After incubation, the amount of peroxidase in the media and tissue homogenate was determined using Amplex Red, as described in the Methods section. Data are the means ± SEM from 3 independent experiments. *Denotes statistically significant difference from spontaneous. #Denotes statistically significant difference from IL-1β + vehicle.B and C. Lacrimal gland lobules were homogenized and the proteins separated by SDS-PAGE followed by western blotting using an antibody against phosphorylated JNK, a non-discriminatory antibody that recognizes the whole pools of JNK, or an antibody against iNOS. Each lane in the blots represents samples prepared from a separate animal. Data in C are expressed as means ± SEM (n = 3). *Denotes statistically significant difference from control.#Denotes statistically significant difference from IL-1β + vehicle.
Western blotting showed that although JNK activity was still higher in SP600125-injected mice compared to controls, the expression of iNOS was completely inhibited (Fig. 5B,C).
These results show that inhibition of JNK activity in vivo with SP600125 partially alleviated the inhibitory effect of IL-1β on lacrimal gland secretion and abolished iNOS expression.
Effect of inhibiting JNK activity on tear production
Female MRL/lpr mice spontaneously develop, in an age-dependent manner; an autoimmune disease characterized by lymphoproliferation, autoantibody formation, ocular inflammatory lesions, and lacrimal gland disease and has been widely used as a model for human Sjögren’s syndrome dry eye. MRL/lpr mice are congenic with MRL/+, which develop a later onset, milder autoimmune disease, including lacrimal gland inflammation. MRL/+ mice can be used as control for the MRL/lpr animals.
We have already shown that the amount of IL-1β is up-regulated in the lacrimal glands of these mice, in a disease specific manner. We also showed that the lacrimal gland epithelial cells produced IL-1β and expressed the signal-transducing receptor for IL-1, the IL-1 receptor type 1. In this study, female MRL/lpr and MRL/+ mice, were randomized into two groups to receive s.c injections of either vehicle (PEG400+DMSO) or SP600125 (0.5 mg). Tear production was measured at baseline and seven days after daily treatment with vehicle or SP600125.
As shown in Figure 6, at baseline, tear production in MRL/lpr mice assigned to receive vehicle or SP600125, was 28% and 34% that of control MRL/+ mice, respectively. Tear production in the SP600125-treated animals significantly increased from 2.8 ± 0.5 mm to 6.7 ± 1.8 mm (p=0.015). In contrast, tear production in vehicle treated animals did not increase (1.9 ± 0.3 mm versus 2.3 ± 0.3 mm, Figure 6).
Figure 6.
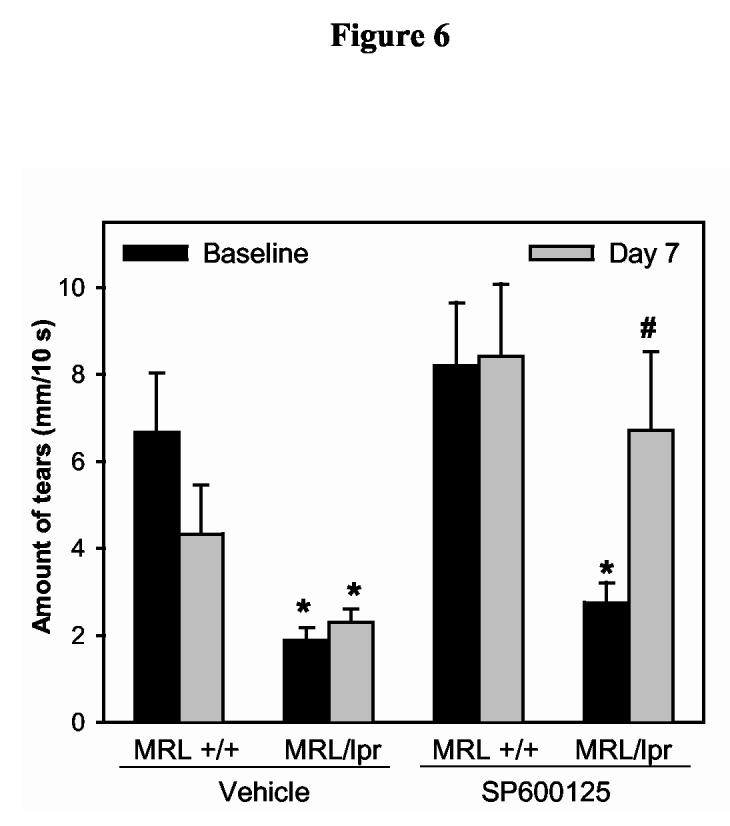
Effect of SP600125 on tear production in a murine model of Sjögren’s syndrome dry eye.Female MRL/lpr (diseased) and MRL/+ mice (control) were randomized into two groups to receive s.c. injections of either vehicle (PEG400+DMSO) or SP600125 (0.5 mg). Tear production was measured with phenol red impregnated cotton threads for 10 seconds at baseline and seven days after daily treatment with vehicle or SP600125. Data are means ± SEM m (n = 6 animals in each group). *Denotes statistically significant difference from MRL +/+. #Denotes statistically significant difference from MRL/lpr treated with vehicle and MRL/lpr at baseline.
These results show that inhibition of JNK with SP600125 increased tear production in a murine model of human Sjögren’s syndrome dry eye.
Discussion
In the present study we showed that concomitant with inhibition of lacrimal gland secretion, IL-1β activated JNK and induced the expression of iNOS. Activation of JNK occurred both in vitro and in vivo and was blocked by a relatively specific inhibitor, SP600125 (Han et al. 2001). SP600125 did not inhibit, at the concentrations tested, the activation of p38 MAPK or ERK by IL-1β. Administration of this inhibitor in vivo alleviated the inhibitory effect of IL-1β on lacrimal gland secretion, completely abolished iNOS expression, and increased tear production in a murine model of human Sjögren’s syndrome dry eye.
Proinflammatory cytokine mediated inhibition of neurotransmitter release is now well documented. For example, several studies have shown that IL-1β, IL-6, and tumor necrosis factor α (TNFα) inhibit acetylcholine and norepinephrine release from myenteric nerves (Hurst and Collins 1993; Main et al. 1993; Ruhl et al. 1994). In a rat model of acute colitis, an inflammatory disease of distal colon, IL-1β was implicated in blocking KCl-induced norepinephrine release from the myenteric plexus (Jacobson et al. 1997). In the hippocampus, IL-1β was shown to inhibit glutamate and norepinephrine release and to decrease the acetylcholine content (Rada et al. 1991; Lynch 1998). TNFα was also shown to alter neurotransmitter release in cultured sympathetic neurons (Soliven and Albert 1992). A recent study reported that IL-1β inhibits acetylcholine release in the esophagus (Cao et al. 2004). Our own studies showed that IL-1β and TNFα inhibit neurotransmitter release in the lacrimal gland resulting in impaired protein secretion and dry eye (Zoukhri et al. 2002).
JNK proteins are widely expressed in mammalian cells and are encoded by three genes JNK1, JNK2, and JNK3 (Davis 2000). These gene are alternatively spliced to create at least ten JNK isoforms.(Paul et al. 1997). Increased JNK activity has been associated with several pathological conditions. In RA, using both genetic as well as pharmacological approaches, it was shown that inhibition of JNK activity ameliorated joint damage induced by IL-1β (Han et al. 2001). Similarly, it was shown that JNK is involved in IL-1β induced apoptosis of pancreatic beta-cells and inhibition of JNK blocked beta-cell death (Ammendrup et al. 2000; Bonny et al. 2001). In the rat hippocampus, IL-1β inhibits long term potentiation by inhibiting glutamate release and both effects were shown to be largely mediated through activation of JNK (Vereker et al. 2000; Curran et al. 2003). Based on these studies, we hypothesize that increased expression of IL-1β, through activation of JNK, inhibits neurotransmitter release causing impaired tear secretion in inflamed lacrimal glands.
There are three well characterized isoforms of NOS expressed by mammalian cells: neuronal NOS (nNOS also known as NOS1), inducible NOS (iNOS or NOS2), and endothelial NOS (eNOS or NOS3) (Nathan 1997; Kubes and McCafferty 2000). While the nNOS and eNOS are constitutively expressed, the expression of iNOS requires protein synthesis. Furthermore, and in contrast to nNOS and eNOS, iNOS activity is not dependent on changes in intracellular Ca2+ levels and is therefore able to produce large amounts of NO for extended periods of time (lasting for days), far exceeding the levels generated by the other isoforms (Kubes and McCafferty 2000).
NO is a short-lived molecule capable of diffusing across cellular membranes and reacting with a variety of targets. In the presence of superoxide (O2-), NO reacts extremely rapidly to produce the highly reactive and toxic peroxynitrite (ONOO-) (Kubes and McCafferty 2000). NO produced by iNOS has been implicated in numerous inflammatory diseases and shown to be involved in tissue destruction, inhibition of hormones and neurotransmitters release, and inhibiting various cellular processes (Konttinen et al. 1997; McDaniel et al. 1997; Al-Mufti et al. 1998; Kubes and McCafferty 2000; van’t Hof et al. 2000; Heneka and Feinstein 2001; Kankuri et al. 2001).
Our results suggest that NO might be implicated in IL-1β mediated inhibition of lacrimal gland secretion since SP600125 completely abolished iNOS expression and restored tear secretion. A recent study showed that SP600125 reduces iNOS expression in activated macrophages by destabilizing its mRNA (Lahti et al. 2003). Furthermore, it is well documented that JNK through activation of the transcription factors AP-1 and NF-kB regulates the expression of iNOS (Ichijo 1999; Heneka and Feinstein 2001). Although further experiments are needed, it is likely that JNK mediates IL-1β induced expression of iNOS in the lacrimal gland which leads to inhibition of neurally- as well as agonist-induced protein secretion.
Acknowledgements
The authors gratefully acknowledge and appreciate Robin Hodges and Barbara Talamo for critical reading of the manuscript, Dr. Craig Reynolds for the generous gift of recombinant human cytokines, Dr. Brydon Bennett for the generous gift of SP600125, and Dr. Fara Sourie for her invaluable contribution to this work. This study was supported by National Eye Institute grant RO1-EY12383.
Footnotes
- IL-1β
- interleukin-1β
- JNK
- cJun NH2-terminal kinase
- ERK
- extracellular signal regulated kinase
- NO
- nitric oxide
- iNOS
- inducible nitric oxide synthase
- KRB
- Krebs-Ringer bicarbonate buffer
- PBS
- phosphate buffered saline
- SDS-PAGE
- sodium dodecyl sulfate polyacrylamide gel electrophoresis.
References
- Al-Mufti RA, Williamson RC, Mathie RT. Increased nitric oxide activity in a rat model of acute pancreatitis. Gut. 1998;43:564–570. doi: 10.1136/gut.43.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammendrup A, Maillard A, Nielsen K, Aabenhus Andersen N., Serup P, Dragsbaek Madsen O., Mandrup-Poulsen T, Bonny C. The c-Jun amino-terminal kinase pathway is preferentially activated by interleukin-1 and controls apoptosis in differentiating pancreatic beta-cells. Diabetes. 2000;49:1468–1476. doi: 10.2337/diabetes.49.9.1468. [DOI] [PubMed] [Google Scholar]
- Bonny C, Oberson A, Negri S, Sauser C, Schorderet DF. Cell-permeable peptide inhibitors of JNK: novel blockers of beta-cell death. Diabetes. 2001;50:77–82. doi: 10.2337/diabetes.50.1.77. [DOI] [PubMed] [Google Scholar]
- Botelho SY, Hisada M, Fuenmayo N. Functional innervation of the lacrimal gland in the cat. Arch. Ophthalmol. 1966;76:581–588. doi: 10.1001/archopht.1966.03850010583019. [DOI] [PubMed] [Google Scholar]
- Cao W, Cheng L, Behar J, Fiocchi C, Biancani P, Harnett KM. Proinflammatory cytokines alter/reduce esophageal circular muscle contraction in experimental cat esophagitis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1131–1139. doi: 10.1152/ajpgi.00216.2004. [DOI] [PubMed] [Google Scholar]
- Curran BP, Murray HJ, O’Connor JJ. A role for c-Jun N-terminal kinase in the inhibition of long-term potentiation by interleukin-1beta and long-term depression in the rat dentate gyrus in vitro. Neuroscience. 2003;118:347–357. doi: 10.1016/s0306-4522(02)00941-7. [DOI] [PubMed] [Google Scholar]
- Dartt DA. Regulation of tear secretion. Adv Exp Med Biol. 1994;350:1–9. doi: 10.1007/978-1-4615-2417-5_1. [DOI] [PubMed] [Google Scholar]
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- DeCarlo DK, Penner SL, Schamerloh RJ, Fullard RJ. Dry eye among males infected with the human immunodeficiency virus. J Am Optom Assoc. 1995;66:533–538. [PubMed] [Google Scholar]
- Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2174. [PubMed] [Google Scholar]
- Dinarello CA. Proinflammatory cytokines. Chest. 2000;118:503–508. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- Drosos AA, Voulgari PV, Psychos DN, Tsifetaki N, Bai M. Sicca syndrome in patients with sarcoidosis. Rheumatol Int. 1999;18:177–180. doi: 10.1007/s002960050081. [DOI] [PubMed] [Google Scholar]
- Fox RI, Törnwall J, Michelson P. Current issues in the diagnosis and treatment of Sögren’s syndrome. Curr. Opin. Rheumatol. 1999;11:364–371. doi: 10.1097/00002281-199909000-00007. [DOI] [PubMed] [Google Scholar]
- Gilbard JP, Farris RL. Ocular surface drying and tear film osmolarity in thyroid eye disease. Acta Ophthalmol (Copenh) 1983;61:108–116. doi: 10.1111/j.1755-3768.1983.tb01401.x. [DOI] [PubMed] [Google Scholar]
- Grus F, Sabuncuo P, Dick H, Augustin A, Pfeiffer N. Changes in the tear proteins of diabetic patients. BMC Ophthalmology. 2002;2:4. doi: 10.1186/1471-2415-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z, Boyle DL, Chang L, Bennett B, Karin M, Yang L, Manning AM, Firestein GS. c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J. Clin. Invest. 2001;108:73–81. doi: 10.1172/JCI12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Feinstein DL. Expression and function of inducible nitric oxide synthase in neurons. J Neuroimmunol. 2001;114:8–18. doi: 10.1016/s0165-5728(01)00246-6. [DOI] [PubMed] [Google Scholar]
- Hodges RR, Dartt DA. Regulatory pathways in lacrimal gland epithelium. Int Rev Cytol. 2003;231:129–196. doi: 10.1016/s0074-7696(03)31004-6. [DOI] [PubMed] [Google Scholar]
- Hurst S, Collins SM. Interleukin-1β modulation of norepinephrine release from rat myenteric nerves. Am. J. Physiol. 1993;264:G30–G35. doi: 10.1152/ajpgi.1993.264.1.G30. [DOI] [PubMed] [Google Scholar]
- Ichijo H. From receptors to stress-activated MAP kinases. Oncogene. 1999;18:6087–6093. doi: 10.1038/sj.onc.1203129. [DOI] [PubMed] [Google Scholar]
- Jacobson K, McHugh K, S.M. C. The mechanism of altered neural function in a rat model of acute colitis. Gastroenterology. 1997;112:156–162. doi: 10.1016/s0016-5085(97)70230-0. [DOI] [PubMed] [Google Scholar]
- Kankuri E, Vaali K, Knowles RG, Lahde M, Korpela R, Vapaatalo H, Moilanen E. Suppression of acute experimental colitis by a highly selective inducible nitric-oxide synthase inhibitor, N-[3-(Aminomethyl)benzyl]acetamidine. J Pharmacol Exp Ther. 2001;298:1128–1132. [PubMed] [Google Scholar]
- Konttinen YT, Platts LA, Tuominen S, Eklund KK, Santavirta N, Tornwall J, Sorsa T, Hukkanen M, Polak JM. Role of nitric oxide in Sjogren’s syndrome. Arthritis Rheum. 1997;40:875–883. doi: 10.1002/art.1780400515. [DOI] [PubMed] [Google Scholar]
- Kubes P, McCafferty DM. Nitric oxide and intestinal inflammation. Am J Med. 2000;109:150–158. doi: 10.1016/s0002-9343(00)00480-0. [DOI] [PubMed] [Google Scholar]
- Lahti A, Jalonen U, Kankaanranta H, Moilanen E. c-Jun NH2-terminal kinase inhibitor anthra(1,9-cd)pyrazol-6(2H)-one reduces inducible nitric-oxide synthase expression by destabilizing mRNA in activated macrophages. Mol Pharmacol. 2003;64:315–308. doi: 10.1124/mol.64.2.308. [DOI] [PubMed] [Google Scholar]
- Lynch MA. Analysis of the mechanisms underlying the age-related impairment in long-term potentiation in the rat. Rev Neurosci. 1998;9:169–201. doi: 10.1515/revneuro.1998.9.3.169. [DOI] [PubMed] [Google Scholar]
- Main C, Blennerhassett PA, Collins SM. Human recombinant interleukin 1 beta suppresses acetylcholine release from rat myenteric plexus. Gastroenterology. 1993;104:1648–1654. doi: 10.1016/0016-5085(93)90641-o. [DOI] [PubMed] [Google Scholar]
- McDaniel ML, Corbett JA, Kwon G, Hill JR. A role for nitric oxide and other inflammatory mediators in cytokine-induced pancreatic beta-cell dysfunction and destruction. Adv Exp Med Biol. 1997;426:313–319. doi: 10.1007/978-1-4899-1819-2_41. [DOI] [PubMed] [Google Scholar]
- Nathan C. Inducible nitric oxide synthase: what difference does it make? J. Clin. Invest. 1997;100:2417–2423. doi: 10.1172/JCI119782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y, Kuwana M. Dry eye as a major complication associated with chronic graft-versus-host disease after hematopoietic stem cell transplantation. Cornea. 2003;22:S19–27. doi: 10.1097/00003226-200310001-00004. [DOI] [PubMed] [Google Scholar]
- Paul A, Wilson S, Belham CM, Robinson CJ, Scott PH, Gould GW, Plevin R. Stress-activated protein kinases: activation, regulation and function. Cell Signal. 1997;9:403–410. doi: 10.1016/s0898-6568(97)00042-9. [DOI] [PubMed] [Google Scholar]
- Punzi L, Betterle C. Chronic autoimmune thyroiditis and rheumatic manifestations. Joint Bone Spine. 2004;71:275–283. doi: 10.1016/j.jbspin.2003.06.005. [DOI] [PubMed] [Google Scholar]
- Rada P, Mark GP, Vitek MP, Mangano RM, Blume AJ, Beer B, Hoebel BG. Interleukin-1 beta decreases acetylcholine measured by microdialysis in the hippocampus of freely moving rats. Brain Res. 1991;550:287–290. doi: 10.1016/0006-8993(91)91330-4. [DOI] [PubMed] [Google Scholar]
- Rios JD, Horikawa Y, Chen LL, Kublin CL, Hodges RR, Dartt DA, Zoukhri D. Age-dependent alterations in mouse exorbital lacrimal gland structure, innervation and secretory response. Exp Eye Res. 2005;80:477–491. doi: 10.1016/j.exer.2004.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhl A, Hurst S, Collins SM. Synergism between interleukin 1 beta and 6 on noradrenergic nerves in rat myenteric plexus. Gastroenterology. 1994;107:993–1001. doi: 10.1016/0016-5085(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Schaumberg DA, Sullivan DA, Dana MR. Epidemiology of dry eye syndrome. Adv Exp Med Biol. 2002;506:989–998. doi: 10.1007/978-1-4615-0717-8_140. [DOI] [PubMed] [Google Scholar]
- Soliven B, Albert J. Tumour necrosis factor modulates the inactivation of catecholamine secretion in cultured sympathetic neurons. J. Neurochem. 1992;58:1073–1078. doi: 10.1111/j.1471-4159.1992.tb09364.x. [DOI] [PubMed] [Google Scholar]
- van’t Hof RJ, Armour KJ, Smith LM, Armour KE, Wei XQ, Liew FY, Ralston SH. Requirement of the inducible nitric oxide synthase pathway for IL-1-induced osteoclastic bone resorption. PNAS. 2000;97:7993–7998. doi: 10.1073/pnas.130511497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereker E, O’Donnell E, Lynch MA. The inhibitory effect of interleukin-1β on long-term potentiation is coupled with increased activity of stress-activated protein kinases. J Neurosci. 2000;20:6811–6819. doi: 10.1523/JNEUROSCI.20-18-06811.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegans ME, Anninger W, Chapman C, Gordon SR. Ocular manifestations of hepatitis C virus infection. Curr Opin Ophthalmol. 2002;13:423–427. doi: 10.1097/00055735-200212000-00014. [DOI] [PubMed] [Google Scholar]
- Zoukhri D, Kublin CL. Impaired neurotransmitter release from lacrimal and salivary gland nerves of a murine model of Sjögren’s syndrome. Invest Ophthalmol Vis Sci. 2001;42:925–932. [PMC free article] [PubMed] [Google Scholar]
- Zoukhri D, Hodges R, Byon B, Kublin L. Role of proinflammatory cytokines in the impaired lacrimation associated with autoimmune xerophthalmia. Invest Ophthalmol Vis Sci. 2002;43:1429–1436. [PMC free article] [PubMed] [Google Scholar]